Research Article
Clear Cell Variant of Follicular Thyroid Carcinoma: A Case Report
5625
Views & Citations4625
Likes & Shares
Background: Clear cell variant of follicular thyroid carcinoma is not frequently found. A few cases are described in the literature, making it difficult to characterize and predict their clinical course.
Clinical case: We describe the case of a 73-year-old man who presented with a 2-month duration dysphonia in the presence of a thyroid nodule. Cytology was compatible with thyroid carcinoma, which was then treated with surgery and radio ablation. The anatomopathological study reported a clear-cell variant of follicular thyroid carcinoma.
Conclusion: Clear cell variant of follicular thyroid carcinoma is exceptionally infrequent. Studies that analyze its biological behavior are lacking, which could determine changes in treatment and subsequent follow up.
Keywords: Clear cell variant, Follicular thyroid cancer, Prognosis
INTRODUCTION
Follicular thyroid carcinoma (FTC) is a malignant tumor derived from the thyroid gland's follicular epithelium, belonging to the group of differentiated cancers. It is the second most frequent thyroid cancer, comprising 12% in iodine-sufficient areas. Uruguay is considered within this percentage, according to the study by Servetto [1]. It is three times more frequent in women, with a peak of presentation between 40-60 years old. Risk factors include head and neck radiation exposure during childhood, thyroid cancer history in a first-degree relative, familial syndrome linked to thyroid cancer (Cowden, Carney Complex Type I, Werner Syndrome) [2-5]. From a histological point of view, they can be classified as well-differentiated carcinoma, characterized by the presence of cuboid cells forming follicles and the presence of colloid, to poorly differentiated, with solid growth pattern, absence of follicles, and marked cell atypia [2]. According to the 2017- World Health Organization Classification of Endocrine Organ Tumors, three main types of FTC (ICD-10: C73) of prognostic importance are established, depending on the intra-extrathyroidal involvement and number of compromised blood vessels: 1) minimally invasive, 2) angioinvasive encapsulated FTC, and 3) extensively invasive. There are less frequent histological variants of FTC, such as ‘signet ring’, glomeruloid pattern, spindle cells, and clear cells. Given the low frequency and the extremely low number of FTC cases described in the literature, their biological behavior is still subject to debate, as well as the need for a therapeutic approach different from that of classical variants of differentiated thyroid cancer [2].We will now describe the case of a patient with a clear-cell variant of follicular thyroid carcinoma, which is very rare, highlighting its significant locoregional extension at diagnosis.
CLINICAL CASE
Symptomatology
A 73-year-old male patient presents with 2-months duration dysphonia, without other elements of locoregional involvement or symptoms of thyroid dysfunction.
Physical exam
During palpation of the neck, a left firm, elastic 3 cm thyroid lobe nodule was detected without delimitating its lower border because it invaded the supraclavicular fossa. The rest of the physical examination was normal.
Imaging
The neck ultrasound showed a 25 x 28 x 28 mm solid, hypoechoic with irregular and lobulated margins nodule occupying the two lower thirds of the left thyroid lobe.
Concerning the superficial sector of the left lobe, a serpiginous hypoechoic structure that made contact with the internal jugular vein was observed, without demonstrable blood flow with Doppler that might correspond to the thrombosed superior thyroid vein.
At sectors IIa and III of the left jugular-carotid chain, two lymphadenopathies of 13 x 11 mm and 33 x 16 x 10 mm width with similar echo structure to the described nodule, and loss of the fatty hilum, were visualized.
Cytology
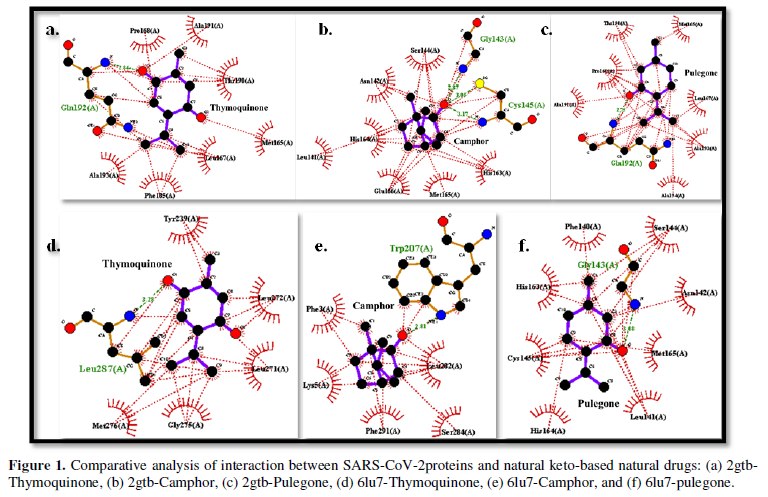
The cytological specimen obtained by fine-needle aspiration-puncture of the nodule and the adenopathy showed a cytogram of high cellularity consisting of atypical cells with marked anisokaryosis (some with a tendency to gigantism) with a significant degree of anaplasia, classified as Bethesda VI (Figure 1).
Lab work
The preoperative TSH value was 2.11 µIU/L (0.27-4.2) because of the aggressive presentation. Medullary thyroid carcinoma was ruled out by measuring blood calcitonin and CEA both within normal values.
Surgical treatment
In the event of high-risk differentiated thyroid carcinoma, a total thyroidectomy plus central and left lateral lymph node dissection was performed.
Anatomical pathology
A carcinoma of 52 mm in the long axis, with characteristics of a widely invasive clear-cell variant follicular carcinoma with poorly differentiated areas, was reported. An extrathyroidal extension involved the adipose tissue and perithyroidal striated muscles. It presents multiple lymphatic vascular embolisms in more than four blood vessels. Micro metastases were observed in 1 of 19 resected nodes in groups II, III, and IV (Figure 2).
Complementary treatment
One month after surgery, a remnant ablation with 180 mCi of radioactive iodine was performed. Post-dose whole-body scan (WBS) showed uptake in 3 remnants within the thyroid bed (one medial and two lateralized to the left of small size), without areas of abnormal uptake in the rest of the organism (Figure 3).
Follow-up
Post-surgical tumor markers thymoglobulin and anti-tg antibodies values were 215 ng/ml and less than 20 U/ml (Normal up to 40), respectively, both of which indicate poor prognosis.
DISCUSSION
Most FTCs are presented clinically as single nodules > 1 cm discovered incidentally by imaging studies (e.g., tomography) performed for non-thyroid pathology. They are generally asymptomatic at diagnosis since symptoms of locoregional involvement such as dysphagia and dyspnea are rare unless it is an extensively invasive FTC [2,6]. Given the clinical presentation at diagnosis, more aggressive variants of thyroid carcinoma (medullary, undifferentiated) were initially considered. Those were ruled out by normal preoperative calcitonin levels and anatomopathological study. Lymph node involvement occurs less frequently in FTC than papillary thyroid carcinoma (13% vs. 50%), and the presence of hematogenous metastases (lung, bone) reaches 10-15% in the case of tumors ≥ 2 cm [2,7]. Within the FTC group, the clear-cells variant (scarce) is characterized by cells with clear or vacuolated cytoplasm, enlarged mitochondria, and endoplasmic reticulum, a centered chromatin-rich nucleus, and a small nucleolus. These cells present morphological similarity with metastatic cells of renal and parathyroid cancer. The immunohistochemical study with positive thyroglobulin is vital, which distinguishes CFT with high sensitivity and specificity [8,9]. As limitations, it should be noted that immunohistochemistry of the resected specimen was not performed. Still, renal pathology and parathyroid tumors were ruled out by urinary tract ultrasound and intraoperative exploration, respectively. Regarding the natural history of this type of tumors, given its low frequency and few reports in the literature, there is no established consensus on whether its behavior and clinical evolution differ from the classic variants of follicular carcinoma [2,8].
CONCLUSION
The clear-cell variant of follicular thyroid carcinoma is highly infrequent. Studies that analyze these tumors' biological behavior are lacking, so treatment and follow-up guidelines other than classic FTC variants are not currently recommended.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
FUNDING INTERESTS
The authors have not received any funding for the present manuscript.
- Servetto C, Rosas B, Da Rosa L, Bidegain E, López E, et al. (2017) Ioduria survey as a control of iodine intake. Acta Bioquímica Clín Latinoam 51(4): 575-579. Available online at: https://www.redalyc.org/pdf/535/53554497002.pdf
- Tuttle M (2020) Follicular cancer (including Hürthle cell cancer). In J Mulder (Ed.). Available online at: https://www.uptodate.com/contents/follicular-thyroid-cancer-including-hurthle-cell-cancer?search=follicular-thyroid-cancer-including-hurthle-cell%20cancer&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Ngeow J, Mester J, Rybicki LA, Ni Y, Milas M, Eng C. Incidence and clinical characteristics of thyroid cancer in prospective series of individuals with Cowden and Cowden-like syndrome characterized by germline PTEN, SDH, or KLLN alterations. J Clin Endocrinol Metab 2011; 96(12):2063-2071.
- Carney JA, Lyssikatos C, Seethala RR, Lakatos P, Perez-Atayde A, et al. (2018) The Spectrum of Thyroid Gland Pathology in Carney Complex. The importance of follicular carcinoma. Am J Surg Pathol 42(5): 587-594.
- Oshima J, Sidorova JM, Monnat RJ (2017) Werner syndrome: Clinical features, pathogenesis and potential therapeutic interventions. Ageing Res Rev 33(206): 105-114.
- Sayar I, Peter K, Gelinek I, Demirtas L, Isik A (2014) Clear cell variant of follicular thyroid carcinoma with normal thyroid-stimulating hormone value: A case report. J Med Case Rep 8(1): 2-4.
- Tuttle M (2020) Papillary thyroid cancer. In J. Mulder (Ed.). Available online at: https://www.uptodate.com/contents/papillary-thyroid-cancer-clinical-features-and-prognosis?search=cancer%20thyroid&source=searchresult&selectedTitle=2~150&usagetype=default&displayrank=2
- Schröder S, Böcker W (1986) Clear-cell carcinomas of thyroid gland: A clinicopathological study of 13 cases. Histopathology 10(1): 75-89.
- Segal K, Har-el G, Avidor I, Shvero J, Lubin E, et al. (1986) Clear cell Carcinoma of the thyroid gland. Head Neck Surg 8: 313-319.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Dermatology Clinics and Research (ISSN:2380-5609)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)





