Research Article
Assessment of ABO System Antibodies Title in Patients Submitted to Bariatric Surgery
4164
Views & Citations3164
Likes & Shares
Introduction: The bariatric surgery has been utilised by individuals who wish to lose weight as fast as possible. It is known that there is an alteration of the gut microbiota after the surgery, and that it helps on weight loss, for example. The gut microbiota is responsible for the production of the ABO system’s antibodies. ABO minor incompatibility arises when transfuse platelet concentrate having ABO incompatible antibodies with antigen receptor. These reactions are the result of abnormally high levels of anti-ABO antibodies thus generating hemolysis. The objective of the study was to evaluate the discrepancy when performed phenotyping ABO.
Methodology: The study consisted of 18 patients undergoing bariatric surgery and 18 control subjects aged 18 to 65 years, the were held phenotyping ABO, titration of the ABO system antibodies and irregular antibody screening.
Results: There was significant decrease in antibody titer for anti-A in blood group “O” and “B” (p = 0.0022) between the bariatric group and control group. Just as there was a significant difference (p = 0.0019) with the anti-B title of individuals "A" between the groups. There was not any discrepancy when done phenotyping ABO but noted a trend in the reduction of anti-ABO securities bariatric individuals.
Conclusion: There was a decrease in the antibody titer for anti-A in titer of anti-B without generating ABO classification discrepancies. However, bariatric group are potential donor platelet apheresis so that it can thus prevent haemolytic reactions due to ABO incompatibility.
Keywords: ABO Blood-Group system, Transfusion reaction, Bariatric surgery, Antibodies
INTRODUCTION
Bariatric surgery is being used by individuals who wish to lose weight more quickly. This surgery is classified according to its technique in restrictive, whose surgery is limited only in procedures in the gastric cavity. Another technique used is the disabsorptive ones, in which it interferes in the digestion, because in it there is a deviation of the intestine reducing its area of absorption. However, there is the mixed classification, in which it is the combination of both the Restrictive and the Disabsortive techniques [1,2].
Bariatric surgery is being used by individuals who wish to lose weight more quickly. This surgery is classified according to its technique in restrictive, whose surgery is limited only in procedures in the gastric cavity. Another technique used is the disabsorptive ones, in which it interferes in the digestion, because in it there is a deviation of the intestine reducing its area of absorption. However, there is the mixed classification, in which it is the combination of both the Restrictive and the Disabsortive techniques [1,2].
Currently the technique that has been most used is mixed, called gastric bypass with Roux-en-Y, because it restricts the gastric cavity and reduces the intestinal surface in contact with food [3]. In this technique, a part of the gastric cavity (about 50 mL) with the ileum, leaving the rest out of food traffic [1].
Changes in digestion and nutrient absorption are important aspects for weight loss. However, these have consequences for the patients, such as anemia, hypoalbuminemia, positive occult blood, presence of reducing substances and fat in feces [4].
In previous studies in which gastric bypass surgery in rats was performed, it was possible to analyze that there was a change in intestinal microbiota after surgery due to intestinal reconfiguration, weight change, diet and intestinal transit. It was possible to notice a significant change one week after surgery [5].
The term “microbiota” refers to a community of living microorganisms in a particular ecological niche [6]. The gastrointestinal tract houses the largest and most diverse species of bacteria colonizing the human body that coexist in a balance in their host. To maintain this balance depends on physiological factors such as mucus, intestinal peristalsis, rate of epithelium exchange, pH, endogenous enzymatic activity [7].
The intestinal microbiota is mainly composed of bacteria of the genus Lactobacillus and Bifidobacteruim, which contribute significantly to the health of its host through its functions: antimicrobial, metabolic/nutritional, protective and immunological since the bacteria provoke a continuous response of the immune system and constitutes an important component of this.
However, the intestinal microbiota is the main responsible for the passive production of antibodies of the ABO system, because the bacteria present in their cell membranes carbohydrates similar to the immunodominant sugars of the antigens A and B [8-10].
The development of antibodies to the ABO blood system can occur in two ways: natural and/or immune [9,11]. Natural anti-ABO antibodies begin to be produced around 3 and 6 months of life with their maximum production occurring at 5 to 10 years and from 65 years the antibody titre begins to decrease. Other substances such as dust, pollen and food also stimulate the production of antibodies Anti-A, Anti-B and/or Anti-AB [8,11].
Such regular antibodies may be of the IgM and IgG classes. Anti-A and Anti-B antibodies are IgM, which react best at room temperature. Anti-AB antibodies are IgG and they react better at 37°C and are able to cross the placental barrier [8,9,11,12].
Immune antibodies, however, are produced through alloimmunization that may occur through gestation or incompatible ABO blood transfusions, or through heteroimmunization of substances of animal or bacterial origin, such as antidifiteracy or anti-tetanus sera [8,9,11].
The determination of the ABO phenotype is performed in two steps: direct test and reverse test. The former uses monoclonal sera A and B to determine the presence or absence of ABO erythrocyte antigens of the subject. On the reverse side, antigen A and B are used to detect the presence or absence of anti-ABO antibodies in the subject's plasma. ABO phenotyping should be performed in all donors as well as at blood recipients [13].
The ABO phenotype is the test often performed in blood banks and there is always a reciprocal relationship between direct and reverse testing. In this way, it serves as a control over the other, since in case there are discrepancies between the expected results of both tests it is not possible to determine the ABO phenotyping of the individual. In these situations of discrepancies, the ABO classification of the individual is called ABO Inconclusivo, until the discrepancy is solved [10,13,14].
Antibody titration of antigens from the ABO System allows the semi-quantification of anti-A and anti-B antibodies. Generally, antibody titration in clinical analysis laboratories has the objective of assisting in the diagnosis of humoral immunodeficiencies and monitoring of hemolytic disease of the fetus and newborn. In blood banks, titration is performed in laboratory routine in most hemotherapy services, as strategies for the prevention of immunohemolytic transfusion reactions and to evaluate the results of ABO-incompatible bone marrow transplants. As well as, anti-ABO antibody titration can be used to aid in the monitoring of solid organ transplant rejection and ABO-incompatible immune response [10].
Immediate transfusion reactions occur during or within 24 h after blood transfusion. Among them, acute haemolytic reaction, non-haemolytic febrile reaction, allergic reactions (mild, moderate, severe), volume overload, bacterial contamination, non-cardiogenic pulmonary edema, hypotensive reaction and nonimmune hemolysis [15].
Acute hemolytic reaction is the most feared in transfusion practice due to its severity and high mortality rate, with an incidence of 0.77 transfusion reactions per 100,000 transfusions. It occurs because of the donor ABO red blood cell transfusion that is incompatible with the ABO anti-blood receptor antibodies. In most cases it occurs mainly due to errors in the identification of patient samples [16].
These ABO incompatibility transfusion reactions may occur when there is no discrepancy in the determination of ABO Blood Typing. As well as, they may occur due to technical failures, weak ABO subgroups, alloimmunization, high anti-ABO antibody titers and others [14].
Caring for platelet transfusion should take into account not only the patient’s weight, but also the presence of high anti-ABO antibody titers in the blood component [13]. There is no consensus in the literature regarding the critical anti-ABO titre which should be used to avoid transfusion reactions with minor incompatibility. These reactions occur when the anti-ABO antibodies contained in the platelet hemocomponetes cause hemolysis of the red blood cell receptor [9,17,23].
Due to the relationship between the production of ABO antibodies and intestinal microbiota, this study aimed to evaluate whether patients who underwent bariatric surgery may present low ABO antibody titers to the point of causing an ABO typing discrepancy in these individuals.
METHODOLOGY
A descriptive cross-sectional survey was performed with 18 individuals undergoing bariatric surgery (Bariatric Group) and 18 individuals who did not undergo surgery, and this was called the Control Group. They were selection of the Bariatric Group and Control Group was through a personal interview after accepting the Informed Consent Form (TCLE) and a questionnaire for the research participation. The questionnaire presented questions such as date of birth, gender, whether or not the bariatric surgery was performed, the surgical technique performed and the year of surgery.
The inclusion criteria for the bariatric group were individuals who were aged between 18 and 65 years and who had undergone bariatric surgery. For the control group they should be aged between 18 and 65 years and who stated that they had not performed bariatric surgery. Individuals above 65 years of age with AB phenotyping, pregnant women or women who had an abortion in the last 12 months, and volunteers who presented the Irregular Antibody Test (EPI) with a Positive result could not be selected in the study.
The research was approved in an Ethics Committee with opinion 1,269,4344 mL of blood was collected in a tube with the anticoagulant Ethylenediaminetetraacetic Acid (EDTA), for ABO phenotyping, titration of anti-A and anti-B antibodies, and PAI. The ABO phenotype was performed according to the legislation in force [13].
Determination of the titer of Anti-A and Anti-B antibodies was done according to Judd et al. [18] by serial serum titration of the subjects. A dilution battery for anti-ABO antibodies of the IgM class was performed up to 1/128 at room temperature (RT), in which the titer was determined when the last tube showed a positive intensity of 1+ intensity of agglutination [18].
The Irregular Antibody Search was performed according to the legislation in force [13]. For the statistical analysis, the percentage for sex and ABO phenotypes was performed. The mean and standard deviation for age were taken. The frequency was determined by fashion for titration of anti-ABO antibodies. For these statistical analyzes the software was used using Excel® software. The D'Agostino test was used to evaluate normality between the age of the populations, since the abnormality between the individuals of the same group could influence the statistical results. The independent T-test was performed to evaluate the level of significance between the ABO system titles and a significant value of p
RESULTS
When analyzing the normality of the populations, both presented normal, and for the bariatric individuals the variation was of 27.93% among this group. The variation in the normality of non-bariatric individuals was 24.84%. In this way, it was observed that both populations were considered normal so that the parameters could be evaluated between the groups.
When the age between the groups was evaluated, there was a significant difference between the age of the two populations (Bariatric x Control) (p = <0.001).
Of the bariatric volunteers, 89.9% were female and 11.1% were male. In the control group, 77.8% were female and 22.2% were male. Regarding the ABO phenotype of the bariatric subjects, 50% presented the “A” phenotype, 22.2% to the "B" phenotype, 27.8% the “O” phenotype and no “AB” phenotype was found. In this way there were no individuals excluded from this phenotype.
However, among the control subjects, 36.8% were from phenotype “A”, 10.5% from “B” phenotype, 47.4% from “O” phenotype and 5.3% from "AB" phenotype, being excluded from the research. In both groups there was no positive EPI, so no sample was discarded without an ABO classification discrepancy.
The mean age of volunteers was 42 ± 12 years for bariatric volunteers and 25 ± 6 years for volunteers in the control group.
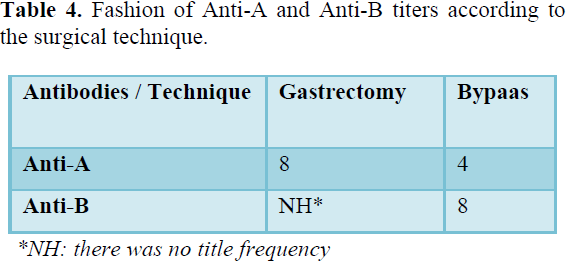
Regarding the surgical technique, 83.3% of the volunteers underwent gastric bypass, 11.1% underwent vertical gastrectomy and 5.6% did not respond.
Concerning anti-ABO antibody titers of bariatric volunteers with "O" phenotype the frequent anti-A titre was 8 and anti-B was 4. However, for phenotype A the usual anti-B titre was 8, in phenotype B the common anti-A titre was 2. Of all the phenotypes that had anti-A the frequent titre of antibodies was 4 and anti-B of 8 (Table 1). In the volunteers, the anti-A titre was 64 and anti-B was 8, in phenotype A the common title was 16, in phenotype B there was no frequent titre because only two individuals had this title phenotype and presented different titers. Among all ABO phenotypes in the control group, the usual anti-A titre was 64 and anti-B 16 (Table 2).
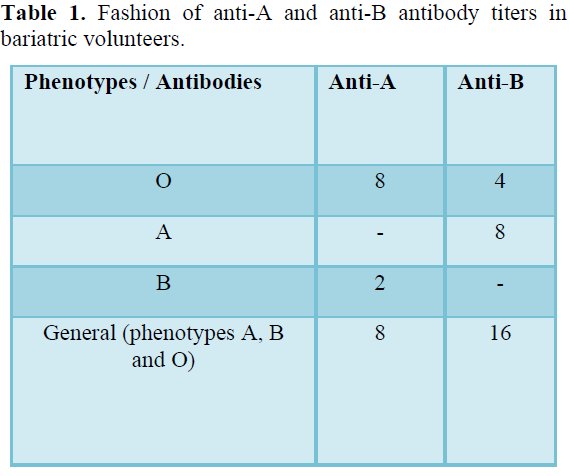
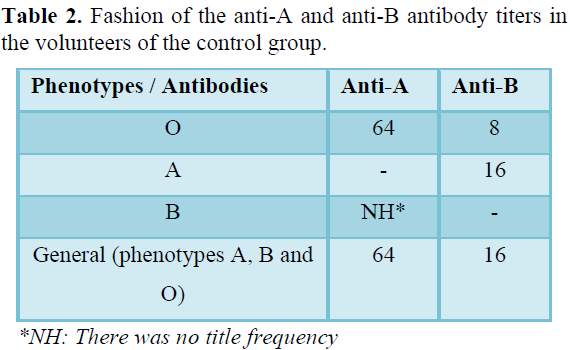


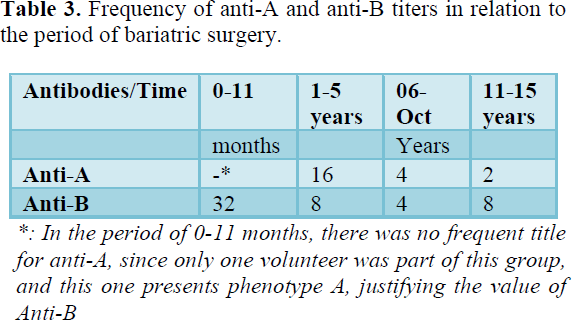
Table 3 shows the frequency of anti-A and anti-B titers in relation to the period of bariatric surgery and Table 4 shows the frequency of anti-A and anti-B titers in relation to the surgical technique performed.


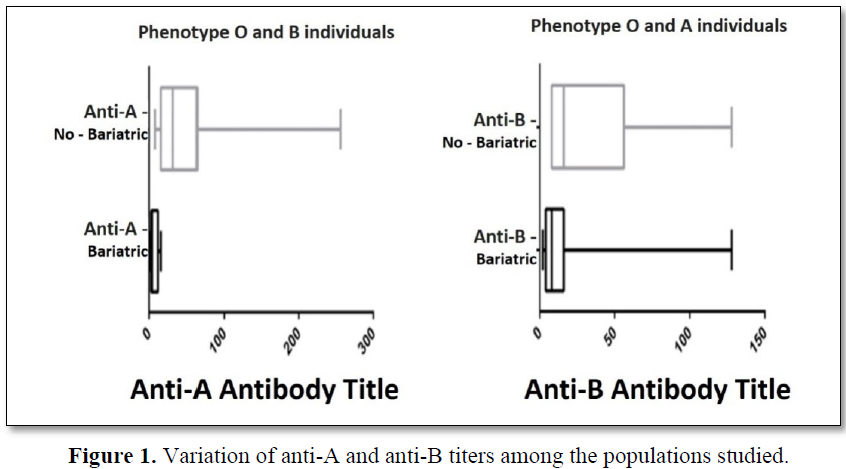
A significant difference between the titre of anti-A antibodies (Phenotype O and B) among the individuals studied was found (p = 0.0022). There was no significant difference between the titers of anti-B antibodies (Phenotype O and A) among the individuals studied (p = 0.5377) (Figure 1).
There was a significant difference between the titre of anti-B antibodies (Phenotype A) among the individuals studied (p = 0.0019).
For the titre of anti-A antibodies (Phenotype B) it was not possible to determine the difference between the groups, since the number of non-bariatric Group B subjects was lower than the bariatric group.
There was no significant difference between the titre of anti-A antibodies (Phenotype O) among the individuals studied (p = 0,2800).
There was no significant difference between the titers of anti-B antibodies (Phenotype O) among the individuals studied (p = 0.0993).

DISCUSSION
In the studies of Joia-Neto et al. (2010), Valezi and Machado (2011), Marchesine and Nicareta (2014) and Silva et al. (2014), it was observed that the majority of the persons undergoing bariatric surgery were female, 63.7%, 77.3%, 72.1% and 81.4% of the population, respectively.2,4,19,20 study was also evidenced a predominance of the female sex being 89.9% of the population.
The mean age in this study was 42 ± 12 years, which is related to other studies such as that of Marchesine (2014), in which the mean age was 41.2 years, in the study by Joia-Neto (2010), whose mean was of 41.13 ± 9.22 years and Valezi (2014) in whom the mean was 35.9 ± 12.2 years. [4,19]
Novaretti et al. (2000) studied the blood groups in Caucasoid and Negroid blood donors in the city of São Paulo and observed the frequency of phenotypes of the ABO System, with phenotype A being 46.52%, phenotype A 39 , 45%, phenotype B 11.51% and phenotype AB 2.52% .20 Baiochi et al. (2007) also observed this frequency of the phenotypes and in the O phenotype the frequency was 50.67%, phenotype A was 32 , 17%, phenotype B was 13.45 and phenotype AB was 3.71% .[22]
This frequency was observed in the control group, in which a frequency of 36.8% of the "A" phenotype, 10.5% of the "B" phenotype, 47.4% to the "O" phenotype and 5, 3% to the "AB" phenotype and was different in the bariatric population, 50% of which were of the "A" phenotype, 22.2% to the "B" phenotype, 27.8% to the "O" phenotype and none belonging to the phenotype "AB". It may be justified because the sample number is small.
In the study by Geraldo (2016) the frequency of anti-A and anti-B was observed in the studied population and it was observed that the frequency of TA in both anti-A and anti-B was 32.23 Already France 2011) compared antibody titers according to sex and age, with similar titers in both sexes, and that the titer of both anti-A and anti-B antibodies tended to be lower in those aged over 30 years. The mean titer between the ages of 19-20 years was 32 and at the age of 40-49 it was 16.12 In that study the frequent titre in the volunteers of the control group was 64 for anti-A and 16 on anti-B. In the bariatric group it was 8 for anti-A and 16 for anti-B. There are no studies or reports on the titre of antibodies in patients who have undergone bariatric surgery.
In this study, there was a significant difference between the titre of anti-B antibodies (Phenotype A) and between the titers of anti-A antibodies (Phenotype O and B) among the individuals studied. However, it was possible to observe a tendency in the bariatric group to have lower titers of anti-ABO antibodies in relation to the control group. It is known that in the elderly the ABO antibody titre decreases after age 65. [8,11]
In individuals over the age of 65, there is a change in the intestinal microbiota due to changes in diet as well as a decrease in the function of the immune system.[24]
It is likely that in bariatric subjects there will be a change in the microbiota or adaptation of the same over the years thus providing a decrease in the ABO antibody titer.
Pentraxin-3 (PTX3) is an innate humoral immune system protein that plays an important role in protecting against infections, controlling inflammation and matrix deposition. Tonial and colleagues (2019) showed that patients returned to decreased (normal) levels in PTX3 concentration after bariatric surgery.[25]
There is a potential risk of transfusion reaction in incompatible ABO platelet units when there are high antibody titers. Bazigou and colleagues (2015) in the study defined that titles greater than 64 are already considered high titles. [15] In the present study, we found that patients who underwent bariatric surgery maintained lower anti-ABO antibody titers than those considered critical. Gambero and colleagues (2011) observed a frequency of 12.8% for donors considered dangerous with a title above 128.8. In this research, there was a tendency for more low titers in bariatric volunteers, who may be potential donors for platelet apheresis. However, it is important to note that in the present study, antibody titers in blood donors were not analyzed. The assignment of bariatric individuals as potential donors of platelets by apheresis must be performed with caution, since blood donation should be an act of solidarity, but that does not pose risks to the blood donor itself.[26]
CONCLUSION
Therefore, there was a decrease in the anti-A antibody titre in the "O" and "B" Phenotype and in the anti-B titre in the Blood Phenotype A but did not generate discrepancies when the ABO phenotype was done. In order to have greater reliability in the results, we must make new studies with the largest sample number. Because individuals undergoing bariatric surgery may be potential donors of platelets for apheresis thus avoiding transfusion reactions and increasing the number of blood components for the blood bank.
- Murara JR, Macedo LLB, Liberali R (2008) Analysis of the efficacy of bariatric surgery in reducing body weight and fighting morbid obesity. Revista Brasileira de Obesidade, Nutrição e Emagacrecimento 2(7): 87-99.
- Silva PRB, Souza MR, Silva EM, Silva AS (2014) Nutritional status and quality of life in patients undergoing bariatric surgery. Brazilian Archiv Digest Surg 27(1): 35-38.
- Bordalo LA, Teixeira TFS, Bressan J, Mourão DM (2014) Bariatric surgery: How and why supplementary. J Brazilian Med Assoc 57 (1): 113-120.
- Jóia-Neto L, Lopes-Junior AG, Jacob CE (2010) Metabolic and digestive alterations in the postoperative period of bariatric surgery. A B C D 23(4): 266-269.
- Liou AP, Paziuk M, Luevano-Junior JM, Machineni S, Turnbaugh PJ, et al. (2010) Conserved shifts in the gut microbiota due to gastric bypass reduces host weight and adiposity. Sci Translat Med (178): 141-178.
- Guarner F (2007) Role of intestinal flora in health and disease. Hospital Nutrition 22 (2): 14-19.
- Sanz Y, Collado MC, Haros M, Dalmau J (2004) Metabolic-nutritive functions of the intestinal microbiota and its modulation through the diet: Probiotics and prebiotics. Spanish Pediatr Act 62(11): 520-526.
- Gambero S, Secco VNDP, Ferreira RR, Deffune E, Machado Pea (2004) Frequency of Anti-A and Anti-B hemolysins in blood donors from the Botucatu blood center. Brazil J Hematol Hemother 26(1): 28-34.
- Girello AL, Kühn Tibb (2011) Fundamentals of erythrocyte immunohematology. 3ª ed. São Paulo: Senac, pp: 304.
- Roback JD, Grossman BJ, Harris T, Hillyer CD (2011) Technical manual - American Association of Blood Banks. 17th edition. Bethesda: AABB Press.
- Cosechen VS (2010) Frequency of anti-A and anti-B agglutinins in blood donors of the “o” group of the Guarapuavahemonucleus (PR). Salus Magazine pp: 15-22.
- France NDG, Poli MCC, Ramos PGA, Borsoi CSR, Colella R (2011) Titers of ABO antibodies in group Blood donors. Brazil J Hematol Hemother 33(4): 259-262.
- Brasil PortariaConsolidação nº 5 de 28 de setembro de (2017) Consolidação das normassobre as ações e osserviços de saúde do Sistema Único de Saúde. DiárioOficial da União, Brasília, DF.
- Kaur G, Kaur P, Basu S, Kaur R (2013) Blood groups discrepancies at a tertiary care center-analysis and resolution. Int J Lab Hematol 36(4): 481-487.
- BRAZIL Ministry of Health (2015) Instruction No. 1. Jaime César De Moura Oliveira. Official Journal of Reason. Brasilia. Accessed on: May 25, 2016. Available online at: http://portal.anvisa.gov.br/wps/wcm/connect/7856ad00484d5627a531a5bdc15bfe28/IN_01-2015_Hemovigilancia_Marco-conceitual.pdf?MOD=AJPERES
- NATIONAL SANITARY SURVEILLANCE AGENCY (2014) Hemovigilance Bulletin. 6th ed. Brasília: Notivisa, 2014. 28 p. Accessed on: May 25, 2016. Available online at: http://portal.anvisa.gov.br/wps/wcm/connect/df1eaf00462dc945bfdfbfec1b28f937/Boletim_Hemovigil_n6_2014.pdf?MOD=AJPERES>
- Bazigou F, Lempesopoulos K, Kavallierou L, et al. (2015) Evaluation of anti-A and anti-B alloisogglutinin titer in group O plateletpheresis donors. Hematol Transfus Int J 1(3): 76-81.
- Judd WJ, Johnson ST, Storry JR (2008) Judd Methods in Immunohematology. 3rd ed. Bethesda: AABB Press, pp: 557-559.
- Marchesine JB, Nicareta JR (2014) Comparison of five techniques for the surgical treatment of morbid obesity with pimples. Arquivos Brasileiro de Cirurgia Digestiva 27 (1): 17-20.
- Valezi AC, Machado VHS (2011) Thinning and cardiac performance. Brazil Archiv Digest Surg 24(2): 131-135.
- Novaretti MCZ, Dorlhiac-Llacer PE, Chamone DAF (2000) Study of blood groups in Caucasoid and Negroid blood donors in the city of São Paulo. Brazil J Hematol Hemother 22(1): 23-32.
- Baiochi E, Camano L, Sass N, Colas OR (2007) Frequencies of blood groups and abo and rhd incompatibilities in puerperae and their newborns. J Brazil Med Assoc 53(1): 44-46.
- Geraldo A, Souza AL, Martinello F (2016) Relationship between intestinal bifidobacteria content and ABO antibody titer. Int J Microbiol Immunol Res, p: 001-008.
- Jeffery IB, O'Toole PW (2013) Diet-microbiota interactions and their implications for healthy living. Nutrients, p: 234-252.
- Tonial AF, Nisihara R, Nassif Pan, Munhoz SI, Cortina AG, et al. (2020) Bariatric surgery results in restoration of physiological plasma levels of pentraxine-3. Biomed Rep 12(2): 68-72.
- Wang HH, Chen PM, Lin CL, Jau RC, Hsiao SM, et al. (2019) Joint effects of risk factors on adverse events associated with adult blood donations. Medicine (Baltimore) 98(44): e17758.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Astronomy and Space Research
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Food and Nutrition-Current Research (ISSN:2638-1095)



