Research Article
Influence of Extractives of the Pre-Pupal Stages of the Black Soldier Fly, Hermetiaillucens L. (Dipteral: Stratiomyidae) on the Growth of Bacterial Species (Salmonella Typhimurium L., Escherichia Coli L. and Pseudomonas Aureginosa L.)
804
Views & Citations10
Likes & Shares
The attempt of the studies was carried with the aim of determination of the inhibition of the growth of three bacterial species (Salmonella typhimurium L., Escherichia coli. and Pseudomonas aureginosa L.) through the use of whole-body extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Dipteral: Stratiomyidae). The whole-body extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Diptera: Stratiomyidae) was prepared through the method of maceration. Completely Randomized Design (CRD) was followed for the experimentation. The attempt of experiments was consisting of six groups of the treatment with three replications each. The groups of the treatment in the attempt include six different concentrations of whole-body extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Diptera: Stratiomyidae) (50 mg/l; 100 mg/l; 150 mg/l; 200 mg/l.; 250 mg/l and 300 mg/l). The antibiotic compound: chloramphenicol (thirty micrograms per disc-paper) was served as a positive control. The dimethyl sulfoxide was served as negative control. The zone of inhibition of the growth of bacterial species, Salmonella typhimurium (L.) through the use of extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Dipteral: Stratiomyidae) with concentration of 50; 100; 150; 200; 250 and 300 ppm in the present was 7.28 mm; 7.91 mm; 9.13 mm; 10.49 mm; 11.73 mm and 12.03 mm respectively. The zone of inhibition of the growth of bacterial species, Salmonella typhimurium (L.) through the use of antibiotic compound: Chloramphenicol was 14.76 mm. The zone of inhibition of the growth of bacterial species, Escherichia coli (L.) through the use of extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Dipteral: Stratiomyidae) with concentration of 50; 100; 150; 200; 250 and 300 ppm in the present was 7.13 mm; 8.92 mm; 9.74 mm; 9.86 mm; 10.03 mm and 10.88 mm respectively. The zone of inhibition of the growth of bacterial species, Escherichia coli (L.) through the use of antibiotic compound: Chloramphenicol was 27.64 mm.
The zone of inhibition of the growth of bacterial species, Pseudomonas aeruginosa (L.) through the use of extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Diptera: Stratiomyidae) with concentration of 50; 100; 150; 200; 250 and 300 ppm in the present was 7.18 mm; 9.96 mm; 11.47 mm; 12.56 mm; 13.44 mm and 14.69 mm respectively. The zone of inhibition of the growth of bacterial species, Pseudomonas aeruginosa (L.) through the use of antibiotic compound: Chloramphenicol was 21.34 mm. The zone of inhibition of growth of the bacterial species was found increasing significantly (P<0.05) according to the concentration of extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Dipteral: Stratiomyidae). The concentration of 300 ppm of extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Dipteral: Stratiomyidae) was found effective for the inhibition of growth of bacterial species in the attempt.
Keywords: Antimicrobial Proteins (AMPs), Hermetiaillucens L., Salmonella typhimurium L., Escherichia coli L., Pseudomonas aureginosa L
INTRODUCTION
With reference to the antimicrobial proteins, the larval and pre-pupal stages of black soldier fly BSF; Hermetiaillucens (L.) are potential source. According to Veldkamp [1], the amino acids composition of proteins in the body of the larval and pre-pupal stages of black soldier fly BSF, Hermetiaillucens (L.) are exhibiting resemblance to composition of the amino acids in the soybean meal and fish meal. In addition to the significantly high content of the proteins, the larval and pre-pupal stages of black soldier fly BSF, Hermetiaillucens (L.) are reported for antimicrobial (antibacterial) activity in the form of antimicrobial peptides (AMP) [2]. Kim and Rhee [3] reported high content of lauric acid in the body of the larval and pre-pupal stages of black soldier fly BSF, Hermetiaillucens (L.). The lauric acid, is a fatty acid recognized as the natural antimicrobial compound. Bovera [4] opined the possibility of the content of chitin (polysaccharides) for the fortification of immunity in animals. Harlystiarini [5] demonstrated the activity of inhibition of growth of the Gram-negative bacteria (E. coli) through the use of extractives of the body of the larval and pre-pupal stages of black soldier fly BSF, Hermetiaillucens (L.). Jayanegara [6] reported the possibility of use of the insects as potential feed ingredients for ruminants: Chemical composition, in vitro rumen fermentation and methane emissions. The activity of bio-compound pertaining “antibacterial” category deserve significant role in wealthy health and the development of organs of digestive system in poultry birds. The activity of bio-compound pertaining “antibacterial” category help for the absorption of nutrients in the animals. The antibiotic compounds used in the animal feeds for the optimal growth are known as “Antibiotic Growth Promotors”. Prohibition of the utilization of “Antibiotic Growth Promotors” is due to their potentials of causing resistance. Increased microbial resistance to antibiotic compound is going to provide a great opportunity to obtain antimicrobial compounds, may be in the form of antimicrobial proteins (AMPs) through the use of the larval and pre-pupal stages of black soldier fly BSF, Hermetiaillucens (L.) like insects. The present attempt is planning to determine the potential of larval and pre-pupal stages of black soldier fly BSF, Hermetiaillucens (L.) for antimicrobial proteins (AMPs).
As the industry of black soldier fly (BSF) progresses into the scale of commercial production, there is possibility of continuity of the trend of increasing sophisticated and controlled growth. It is utmost important to note that, the facility of advanced nature with capability of prevention of infection from entering and to stop any spread of infections within facility. The defense against the insect pathogens and the solutions of the control established may eventually trickle down in part to the facilities of “less‐advanced” category. However, the facilities of “Semi‐open” category may be preferable provided that, it should be designed properly. The insects use to multiply at higher rate in simple, semi-open facilities. The most significant and immediate responsibility of the farmers rearing the black soldier fly (BSF) is management of possible diseases through the garbage (waste material). The farmers rearing the black soldier fly (BSF) must send the samples of waste material and the samples of black soldier fly to the laboratory for diagnosis of pathogenic situations and further specific advices.
According to Wang & Shalom (2017), the attempts of researches of fundamental class on the pathogenesis through the insects is vital. North and/or South America are supposed to be the native countries of Black soldier fly (BSF). The Black soldier fly (BSF) can now be found across the regions of tropical and temperate all over the world. Although the natural history of the black soldier fly (BSF) precludes them from vectoring specific known pathogens to the human being, there remains a risk of transmission of pathogens from wild life to the socially cultured human-populations. Therefore, it is a need of assessment of the risk in more depth. The attempts of the researches should focus on the discovery of pathogens with potentials of transfer from the wild species of black soldier fly itself or from the wild life and or from other members of the family: Stratiomyidae of class: Insect. This may predict more accurately the dangers in the establishment of facilities of rearing beds for the life of black soldier fly (BSF). For the purpose to discover the pathogens on insects, it is a need to establish the specialized laboratories. It may possible for such diagnostic laboratories to carry out the testing pertaining presence of and transmission of potential pathogens from other (pest) insects to the black soldier flies (BSFs). The substrates (in the form of waste material) used to feed the black soldier flies (BSFs) could themselves harbor potential pathogens and creating unfavorable situation for the productivity. Application of or promotion of microbial species of specific and beneficial category appears to be interesting and most relevant of prevention or suppression of transfer of the pathogens from the wild animals to the human population through the black soldier fly (BSF). Grau, et al. (2017) recommend the introduction of microbes of beneficial categories for the purpose to create a “Man-Made-Biome” for the larval stages of black soldier fly (BSF). The “Probiotic Treatment” is going to reduce the population of microbials in the rearing bed of the larval stages of black soldier fly (BSF). Overall, farmers rearing the black soldier fly (BSF) would like to stress the significance of collaborations and the co-ordinations of international effort for the purpose of diagnosis and management of potential microbial diseases in black soldier fly (BSF). diseases in BSF production systems.
On this much background, the attempt of studies on assessment of the influence of extractives of the pre-pupal stages of the Black Soldier Fly (BSF), Hermetiaillucens L. (Dipteral: Stratiomyidae) on the Growth of Bacterial Species (Salmonella typhimurium L., Escherichia coli L. and Pseudomonas aureginosa L.) has been planned.
MATERIALS AND METHODS
- Rearing of the black soldier fly, Hermetiaillucens (Linnaeus) (Dipteral: Stratiomyidae):
The present attempt on the rearing of the black soldier fly, Hermetiaillucens (Linnaeus) (Dipteral: Stratiomyidae) in local environmental conditions of Baramati (India)was carried during 4 November, 2020 - 28 February, 2021 in the insectary (Green House) of Shardabai Pawar Mahila Arts, Commerce and Science College, Shardanagar Tal. Baramati, Pune, India. The culture was initiated through keeping household organic waste (Kitchen Waste). The content of the organic waste (Kitchen waste) was with sour milk, waste tea powder, vegetable waste (cabbage and fruits of papaya). This content of the organic waste (Kitchen waste) was taken in a box and labelled as “tray with rearing bed” (or Larval Rearing Bin). This box (Larval Rearing Bin) was designed in the shape of a rectangular wooden box with the dimensions of 2x1.5x1.5 feet with ventilation holes on the top lid, and a rectangular plank was placed at an inclined position making an angle of 45° with the bottom so as to facilitate the self-harvesting of mature larva as they turn into pre-pupa. Little amount of water was used to spray on the contents in a tray. Spraying the water on organic waste initiates the process of decomposition through bacteria. After a few days as the wastes began to decompose (Pedro, et al., 2014). The fertilized egg mass of the black soldier fly, Hermetiaillucens (Linnaeus) (Dipteral: Stratiomyidae) was procured from Mangal Agro Farm Mire Rd, Maka, Maharashtra 414501 India. The egg mass was kept suspended over fresh food (slices of fruits of papaya, Carica papaya L.). For uniform hatching, it requires a humid and cool place with fresh airflow. Hatching of the eggs take place within 24 h of provision of favorable conditions to the fertilized eggs.
On fifth day after hatching, the larvae from incubation box were transferred to box with rearing bed (Larval Rearing Bin). The larvae were allowed for feeding and their development. The mature stages of prepupa of the black soldier fly, Hermetiaillucens (Linnaeus) (Diptera: Stratiomyidae) were collected from this stock culture. The mature stages of prepupa of the black soldier fly, Hermetiaillucens (Linnaeus) (Dipteral: Stratiomyidae) were transferred to the rearing cages once in three days. This transfer of the mature stages of prepupa of the black soldier fly, Hermetiaillucens (Linnaeus) (Dipteral: Stratiomyidae) is to the rearing cages observe different life stages. The cages with the mature stages of prepupa of the black soldier fly, Hermetiaillucens (Linnaeus) (Dipteral: Stratiomyidae) were placed inside the insectary (Green House) of Shardabai Pawar Mahila Mahavidyalaya, Shardanagar Tal. Baramati, Pune, India. After three days, the stages of prepupa of the black soldier fly, Hermetiaillucens (Linnaeus) (Dipteral: Stratiomyidae) from the rearing cages were placed in a small plastic bucket containing soil and kept inside the rearing cage in order to provide a place for pupation. The condition of humidity of the cage was maintained at 70-80 %. This was achieved through keeping a water source with sponges soaked in it as well as by spraying water three to four times a day (Briscoe and Chittka, 2001; Pedro, et al., 2014; Zhang, et al., 2010). The source of lighting was provided daily for 12 h. The light provision is to stimulate adult mating. The card boards were made hung in various locations. The provision of the card boards is to mimic sites for laying the eggs by the adult female black soldier fly, Hermetiaillucens (Linnaeus) (Dipteral: Stratiomyidae) (Pedro, et al., 2014; Sheppard, et al., 1994; Sheppard, et al., 2002; Shields, 1982; Tomberlin Jeffery and Craig Sheppard, 2002). The observations were recorded on egg hatching; the period of development of the larval, pupal and adult stages and the morphology of the life stages. The sex ratio was determined through random sampling performance and observations of the genitalia of randomly collected adults.
For the purpose of determination of frequency of mating of newly emerged adult flies of the black soldier fly, Hermetiaillucens (Linnaeus) (Dipteral: Stratiomyidae) and egg laying capacity (fecundity), ten sets of identical plastic containers capacity: 2 L) were taken. Newly emerged adult male and adult female were kept in pairs plastic containers. All these setups were then placed in the insectary (Green House) of Shardabai Pawar Mahila Mahavidyalaya, Shardanagar Tal. Baramati, Pune, India. They were provided with artificial lighting (60W) and humidity (70-80 %) (Briscoe and Chittka, 2001; Zhang, et al., 2010; Savonen Carol, 2005; Spranghers, et al., 2017; Laplander, et al., 2018; Wang, et al., 2017). The observation on the determination of the egg laying sites (ovipositional sites); egg laying period (ovipositional period) and the life span was carried every 12 h. The eggs were collected from this set up. The eggs were allowed to hatch under varying conditions. This attempt was for the purpose to determine period of incubation and the ability of the eggs to tolerate unfavorable temperatures. The eggs and larvae were collected daily from the rearing bin. Larvae were taken back to laboratory. The larvae were washed thoroughly to remove impurities. The larvae were knocked out by freezing and measured using coulometer to record total body length, body width, and length of mouth hook. The Dyar’s rule was followed for the purpose of determination of the morphometry of the larvae. It was carried through the determination of number of larval instars in its life cycle of the black soldier fly, Hermetiaillucens (Linnaeus) (Dipteral: Stratiomyidae) (Craig Sheppard, et al., 2002; Goldson, et al., 2001; Mohammed, 2011; Borelli Marco, et al., 2020; Bruno Daniele, et al., 2018; Holmes, et al., 2013).The olfactometer was utilized for the determination of behavior of the feeding and the preference of food waste by the larval stages of the black soldier fly, Hermetiaillucens (Linnaeus) (Dipteral: Stratiomyidae). For consistency in the results, each attempt was repeated at least for three times. The collected data was subjected for analysis through the statistical method.
- Preparation of Extractives of the Pre-pupal Stages of the Black Soldier Fly (BSF), Hermetiaillucens (Dipterans: Stratiomyidae):
The mature pre-pupal stages of the Black Soldier Fly (BSF), Hermetiaillucens L. (Diptera: Stratiomyidae) were selected randomly from the stock culture. They were kept in freezer at -35°C for 24 h. After 24 h of freezing, they were subjected for thawing followed by washing thoroughly. The content was then processed for drying for 48 h in oven (60°C). Through the use of blender, the oven dried pre-pupal stages of the Black Soldier Fly (BSF), Hermetiaillucens L. (Dipteral: Stratiomyidae) were subjected for grinding until smooth. The content thus obtained was titled as, “Black Soldier Fly Meal” (BSF Meal). For the purpose to prepare the extractives from “Black Soldier Fly Meal” (BSF Meal), methanol was selected as solvent. 10 ml of “Black Soldier Fly Meal” (BSF Meal) were mixed in 100 ml of methanol. The contents were kept for 24 h at room temperature for maceration. The method of obtaining extractives through the maceration belong to Choi, et al. (2012). After 24 h of maceration, the content was filtered through the use of common laboratory filter paper. For the purpose to obtain extractives in concentrated form, the filtrate was subjected for evaporation with a reduced pressure rotary evaporator at 40°C.
- Assay of Antimicrobial (Antibacterial) Activity of the Extractives of the Pre-pupal Stages of the Black Soldier Fly (BSF), Hermetiaillucens (Dipteral: Stratiomyidae):
The assay of antimicrobial (antibacterial) activity of the extractives of the pre-pupal stages of the Black Soldier Fly (BSF), Hermetiaillucens L. (Dipteral: Stratiomyidae) was carried-out in vitro. The bacterial species selected for the study include: Salmonella typhimurium (L.), Escherichia coli (L). and Pseudomonas aureginosa (L.). The cultures of bacterial species selected for the study (Salmonella typhimurium L., Escherichia coli L. and Pseudomonas aureginosa L.) are from Medical Microbiology Laboratories of Baramati Medical College, Baramati India. The method explained by Nagappan, et al. (2011) was followed for the assay of antimicrobial (antibacterial) activity of the extractives of the pre-pupal stages of the Black Soldier Fly (BSF), Hermetiaillucens L. (Dipteral: Stratiomyidae) through the method of agar diffusion by using paper discs. The bacterial species to be tested for assays were subjected for the subculturing first on the medium of “Trip tic Soya Agar” (TSA) medium. Incubation of petri-plates at 35°C (± 1°C) for 24 h. The bacterial species that have been sub-cultured that have grown on TSA medium are made suspensions until a population of 107 cfu is obtained.
Through the use of volumetric pipette, total suspension of 0.1 ml of was taken. The suspension was transferred into the growth medium of Muller Hilton Agar (MHA). The suspension was made flattened on the surface of the growth medium of Muller Hilton Agar (MHA) with the help of the bent glass rods. The Muller Hilton Agar (MHA) medium was kept at room temperature for about fifteen. Through the process of dissolving appropriate quantity of extractives of the pre-Pupal stages of the Black Soldier Fly (BSF), Hermetiaillucens L. (Dipteral: Stratiomyidae) in known volume of dimethyl sulfoxide (DMSO), solution of six different strength were prepared. The desired strengths (concentrations) of the extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Diptera: Stratiomyidae) in the attempt include: 50 mg/l; 100 mg/l; 150 mg/l; 200 mg/l; 250 mg/l and 300 mg/l. 60µl of the extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Diptera: Stratiomyidae) from each strength were dripped on each disc-paper with the help of micropipette and placed in petri-dish with sterile Muller Hilton Agar (MHA). Antibiotic: chloramphenicol (thirty micrograms per disc paper) was utilized as a group of positive control. The Muller Hinton Agar (MHA) in each petri dish was subjected to a bacterial suspension (of about 1.5 x 108) to the entire surface. Care was taken for uniformness. Then all the petri-dishes were allowed to become dry through waiting for about an hour. All the petri-dishes were then subjected for incubation at 37°C for 24 h. The observations on antimicrobial (antibacterial) activity of the extractives of the pre-pupal stages of the Black Soldier Fly (BSF), Hermetiaillucens L. (Dipteral: Stratiomyidae) were carried out at 24 h after the period of incubation. Sensitivity of the microbial (bacterial) species to the extractives of the pre-pupal stages of the Black Soldier Fly (BSF), Hermetiaillucens L. (Diptera: Stratiomyidae) was made confirmed with the observation. Sensitivity of the microbial (bacterial) species to extractives of the pre-pupal stages of the Black Soldier Fly (BSF), Hermetiaillucens L. (Dipteral: Stratiomyidae) was expressed by the wide diameter of zone of inhibition. The diameter of inhibitory zone diameters was measured through the use of digital calipers in millimeters (mm). For the purpose to get consistency in the results, the whole experimentation was repeated for three times.
- Statistical Analysis of the data:
The whole experimentation was repeated for three times. The data was collected and subjected for analysis through the statistical methods. In vitro-antibacterial activity of the extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Dipteral: Stratiomyidae) test data was analyzed through the use of “Analysis of Variance” (ANOVA). The level of confidence for the “Analysis of Variance” (ANOVA) was ninety-five percent (α = 0.05). Duncan test was followed for the data exhibiting significant difference.
RESULTS AND DISCUSSION
The results on the invitro antimicrobial (antibacterial) activity of the extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Dipteral: Stratiomyidae) are summarized in Table 1 and presented in Figures 1-3.
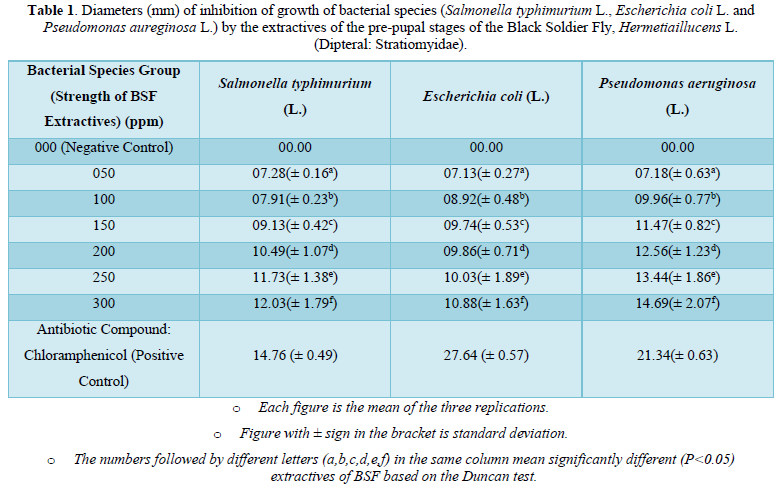
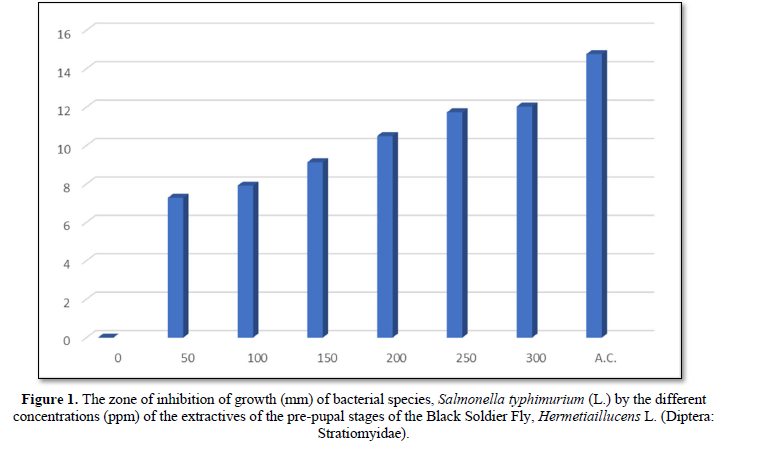




The diameter (mm) of zone of inhibition of the growth of bacterial species, Salmonella typhimurium (L.) through the use of extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Dipteral: Stratiomyidae) with concentration of 50 ppm; 100 ppm; 150 ppm; 200 ppm; 250 ppm and 300 ppm in the present was 7.28 mm; 7.91 mm; 9.13 mm; 10.49 mm; 11.73 mm and 12.03 mm respectively. The diameter (mm) of zone of inhibition of the growth of bacterial species, Salmonella typhimurium (L.) through the use of antibiotic compound: Chloramphenicol was 14.76 mm (Table 1 and Figure 1).
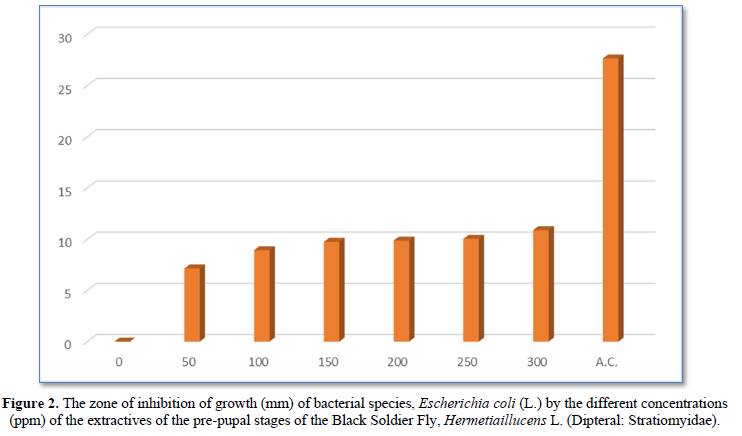
The diameter (mm) of zone of inhibition of the growth of bacterial species, Escherichia coli (L.) through the use of extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Dipteral: Stratiomyidae) with concentration of 50 ppm; 100 ppm; 150 ppm; 200 ppm; 250 ppm and 300 ppm in the present was 7.13 mm; 8.92 mm; 9.74 mm; 9.86 mm; 10.03 mm and 10.88 mm respectively. The diameter (mm) of zone of inhibition of the growth of bacterial species, Escherichia coli (L.) through the use of antibiotic compound: Chloramphenicol was 27.64 mm (Table 1 and Figure 2).
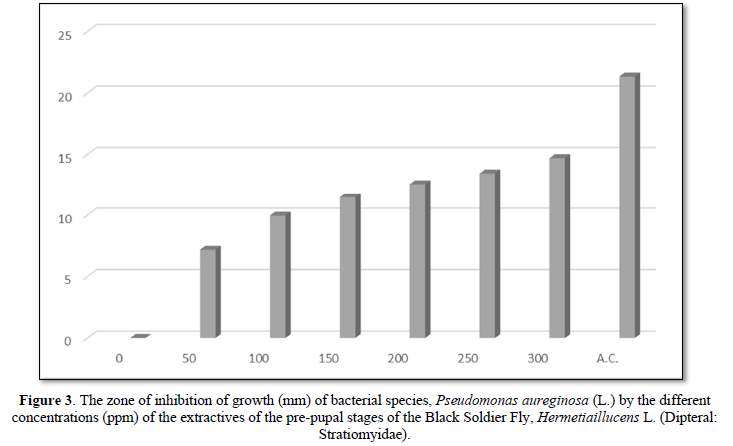
The diameter (mm) of zone of inhibition of the growth of bacterial species, Pseudomonas aeruginosa (L.) through the use of extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Diptera: Stratiomyidae) with concentration of 50 ppm; 100 ppm; 150 ppm; 200 ppm; 250 ppm and 300 ppm in the present was 7.18 mm; 9.96 mm; 11.47 mm; 12.56 mm; 13.44 mm and 14.69 mm respectively. The diameter (mm) of zone of inhibition of the growth of bacterial species, Pseudomonas aeruginosa (L.) through the use of antibiotic compound: Chloramphenicol was 21.34 mm (Table 1 and Figure 3).
The zone of inhibition of growth of the bacterial species was found increasing significantly (P<0.05) according to the concentration of extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Dipteral: Stratiomyidae). The concentration of 300 ppm of extractives of pre-pupal stages of the black soldier fly, Hermetiaillucens L. (Dipteral: Stratiomyidae) was found effective for the inhibition of growth of bacterial species in the attempt.
The diameter of zone of inhibition of growth of bacterial species, Salmonella typhimurium (L); Escherichia coli (L.) and Pseudomonas aeruginosa (L) for the negative control group in the present attempt exhibited significant differences with reference to the positive control group. The negative control group in the present attempt exhibited the absence of zone of inhibition of growth of the bacterial species. The positive control group in the present attempt exhibited the most significant of zone of inhibition of growth of the bacterial species.
Choi, et al. (2012) reported higher levels of sensitivities of the methanol extractives of black soldier fly (BSF) by the bacterial species of the “Gram Negative” group in comparison with the bacterial species of the “Gram Positive” group. The possibility of presence of specific ability of interaction of the wall of bacterial species to the active compounds present in the methanol extractives of black soldier fly (BSF) is maximum.
According to Davis and Stout (1971), the inhibition of growth of the bacterial species for the compound may be classified according the dimension of the response. Accordingly, the diameter (mm) of zone of inhibition of growth of bacterial species ranging from one milli meter (mm) to five milli meters (mm) may be classified as, “weak”. The diameter (mm) of zone of inhibition of growth of bacterial species ranging from five milli meters (mm) to ten milli meters (mm) may be classified as, “medium”. The diameter (mm) of zone of inhibition of growth of bacterial species ranging from ten milli meters (mm) to twenty milli meters (mm) and / or more may be classified as, “strong”. Intensity of capabilities of inhibition of growth of microbials by the extractives of black soldier fly (BSF) depends on concentration of the extractives and the type of microbial or bacterial species. It is utmost important to note that, the facility of advanced nature with capability of prevention of infection from entering and to stop any spread of infections within facility. The defense against the insect pathogens and the solutions of the control established may eventually trickle down in part to the facilities of “less‐advanced” category. However, the facilities of “Semi‐Open” category may be preferable provided that, it should be designed properly. The insects use to multiply at higher rate in simple, semi-open facilities. The most significant and immediate responsibility of the farmers rearing the black soldier fly (BSF) is management of possible diseases through the garbage (waste material). The farmers rearing the black soldier fly (BSF) must send the samples of waste material and the samples of black soldier fly to the laboratory for diagnosis of pathogenic situations and further specific advices.
According to Wang & Shelomi (2017), the attempts of researches of fundamental class on the pathogenesis through the insects is vital. North and/or South America are supposed to be the native countries of Black soldier fly (BSF). The Black soldier fly (BSF) can now be found across the regions of tropical and temperate all over the world. Although the natural history of the black soldier fly (BSF) precludes them from vectoring specific known pathogens to the human being, there remains a risk of transmission of pathogens from wild life to the socially cultured human-populations. Therefore, it is a need of assessment of the risk in more depth. The attempts of the researches should focus on the discovery of pathogens with potentials of transfer from the wild species of black soldier fly itself or from the wild life and or from other members of the family: Stratiomyidae of class: Insect. This may predict more accurately the dangers in the establishment of facilities of rearing beds for the life of black soldier fly (BSF). For the purpose to discover the pathogens on insects, it is a need to establish the specialized laboratories. It may possible for such diagnostic laboratories to carry out the testing pertaining presence of and transmission of potential pathogens from other (pest) insects to the black soldier flies (BSFs). The substrates (in the form of waste material) used to feed the black soldier flies (BSFs) could themselves harbor potential pathogens and creating unfavorable situation for the productivity. Application of or promotion of microbial species of specific and beneficial category appears to be interesting and most relevant of prevention or suppression of transfer of the pathogens from the wild animals to the human population through the black soldier fly (BSF). Grau, et al. (2017) recommend the introduction of microbes of beneficial categories for the purpose to create a “Man-Made-Biome” for the larval stages of black soldier fly (BSF). The “Probiotic Treatment” is going to reduce the population of microbials in the rearing bed of the larval stages of black soldier fly (BSF).
CONCLUSION
The extractives of the pre-pupal stages of the black soldier fly (BSF), Hermetiaillucens L. (Dipteral: Stratiomyidae)has antibacterial activity against the growth of bacterial species in the attempt of studies (Salmonella typhimurium L., Escherichia coli L. and Pseudomonas aureginosa L.).Increase in the concentration of extractives of the pre-pupal stages of the black soldier fly (BSF), Hermetiaillucens L. (Diptera: Stratiomyidae) resulted in the tendency in increase in the diameter of zone of inhibition of growth of the bacterial species. The extractives of the pre-pupal stages of the black soldier fly (BSF), Hermetiaillucens L. (Diptera: Stratiomyidae) with the concentration of 300 ppm was found most significant and effective for inhibition of growth of the bacterial species: Salmonella typhimurium (L.), Escherichia coli (L). and Pseudomonas aureginosa (L.).
ACKNOWLEDGMENTS
The authors would like to express sincere thanks to the administrative staff of the Agricultural Development Trust, Baramati for the permission of the rearing of larval stages of the black soldier fly (BSF), Hermetiaillucens L. (Dipteral: Stratiomyidae) at the green house in the campus of Shardanagar educational campus. The support from department of Microbiology, Shardabai Pawar Mahila Arts, Commerce and Science College, Shardanagar Tal. Baramati Dist. Pune - 413115 India deserve appreciations and exert a grand salutary influence. Vitthalrao B. Khyade has received research fellowship through Indian Council of Medical Research (V. Ramalingaswami Bhawan, P.O. Box No. 4911 Ansari Nagar, New Delhi - 110029, India) (for the academic year: 20180 2019; 2019-2020 and 2020 - 2021) and laboratory support from Mangal Agro Farm Miri Rd, Maka, Maharashtra 414501 India.
- Veldkamp T, van Duinkerken G, van Huis A, Ottevanger E, Bosch G, et al. (2012) Insects as a sustainable feed ingredient in pig and poultry diets: A feasibility study. Food Chem 50: 192-195.
- Park SI, Chang BS, Yoe SM (2014). Detection of antimicrobial substances from larvae of the black soldier fly, Hermetiaillucens (Dipteral: Stratiomyidae) Entomol Res 44: 58-64.
- Kim SA, Rhee MS (2016) Highly enhanced bactericidal effects of medium chain fatty acids (caprylic, capric, and lauric acid) combined with edible plant essential oils (caracole, eugenol, β-resorcylic acid, trans-cinnamaldehyde, thymol, and vanillin) against Escherichia coli Food Control 60: 447-454.
- Bovera F, Loponte R, Marono S, Piccolo G, Parisi G, et al. (2016) Use of Tenebrio Molitor larvae meal as protein source in broiler diet: Effect on growth performance, nutrient digestibility, and carcass and meat traits J Anim Sci 94: 639-647.
- Harlystiarini (2012) Pemanfaatantepung larva black soldier fly (hermetiaillucens) sebagaisumber protein penggantitepung ikan pada ransumpuyuhpetelur (cortunixcortunix japonica) Ipb pp: 0-10.
- Jayanegara A, Yantina N, Novandri B, Laconi EB, Nahrowi, et al. (2017) Evaluation of some insects as potential feed ingredients for ruminants: Chemical composition, in vitro rumen fermentation and methane emissions. J Indones Trop Anim Agric 42: 247-254.
- Yu-Shiang W, Matan S (2017) Review of Black Soldier Fly (Hermetiaillucens) as Animal Feed and Human Food. Foods 6(10): 91.
- Briscoe AD, Chittka L (2001) The evolution of color vision in insects. Ann Rev Entomol 46: 471-510.
- Sheppard CD, Jeffery KT, John AJ, Barbara CK, Sonya MS (2002) Rearing Methods for the Black Soldier Fly (Diptera: Stratiomyidae). J Med Entomol 39(4): 695-698.
- Goldson SL, Neill MRM, Proffitt JR, Baird DB (2001) Seasonal variation in larval - instar head - capsule sizes of Argentine stem weevil, Listronotusbonariensis (kuschel) (Coleoptera; Curculionidae). Aust J Entomol 40(4): 371-375.
- Mohammed SMG (2011) Determination of Larval Instars of Black Cutworm Agrotisipsilon (Hufnagel) (Lepidoptera, Noctuidae). Jordan J Biol Sci 4(3): 173-176.
- Pedro CD, Mehab H, Guillermo FDJ, Selin Y (2014) Development of a Food waste Composting system using Black Soldier Fly larvae. 3rd annual R&D Student Competition-Greenovate NYS. Accessed on: September 30, 2018. Available online at: https://www.rit.edu
- Sheppard C, Newton GL, Thompson SA, Savage S (1994) A value added manure management system using the black soldier flies. Bioresour Technol 50: 275-279.
- Sheppard DC, Jeffery K, Tomberlin J, Joyce JA, Kiser BC, et al. (2002) Rearing Methods for the Black Soldier Fly (Diptera: Stratiomyidae). J Med Entomol 39(4): 695-698.
- Shields EB (1982) Raising earthworms for profit. Eagle River, WI: Shields Publications.
- Jeffery T, Sheppard CD (2002) Factors Influencing Mating and Oviposition of Black Soldier Flies (Diptera: Stratiomyidae) in a Colony. J Entomol Sci 37: 345-352.
- Zhang J, Huang L, He J, Tomberlin JK, Li J, et al. (2010) An Artificial Light Source Influences Mating and Oviposition of Black Soldier Flies, Hermetiaillucens. J Insect Sci 10: 202.
- Carol S (2005) Big maggots in your compost? They’re soldier fly larvae. OSU Extension Service - Gardening. Oregon State University.
- Thomas S, Matteo O, Cindy K, Anneke O, Stefaan D, et al. (2017) Nutritional composition of black soldier fly (Hermetiaillucens) prepupae reared on different organic waste substrates. J Sci Food Agric 97(8): 2594-2600.
- Lalander C, Diener S, Zurbrügg C, Vinnerås B (2019) Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetiaillucens). J Clean Product 208: 211-219.
- Marco B, Daniele B, Matteo B, Novella G, Ling T, et al. (2020) Black Soldier Fly Larvae Adapt to Different Food Substrates through Morphological and Functional Responses of the Midgut. Int J Mol Sci 21(14): 4955.
- Daniele B, Marco B, De Filippis F, Di Lelio I, Gianluca T, et al. (2018) McBain, Andrew J. (ed.). The Intestinal Microbiota of Hermetiaillucens Larvae Is Affected by Diet and Shows a Diverse Composition in the Different Midgut Regions. Appl Environ Microbiol 85(2): e01864.
- Holmes LA, Vanlaerhoven SL, Tomberlin JK (2013) Substrate Effects on Pupation and Adult Emergence of Hermetiaillucens (Diptera: Stratiomyidae): Table 1. Environ Entomol 42(2): 370-374.
- Choi W, Yun J, Chu J, Chu K (2012) Antibacterial effect of extracts of Hermetiaillucens (Diptera: Stratiomyidae) larvae against Gram-negative bacteria. Entomol Res 42: 219-226.
- Nagappan T, Ramasamy P, Wahid MEA, Segaran TC, Vairappan CS (2011) Biological activity of carbazole alkaloids and essential oil of Murrayakoenigii (L.) against antibiotic resistant microbes and cancer cell lines. Molecules 16: 9651-9664.
- Davis WW, Stout TR (1971) Disc plate method of microbiological antibiotic assay. II. Novel procedure offering improved accuracy. Appl Microbiol 22: 666-670.
- Grau T, Vilcinskas A,Joop G (2017) Sustainable farming of the mealworm Tenebrio Molitor for the production of food and feed. ZeitschriftfürNaturforschung C 72: 337-349.
- Sokal RR, Rohlf FJ (1995) Biometry: The Principles and Practice of Statistics in Biological Research. (W.H. Freeman & Company, New York). pp: 887.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Astronomy and Space Research
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)




