Mini-Review
Monge’s Disease
4645
Views & Citations3645
Likes & Shares
INTRODUCTION
Monge’s disease is a high-altitude disease, initially described by Carlos Monge in 1928. This is characterized by increasing cyanosis, polycythemia, finger clubbing, nail hemorrhages, severe pulmonary arterial hypertension and cardiac failure. The symptoms include headache, dizziness, fatigability, paresthesia’s, somnolence and decreased mental alertness. These features are reversible and the disease is found to remit when the patient is brought to lower attitudes, and recurs if he or she returns to high altitude [1-3]. Arterial oxygen saturation of 70% or less, a hematocrit of 80% and hemoglobin of around 25g/dl are representative of the illness [3].
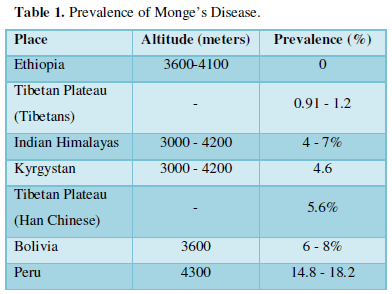
PREVALENCE
Although the disease generally affects people native to altitudes higher than 3000 meters, it does not affect populations around the world equally. A recent study conducted from Himachal Pradesh, India in 2013 has reviewed the global prevalence with highest rates in Andean countries of South America and the lowest rates in people native to East African Mountains of Ethiopia (Table 1).
We, in the Jammu and Kashmir state have seen such patients coming from Ladakh region to seek medical care at our tertiary care center and no study in this regard has been conducted from this state of India so far. An epidemiological survey for studying the overall profile of the disorder is emphasized.

PATHOPHYSIOLOGY AND PATHOLOGY
The basic abnormality is the polycythemia following chronic hypoxemia at high altitudes that leads to increased blood viscosity and uneven blood flow through the lungs leading to ventilation perfusion mismatching. The hyperventilation which usually maintains the alveolar PO2 around 50mmHg at high altitudes ceases to occur due to relative failure of the respiratory center to respond to CO2- stimulation [3,4]. In other words, the primary physiological abnormality appears to be a loss of adaptive altitude hyperventilation with insensitivity of the ventilator response to carbon dioxide [1]. It has also been observed that patients with Monge’s disease are almost devoid of ventilatory response to hypoxia [5]. Recavarren and Arias-Stella [6] in 1962 published a study of right ventricular hypertrophy among two series of necropsies of children, at sea-level and at the high altitude and found, anatomical existence of right ventricular hypertrophy in children born and living at 12, 375 to 14,300 feet above sea-level [6]. In 1964, they again studied 112 subjects aged 11 to 80 years, comprising of control group living at sea-level compared to a group residing at high altitude, and found the evidence of right ventricular hypertrophy among the subjects living at high altitude, the localization of hypertrophy was mainly at the base of the ventricle [7]. Subsequently in 1973, the workers came up with three clinicopathological types of Monge’s disease:
- Type 1: This occurs in subjects who, for professional or other reasons move from sea-level to reside at high altitude and do not adopt to this change. A similar illness known as ‘brisket disease’ is found among cattle living at high altitude. The basic pathogenic mechanism for this type of disease is the failure of homeostatic adjustment to the atmosphere at high altitude
- Type II: This form of disease is seen among people from sea levels who have already adopted to live in a good health at high altitude in whom organic disease exaggerates the hypoxemic state. The co morbid disorders include obesity, kyphoscoliosis and neuromuscular disorders affecting the thoracic cage or illnesses influencing pulmonary function such as emphysema, tuberculosis and pneumoconiosis. Monge classified such cases as secondary mountain sickness
- Type III: seen in subjects who are native to-or have adopted to live at high altitude and later develop Monge’s disease without any apparent co morbid organic disease without any apparent co morbid organic illness. The proposed mechanism being a decreased sensitivity of the respiratory center to CO2 and/or chemoreceptors to hypoxemia [8]. The pulmonary vessels of subjects at high altitude show the characteristic changes associated with prolonged hypoxia that include muscularization of the pulmonary arterioles, development of distinct media, and appearance of longitudinal intimal muscle. Proliferative changes are not characteristic in keeping with the potentially reversible nature of the disorder9. The main pathological findings among cases of fatal chronic mountain sickness include peripheral pulmonary arterial intimal and medial fibrosis, fresh and organized pulmonary arterial thrombi, chronic bronchiolitis and emphysema [8].
TREATMENT
The pulmonary arterial pressure in Monge’s disease is twice as high as that of healthy individuals living at the same high altitude. After two months at sea level, considerable reduction of pulmonary arterial pressure occurs, and the initially increased cardiac output returns to normal [3]. Treatment with respiratory stimulants like progesterone leads to clinical improvement in the ventilator response to hypoxia [10]. Acetazolamide, an inhibitor of carbonic anhydrase has recently shown to reduce erythropoiesis in patients with Monge’s disease. Two doses of 250mg and 500mg daily for 3 weeks have been evaluated. 250mg of acetazolamide per day reduces hematocrit, serum erythropoietin, and plasma soluble transferring receptors as markers of excessive erythropoiesis. No additional benefit has been found with the dose of 500mg per day. Mechanism of action involves metabolic acidosis and secondarily increased ventilation and reduced hypoxemia. However, its effects on chronic hypoxemia induced pulmonary hypertension are not known [11].
FUTURE PROJECTIONS
As this disorder has yet been under-diagnosed at our place, it needs further studies of large sample. As yet it is not known that why some individuals do develop Monge’s disease while most exposed to the same atmosphere does not [9]. The observations made by Kryger and co-workers [10] lead to the evolution of thinking about the presence of sleep disordered breathing among subjects of Monge’s disease. It is also possible to have co-existent sleep related breathing disorders like obstructive and central sleep apnea syndromes, hypoventilation syndromes and overlap syndrome of obstructive sleep apnea and chronic obstructive lung disease [12] among the subjects of Monge’s disease, and the subjects may need to be evaluated on the lines of same to make the spectrum of exact etiopathogenesis clearer.
- Monge CC, Whittemburg J (1976) Chronic Mountain sickness. Johns Hopkins Med J 139(Suppl): 87-89.
- Hurtado A (1960) Some clinical aspects of life at high altitude. Ann Med 53: 247.
- Seaton A, Seaton D, Leitch GA (1989) Functions of the respiratory tract. In: Crofton and Doughlas’s Respiratory Diseases, 4th Blackwell Scientific Publications. London. pp: 28-75.
- Zubieta-Castillo G Sr, Zubieta-Calleja GR Jr, Zubieta-Calleja L (2006) Chronic Mountain sickness: The reaction of physiological disorders to chronic hypoxemia. J Physiol Pharmacol 57(Suppl): 431-442.
- Lefrancois R, Gautier H, Pasquis P (1968) Ventilatory oxygen drive in acute and chronic hypoxia. Resp Physiol 4: 217.
- Arias-Stella J, Recavarren S (1962) Right ventricular hypertrophy in native children living at high altitude. Am J Pasthol 41: 55.
- Recavarren S, Arias-Stella J (1964) Right ventricular hypertrophy in people born and living at high altitudes. Br Heart J 26: 806.
- Arias-Stella J, Kruger H, Recavarran S (1973) Pathology of chronic mountain sickness. Thorax 28: 701.
- Seaton A, Seaton D, Leitch GA (1989) Pulmonary hypertension: In: Crofton and Douglas’s Respiratory Diseases, 4th Blackwell Scientific Publications: London. pp: 567-583.
- Kryger M, Weil J, Grower R (1978) Chronic Mountain polycythemia: A disorder of the regulation of breathing during sleep. Chest 73(Suppl): 303.
- Richalet J-P, Rivera M, Bouchet P, Chirinos E, Onnen I, et al. (2005) Acetazolamide: A treatment for chronic mountain sickness. Am J Respir Crit Care Med 172: 1427-1433.
- Lee-Chiong T (2008) Sleep related breathing disorders. In: Sleep Medicine: Essentials and Review. Oxford University Press. pp: 171-246.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Astronomy and Space Research


