202
Views & Citations10
Likes & Shares
Even today, plants are not only indispensable in health care but form the best hope of source for safe future medicines [2]. In spite of the fact that now we have at our command a number of modern drugs, it is still genuinely urgent to discover and develop new therapeutic agents. It has been estimated that acceptable therapy is available only for one third of the known human ailments. Therefore, the fight against diseases must be carried on relentlessly. Traditional plant medicines still enjoy significant position in the modern-day drug industries due to the minor side effects as well as the synergistic action of the combination of compounds [3].
Newbouldia laevis - one of the plants with magical effects, which, is commonly, called boundary tree or ‘Tree of Life.’ [4] N. laevisis is a medium sized angiosperm which belongs to the Bignoniaceae family and grows to a height of about 7-8 (up to 15) meters [5]. It is called Obum-Nnadi Charity Ndidi et. al. Phytochemical and a ‘Aduruku’ in Hausa; ‘Ogirisi’ in Igbo; ‘Ikhimi’ in Edo and ‘Akoko’ in Yoruba languages [6]. N. Laevis is widely used in African folk medicine for the treatment of malaria and fever, stomachache, coughs, sexually transmitted diseases, tooth ache, breast cancer, and constipation [7]. In Southeastern part and part of the Mid-western Nigeria, the plant is used for the treatment of septic wounds and eye problems [5]. Scientific reports on the phytochemical constituents of the plant revealed the presence of alkaloids and phenylpropanoids in the root, flavonoids, and tannins in the leaf as revealed by [8].
STATEMENT OF PROBLEMS
Research shows that Newbouldia Leavis Leaves is widely used in Nigeria as developing country (especially rural towns) as drugs for treatments of many diseases like malaria and fever, these leaves are widely used without prescription, frequent used can cause liver failure and other organs failure, the challenges of Newbouldia Leavis Leaves lack scientific proof for authentication. Also, these towns do not have access to hospitals and other modern medical facilities for their treatments. Thus, it becomes necessary to investigate the phytochemicals present. Phytochemical screenings are now seen as the first step toward the discovery of useful drugs that the nature has been identified as a potential source due to its diverse presence in plants.
JUSTIFICATION
Results from this research will enlighten us on the phytochemicals presents, the amount of Newboudia Laevis leaves that can be used as first aid when if consumed will have no or low effects on human health, findings from this research can be used as guide for other work.
AIM
The aim of this study is determined the quantitative and qualitative presents in Newboudia Laevis Leaves.
OBJECTIVES
To determine the phytochemicals, present in Newboudia Laevis leave
To determine the qualitative and quantitative analysis on Newboudia Laevis leave
To identify the concentration of secondary metabolites, present in Newbouldia laevis leaves.
MATERIALS
Leave sample, beaker, conical flask Wagner's reagent, 42 (125 mm) Whatman filter paper
COLLECTION, PREPARATION
The leaf samples were collected from Zaria, kaduna state of Nigeria, the samples were dried for two weeks at room temperature. The powdered plant sample part was stored in an airtight labeled plastic container and were used for extraction purposes. The method of cold maceration was used in the extraction by serial exhaustive extraction method. This involves successive extraction with (ethanol). The leaf extract was prepared by soaking 50g of the sample in 500ml ethanol for three days with frequent agitation until soluble matter was dissolved. The resulting mixture was filtered using filter paper and the filtrate was concentrated by evaporation using rotary evaporator.
PHYTOCHEMICAL SCREENING
Phytochemical examinations were carried out for all the extracts using standard procedures to identify the constituents. Qualitative and Quantitative analysis of the crude extracts was carried out to identify the presence of the classes of Secondary Metabolites (alkaloids, flavonoids, tannins, saponins and phenol).
QUALITATIVE ANALYSIS OF THE PHYTOCHEMICALS OF THE NEWBOULDIA LAEVIS LEAVES
Test for Tannins
The leaf powder sample (0.50 g) was weighed into a test tube and boiled for 15 minutes in a water bath containing 40 cm3 of water. Filtration was carried out after boiling using number 42 (125 mm) Whatman filter paper. To 6 cm3 of the filtrate was added 3 drops of 0.1% ferric chloride. A brownish green coloration present
Test for Saponin
Distilled water (50cm3) was added to leave powder samples (0.50g) and boiled for 15 minutes in water bath and filtered using Whatman filter paper number 42 (125mm). A mixture of distilled water (6cm3) and filtrate (12cm3) was agitated vigorously for a stable persistent froth. The formation of emulsion on addition of three drops of olive oil showed positive result.
Test for Flavonoids
The sample (0.50g) was weighed into a beaker was extracted with 50cm3 of distilled water for 2 hours and filtered with Whatman filter paper number 42 (125mm). To 15cm3 of the aqueous filtrate of the leave extract was added 10cm3 of 1.0M dilute ammonia solution followed by the addition of 6cm3 of concentrated tetraoxosulphate (VI) acid. Appearance of yellow coloration which disappeared on standing shows the presence of flavonoids.
Test for Alkaloids
Extraction of component from 4 grams of leave powder sample was carried out using 7% tetraoxosulphate (VI) acid (H2SO4) (30cm3) in 50% ethanol by boiling for 2 minutes and filtered through Whatman filter paper number 42 (125mm). The filtrate was made alkaline using 7 cm3 of 28% ammonia solution (NH3) in a separating funnel. Equal volume of chloroform (6.0cm3) was used in further solution extraction in which chloroform solution was extracted with two 5cm3 portions of 1.0M dilute tetraoxosulphate (VI) acid. This final acid extract was then used to carry out the following test: 0.5cm3 of Dragendorff’s reagent (Bismuth potassium iodide solution) was mixed with 2cm3 of acid extract and precipitated orange colour.
Test for phenol
To 4ml of the extract 4ml of 5% aqueous ferric chloride was added, a blue color was seen.
QUANTITATIVE DETERMINATION OF PHYTOCHEMICAL OF NEWBOULDIA LAEVIS LEAVES
Determination of tannins
Analytical method for quantitative determination of tannin was according to [9] and [10]. By dissolving 50 g of sodium tungstate (Na2WO4) in 37 cm3 of distilled water, Folin-Denis reagent was made. To the reagent prepared above, 10 g of phosphomolybdic acid (H3PMo12O40) and 25 cm3 of orthophosphoric acid (H3PO4) were added. Two-hour reflux of the mixture was carried out, cooled, and diluted to 500 cm3 with distilled water. One gram of each wood powder (sample) in a conical flask was added to 100 cm3 of distilled water. This was boiled gently for 1 hour on an electric hot plate and filtered using number 42 (125 mm) Whatman filter paper in a 100 cm3 volumetric flask. Addition of 5.0 cm3 Folin-Denis reagent and 10 cm3 of saturated Na2CO3 solution into 50 cm3 of distilled water and 10 cm3 of diluted extract (aliquot volume) was carried out after being pipetted into a 100 cm3 conical flask for colour development. The solution was allowed to stand for 30 minutes in a water bath at a temperature of 25°C after thorough agitation. With the aid of a Spectrum Lab 23A spectrophotometer optical density was measured at 700 nm and compared on a standard tannic acid curve. Dissolution of 0.20 g of tannic acid in distilled water and dilution to 200 cm3 mark (1 mg/cm3) were used to obtain tannic standard curve. Varying concentrations (0.2–1.0 mg/cm3) of the standard tannic acid solution were pipetted into five different test tubes to which Folin-Denis reagent (5 cm3) and saturated Na2CO3 (10 cm3) solution were added and made up to the 100 cm3 mark with distilled water. The solution was left to stand for 30 minutes in a water bath at 25°C. Optical density was ascertained at 700 nm with the aid of a Spectrum Lab 23A spectrophotometer. Optical density (absorbance) versus tannic acid concentration was plotted.
The following formula was used in the calculation:
% Tannin = Weight of tannin/ Weight of sample ×100
Determination of Alkaloids
Exactly 200cm3 of 10% acetic acid in ethanol was added to leave powder sample (5g) in a 250cm3 beaker and allowed to stand for 4 hours. The extract was concentrated on a water bath to one-quarter of the original volume followed by addition of 15 drops of concentrated ammonium hydroxide dropwise to the extract until the precipitation was complete immediately after filtration. After 3 hours of mixture sedimentation, the supernatant was discarded and the precipitates were washed with 20cm3 of 0.1M of ammonium hydroxide and then filtered using Gem filter paper (12.5cm). Using electronic weighing balance Model B-218, the residue was dried in an oven and the percentage of alkaloid is expressed mathematically as
% Alkaloid = Weight of alkaloid/ Weight of sample × 100
Determination of Flavonoid
Exactly 50cm3 of 80% aqueous methanol added was added to 5g of sample in a 250cm3 beaker, covered, and allowed to stand for 24 hours at room temperature. After discarding the supernatant, the residue was reextracted (three times) with the same volume of ethanol. Whatman filter paper number 42 (125mm) was used to filter whole solution of leave sample. The leave sample filtrate was later transferred into a crucible and evaporated to dryness over a water bath. The content in the crucible was cooled in a desiccator and weighed until constant weight was obtained. The percentage of flavonoid was calculated as
% Flavonoid = Weight of flavonoid/ Weight of sample × 100
Determination of Saponin
Exactly 100cm3 of 20% aqueous ethanol was added to 5 grams of leave powder sample in a 250cm3 conical flask. The mixture was heated over a hot water bath for 4 hours with continuous stirring at a temperature of 55°C. The residue of the mixture was reextracted with another 100 cm3 of 20% aqueous ethanol after filtration and heated for 4 hours at a constant temperature of 55°C with constant stirring. The combined extract was evaporated to 40 cm3 over water bath at 90°C. 20 cm3 of diethyl ether was added to the concentrate in a 250 cm3 separator funnel and vigorously agitated from which the aqueous layer was recovered while the ether layer was discarded. This purification process was repeated twice. 60 cm3 of n-butanol was added and extracted twice with 10 cm3 of 5% sodium chloride. After discarding the sodium chloride layer the remaining solution was heated in a water bath for 30 minutes, after which the solution was transferred into a crucible and was dried in an oven to a constant weight. The saponin content was calculated as a percentage:
% Saponin = Weight of saponin/ weight of sample ×100
Determination of Phenols
The reaction mixtures were kept in dark for 90minutes and absorbance was read in Spectrophotometer at 650 nm. The standard solution of phenol was prepared with 200 mg of catechol dissolved in distilled water and made upto 100 ml. The working solution (10mg, 20mg,30mg---140mg) was prepared by diluting the stock solution with distilled water in the proportion 1: 10. where 'Y' is the concentration of total phenols in µg and 'X' is the optical density. The phenol concentration in the extract was calculated from the graph and expressed as mg of catechol equivalent per mg of the extract.
% Phenol = Weight of phenol / Weight of sample ×100
RESULTS
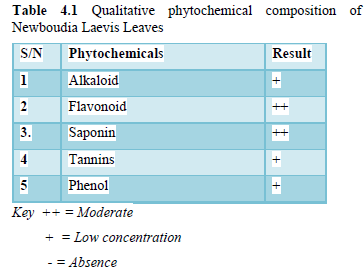
(Table 4.1) shows the Qualitative Phytochemicals in Newbouldia laevis leaves, the result shows the presents of low concentration of Alkaloid. Alkaloid are found to be important in pharmaceutical industries of which are widely used in production of many drugs, anti-malaria, anti-inflammatory, anticancer, antimicrobial effects. Alkaloid can be used as pain relief as first aid but should not be above standard. The result shows the high presents of flavonoid several authors reported on flavonoid groups exhibited a wide range of biological activities such as antioxidant, anti-inflammatory, anticancer and anti-allergic. Also, the result revealed the present of Tannis, their medical properties include antioxidant, anti-inflammatory, antimicrobial effects. Some research indicate tannis may help protect the heart reducing cholesterol build up, preventing the oxidation of LDL cholesterol and modulating blood pressure. Phenol is present in low concentration which normally be involved in defense against ultraviolet radiation or aggression by pathogens, parasite and predators as well as causative to plant colours.
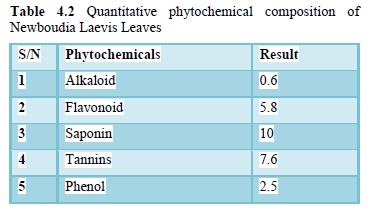
(Table 4.2) shows the result for Quantitative phytochemicals in Newbouldia laevis leaves. The total alkaloid content was 0.6% which was the lowest it implies that the plant does not contain much properties of Alkaloid,so it cannot be used for analgesia. Flavonoid content was 5.8% which tends to be most commonly known with regards to antioxidant nature, they are transformers which alter the body biochemical reactions to carcinogenic chemicals, viruses and things that trigger allergies. Phenol content was 2.5% which compounds are some of the most widespread molecules among plant secondary metabolites, are known to act as normal antioxidant. Saponin content was 10% which is the highest, it is extensively utilized in veterinary vaccines because their character as an adjuvant and helps in the improvement of immune response. Many of them are useful in intracellular histo-chemistry staining permitting antibody access to intranet protein molecules. Tannins content was 7.6%, consuming too many tannins could be harmful to humans. This is due to the fact that tannins are chelator for metal ion and because tannins - chelators for metal ions are not bioavailability and cause anemia.
CONCLUSION
Newbouldia laevis leaves contains many phytochemicals which are used in many pharmaceutical industries in the production of drugs in the world, Alkaloid, flavonoid, saponin, tannins and phenol were found to be present. The diversity of phytochemical present suggested that N. laevis could serve as a source of drugs.
RECOMMENDATION
- Advanced analysis should be used to accurately know the phytochemicals presents like the use of Higher performance liquid chromatography (HPLC).
- Consumption of Newbouldia Laevis should be below standard to avoid any damage
- Newboudia laevis leaves can be use in pharmaceutical industries in the production of drugs; more research should be done.
- WHO (1998) Regulatory situation of herbal medicines. A worldwide review. Pp: 1-5. Geneva, Switzerland.
- Hamburger M, Hostettmann, K (1991) Bioactivity in plants: the link between phytochemistry and medicine. Phytochemistry 30: 3864- 3874.
- Refaz Ahmad Dar, Mohd Shahnawaz, Parvaiz Hassan Qazi (2017) General overview of medicinal plants: A review: The J Phytopharmacology 6(6): 349-351.
- Idu M, Obaruyi GO, Erhabor JO (2009) Ethnobotanical uses of plants among the binis in the treatment of ophthalmic and ENT (Ear, Nose and Throat) ailments. Ethnobotanical Leaflets. 13:480-496
- Usman H, Osuji JC (2007) Phytochemical and in vitro Newbouldia laevis. African J Traditional Compliance and Alternate Medicine 4(4): 476-480.
- Hutchinson J, Dalziel JM (1963) Flora of West Tropical Africa vol II. London: Crown Agents for Oversea Government and Administration Pp: 435-436.
- Arbonnier M (2004) Trees, shrubs and lianas of West African dry zones. CIRAD, Margraf Publishers, GMBH MNHN, Cote d’Ivoire. 194
- Germann K, Kaloga M, Ferreira D, Marais JP, Kolodziej H (2006) Newbouldioside A-C phenylethanoid glycosides from the stem bark of Newbouldia leavis. Phytochem. 67: 805–811.
- Ahmed O, Mohammad Ali (2014) Qualitative and Quantitative Analysis of Phytochemicals of Loranthus bengwensis leaf. Int. R J Pharm. Sci 05(01): 1-3.
- Ejele AE, Duru IA, Ogukwe CE, Iwu IC (2012) Phytochemistry and antimicrobial potential of basic metabolities of Piper umbellatum, Piper guineense, Ocimum gratissimium and Newbouldia laevis extracts. J Emer Trends Eng. Appl. Sci 3(2): 309-314.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Pathology and Toxicology Research
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- International Journal of Diabetes (ISSN: 2644-3031)


