1499
Views & Citations499
Likes & Shares
In this work, a three-dimensional model of dermal equivalent was used with the purpose of differentiating of HFBSCs, obtained in a previous study, towards the epithelial lineage in humans and in mice by means of histological techniques. As possible therapeutic potential of keratinocytes to be used in tissue regeneration of bloody areas of the skin, such as chronic ulcers and burns [4, 5].
In recent years, progress has been made in the knowledge of the differentiation of SCs, to which the epithelium- mesenchyme interaction that generates the microenvironment that promotes the maintenance of stem cells is mainly attributed [6]. The decision between self-renewal and differentiation is influenced by a specialized microenvironment called the cell niche, defined as the smallest dynamic multicellular and structural unit that regulates stem cell self- renewal and differentiation decisions [7,8] considered physiologically dynamic domains [9] where physical and molecular interactions with the cell niche and the orientation of the cell division plane during SCs stem cells mitosis control the balance between symmetric and asymmetric division of SCs stem cells [10]. Finding at least three niches located in the skin: the region closes to the prominence of the hair follicle, at the base of the sebaceous glands, and in the basal cells of the epidermis. Emphasizing that the three niches are in close proximity to the basement membrane, which is known to be rich in extracellular matrix, ligands, and growth factors that provide a series of external stimuli for which it has been indicated as a determining factor in obtaining active SC stem cells, in addition to playing an important role in the control of the surrounding dermis since they can migrate and participate in the repair of wounds in the epidermis [7,8,11-17].
In order to direct, our hypothesis states that the maintenance of SC stem cells is influenced by the microenvironment. If key factors are supplied in in vitro studies in cultures that allow similar conditions to the tissue of interest, then the cells could be induced to differentiate into different lineages depending on the conditions supplied.
MATERIALS AND METHODS
Tissue Samples
Follicles were obtained from the bodies of newborn (2 to 3 day old) mice (n = 10). All animals were acquired from the animal facility of the Instituto Nacional de Higiene Rafael Rangel. Also, excess healthy human scalp skin was collected from 30 to 58year old facelift patients (n = 10). All studies had the approval of the ethical committee of the Faculty of Sciences of the Universidad Central de Venezuela and written informed consent.
Tissue Isolation and Cultivation
The newborn mice were sacrificed, kept in 70% alcohol for 10 min, and washed twice for 5 min with phosphate- buffered saline (PBS). The tissues were trimmed into small pieces (4 mm × 4 mm), and the skin fragments were incubated in 0.25% dispase II (Bacillus polymyxa, Gibco, BRL) in DMEM/F12 (1:1; Gibco-BRL) for 12-18 h at 4˚C. Scalp tissues were first rinsed in lactated Ringer’s solution, and excess adipose tissue was removed. The hair follicles were squeezed out carefully in anagen phase, identified by the visible bulb and intact ORS, and carefully selected under the dissecting using micro forceps and a stereoscope. After two rinses with F-12, the follicles were transferred into a 35-mm dish. Then the bulge region was amputated from the upper follicle by making two transverse cuts at the site of the enlargement spots of the ORS with a fine needle. All surgical procedures were performed in a sterile environment. After an additional two rinses, the bulges were transferred into a new dish at a density of 20 per dish, immersed in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) and Ham’s F12 medium (3:1; Gibco), supplemented with 10% fetal bovine serum (FBS; Gibco), 10 ng/ml epidermal growth factor (Invitrogen), 5 g/ml hydrocortisone, 5 g/ml insulin (Sigma-Aldrich), 200 mmol/L L-glutamine (Gibco), and antibiotics (100 U/ml penicillin and 100 g/ml streptomycin). The culture was incubated at 37 ˚C with 5% CO2 in air, and the medium was changed twice a week. Human scalp tissues were first rinsed in lactated Ringer’s solution and Ham’s F12 with a double concentration of antibiotics (penicillin G, 100 μg/ml, and streptomycin, 100 μg/ml). This solution was used as a transport medium to maintain aseptic conditions and optimal physiological conditions. Next, the samples were rapidly washed in 70% alcohol and sectioned in the same way as the samples of mouse skin.
Subculture and Amplification
Colony formation was performed after 3-4 days in primary culture; cells were collected by incubation with a 1:1 mixture of 0.125% trypsin (Sigma) and 0.02% EDTA (Sigma) for 5-10 min at 37˚C. The dispersed cells were centrifuged for 5-10 min at 2500 rpm and replated in 35-mm dishes coated with gelatin (mouse) and type I collagen (human) in tissue culture flasks with a medium change every 3-4 days. Cells were routinely passaged every 7 days. Every week, the cells were trypsinized (Gibco), and mass cultures were serially passaged until growth capacity was exhausted. Maintained an undifferentiated phenotype of the stem cells as described [5].
Immunohistochemical Staining
Cells, plated in 35-mm dishes, were washed twice for 5 min with PBS and fixed in methanol for 5 minutes. Peroxidase and nonspecific antibody binding were blocked by incubation with 1% H2O2/methanol and serum for 10 min at room temperature, followed by incubation with primary antibodies (anti-mouse CK15, clone LHK15; Thermo Fisher) in mouse and human, anti-mouse cluster of differentiation CD34 IgG (eBioscience) in mouse, and anti-human CD200 IgG (eBioscience) in human, diluted 1:100 overnight at 4˚C for 30 min. The cells were then washed 3× for 5 min to remove the unbound primary antibody and incubated with a secondary antibody (kit) for 10 min at 37˚C; unbound second antibody was removed by washing 3× for 5 min and visualized by DAB kit (Dako). Cells whose cytoplasm appeared brown were taken to be positive cells. Counterstaining with hematoxylin was performed when it was necessary to identify the bulge area. Also, for dermal equivalent and co-culture models was employed specific proteins for epidermal keratinocytes were used, such as pan-cytokeratin (1:100; B311.1, Santa Cruz Biotechnology) and E- cadherin (1:100; sc-8426, Santa Cruz Biotechnology).
Differentiation into Epidermal Cells
HFBSCs were cultured, and fibroblasts were used to carry out the cell differentiation tests towards the epithelial lineage, from which the dermal equivalent and co-culture were reconstituted. Two different cultures of DMEM medium were used; the first was supplemented with 10 ng/ml (EGF) as a control, and the second with a double concentration of epidermal growth factor (20 ng/ml) as an experimental model.
To obtain fibroblasts, the skin dermis was obtained from some samples obtained from humans and mice. The dermis was cut into fine fragments (1 mm) and placed in culture plates, then the nutrient medium DMEM supplemented with 10% of FBS was added. The cultures were then incubated at 37 °C in a humid atmosphere with 5% CO2. Subsequently, once the cells were irradiated from the explants, subculture was performed. These cells were used for the establishment of monolayer culture and co-culture.
Dermal Equivalent
To make the dermal equivalents, collagen obtained from the tail of a rat was used according to the methodology of Van Bockmeer [18]. The dermal equivalents consisted of a type I collagen matrix, a three- dimensional substrate formed by an intricate network of fibers with the consistency of a flexible and transparent gel where human and mouse dermal fibroblasts were embedded (Figure 1). The fibroblasts were placed in a collagen stock and a solution of DMEM medium and sodium hydroxide (DMEM 10X: NaOH 1M, ratio 2:1) at a density of 5,000 cells/ml, and then the collagen was allowed to polymerize for a few minutes in the CO2 oven. These equivalents were used after 3 to 4 days of preparation. Subsequently, HFBSCs were cultured on the dermal equivalent, seeded at a density of 1x104 cells per well and left for around 2 to 3 h. After this time had elapsed, the nutrient medium was added, and it was left under submerged conditions for 2 to 3 days until semi-confluence was achieved. Subsequently, the differentiation inducing medium was added to this culture, and it was placed in an air-liquid interface. To induce differentiation, two culture systems were performed:

In the first system, the cells of the prominence region of the follicles of mice (M) and humans (H) were cultured in the type I collagen membrane with fibroblasts and a double concentration of base medium (M/H + F + 20 ng/ml EGF) and for their control, the cells were seeded in the absence of fibroblasts (M/H + 20 ng/ml EGF) (Figure 1).
In the second system, the cells of the prominence region of the follicles of mice and humans were seeded in the type I collagen gel with fibroblasts and base medium (M/H + F + 10 ng/ml EGF); for control, the cells were seeded in the absence of fibroblasts with base medium (M/H + 10 ng/ml EGF) (Figure 1).
These dermal-epidermal equivalents were fixed in Rossman for 15 min at room temperature and then placed in 70% ethanol for further evaluation.
Co-culture models
Once the homogeneous populations of mouse (M) and human (H) HFBSCs were obtained from the primary cultures, they were seeded in secondary cultures at a density of 1x104 cells per well on the inserts with a Millipore membrane. Simultaneously, the fibroblasts were cultured in 45-mm dishes, and later the insert was placed inside a plate containing the fibroblasts and double concentration of base medium was added in the M/H + F + 20 ng/ml EGF model and the base medium in the model (M + F + 10 ng/ml EGF) (Figure 2).

In the control co-culture models were performed without fibroblasts with double concentration of base medium in the model was added (M/H + 20 ng/ml EGF), the base medium in the model (M + 10 ng/ml EGF) (Figure 2). Cultures were incubated at 37°C and 5% CO2 with periodic medium changes. The induction of cell differentiation was evaluated using phase contrast microscopy and immunocytochemical methods.
RESULTS
Dermal equivalents
In differentiation assays using dermal equivalents during culture follow-up, they showed a stable and manageable consistency. The cultured fibroblasts appeared in a few hours with a great ability to emerge and acquired their typical morphology emitting cytoplasmic prolongations within the collagen gel and the progressive increase in the number of fibroblasts present could be observed under an inverted microscope.
Once the homogeneous populations of cells from the region of the prominence of mouse and human follicles were obtained from the primary cultures, they were seeded in secondary cultures on the dermal equivalents showing colony formation and reached semi-confluence in 15 days.
When analyzing the morphological aspect of the cells and their behavior in vitro in the dermal equivalent, it was possible to observe, in the initial stages, a heterogeneous population consisting mainly of 4 different cellular phenotypes, which were evidenced with phase contrast microscopy as described in the following: polygonal - shaped cells with a centrally located spherical nucleus, growing in mosaic, forming homogeneous colonies (keratinocyte-like cells); refringent cells, small, rounded, with scant cytoplasm and a central nucleus with a prominent nucleus (stem- like cells); cells with spindle-shaped morphology with a central oval nucleus, several nuclei, and thin cytoplasmic extensions (fibroblast-like cells); and cells with a central nucleus, with a large cytoplasmic volume, with dendritic extensions that were seen on the periphery of the monolayer (similar to neuronal lineage cells).
In the first culture system (M + F + 20 ng/ml EGF) initially the round cells grew, forming colonies from which cells of different morphologies irradiated, with a gradual increase in the population giving rise to the formation of a monolayer of small polygonal cells in a period of 2 days, reaching confluence 7 days after starting the culture with a predominance of cells with a polygonal morphology similar to keratinocytes (Figures 3) where a monolayer with a typical pattern of mosaic epithelial cells could be seen and the cell colonies tended to decrease, transforming into polygonal cells between 14 and 16 days of culture. In addition, it is important to highlight the presence of numerous cells in mitosis. Also, under these culture conditions, cells with dendritic extensions were observed, a morphology compatible with melanocytes (Figure 3H).
In the second culture system (M + F + 10 ng/ml EGF) confluence, aggregation, and formation of monolayers were observed (Figure 3C). The round cell colonies grew, maintaining their phenotype. After around 7 days, cells that began to irradiate from the colonies could be seen with a polygonal morphology, similar to keratinocytes. However, in this system, colonies of round cells were maintained, and the differentiation towards a keratinocyte-like phenotype was less evident at the beginning of cultivation, compared to the other system (M + F + 20 ng/ml EGF). In the control culture systems (M + 20 ng/ml EGF) and (M + 10 ng/ml EGF), a cell population made up mainly of rounded cells forming colonies (Figures 3B and D) was initially observed. After 9 days of culture, differentiation into cells with a polygonal phenotype conforming to the monolayer.
In humans, the model cultures (H + F + 20 ng/ml EGF) will be followed by some colonies after 3 to 4 days of culture, which grew and transformed into polygonal cells after 7 days of culture, almost completely decreasing the round cell colonies. However, the growth of these cells was slow when compared to that of the mouse; semi-confluence appeared 12 days after starting the culture, and similarly, they grew in a monolayer with a typical pattern of mosaic polygonal cells (Figures 4A and B). The control culture system, in the absence of fibroblasts (H + 20 ng/ml EGF) (H + 10 ng/ml EGF), managed to obtain cell monolayers made up mainly of two types: round cells and polygonal cells.


In the dermal equivalents, morphological changes of the HFBSCs were evidenced, and they were evaluated by determining the expression of characteristic markers of the epidermal cells. In the process, cells that changed their morphology from rounded to polygonal with a rounded nucleus and central location were observed; in some cases, a population of cells with a reduced nucleus and cells with apparently more prominent spherical nuclei were observed. It was possible to demonstrate positive expression of Pan-CK in the superficial cells of the equivalent model (M + F+ 20 ng/ml EGF) (Figure 5A), (M + 20 ng/ml EGF) (Figure 5B), (M + F + 10 ng/ml EGF) (Figure 5C), and (M + 10 ng/ml EGF) (Figure 5D) (Table 1).
Considering the results obtained, frozen sections of some equivalents were made to describe the histological characteristics of the cells. For comparative purposes, skin samples taken directly from one of the donors (Figure 5E) showing high expression for Pan-CK were used as a positive control. The fibroblasts were distributed in a kind of network, reminiscent of their arrangement in the dermis, thus remaining inside the gel without colonizing the space occupied by keratinocyte-like cells. However, a growth of round cells that migrated within the equivalent was observed (Figure 5F).
Demonstrating reaction for Pan-CK in cells with a phenotype similar to that of cells of the epithelial lineage (Figures 5F, G) located on the external surface of the gels, which grew and organized remembering the two main layers that make up the integument, the dermis and the epidermis, as well as a positive reaction of E-cadherin at the level of the cytoplasm and the cellular interaction sites in the subcultures (Figure 5H), constituting together the epidermal equivalents. It is important to mention that the controls for both determinations were negative (Table 1).

Similar to the results were obtained in human, positive expression of varied intensity for Pan-CK in the apical cells of the dermal equivalent (Figures 6A-C) and in longitudinal sections two layers of spindle cells with a central nucleus are shown resting on rounded cells, an organization that resembles a partially stratified epithelium (Figure 6B). E- cadherin expression was also evidenced (Figures 6A, B). For comparative purposes, skin samples taken directly from one of the donors showing high expression for Pan-CK were used as a positive control (results not shown).
Co-culture models
In the differentiation assays, the fibroblasts were cultured, which proliferated with a progressive increase in the population and acquired their typical morphology.
Once the homogeneous populations of HFBSCs of mouse and human follicles were obtained from the primary cultures, they were seeded in secondary cultures, reaching confluence at 15 days, which allowed obtaining cell monolayers made up mainly of two types: polygonal cells and rounded cells. To induce differentiation, two culture models were standardized with their respective controls, described in the "Materials and Methods" section (Figures 1 and 2).


In the co-cultures of mouse HFBSCs CRPFPR with fibroblasts and in the inducing medium (M + F + 20 ng/ml EGF), it was observed that the cells adopted a polygonal morphology, organizing one next to the other and forming a monolayer with morphological characteristics similar to those of the simple epithelium in the first days of culture (Figures 7A-D). After one week of starting the co-cultivation, most of the CRPFPs were transformed into cells with typical keratinocyte morphology. Immunocytochemistry revealed the expression of Pan-CK in the cytoplasm (Figure 7A). Similar results were found in co-cultures of CRPFP with fibroblasts and base medium (H/M + F + 10 ng/ml EGF), with greater proliferation of rounded cells (Figure 7C).
In the control co-cultures without fibroblasts, morphological changes were observed where the cells presented a transition state between rounded and polygonal (Figures 7B, D), and polygonal and rounded cells showed a positive reaction for Pan-CK (Table 1). Due to the limitations in obtaining hair follicle stem cells for human HFBSCs, a co- cultivation assay was carried out with two replicates and their respective controls.

In the co-cultures of human HFBSCs with fibroblasts and inducing medium, morphological changes from round cells to polygonal cells were observed on the third day of culture. After 8 days, the cells were arranged side by side (Figures 8A, C, E and G), with a predominance of polygonal cells over round cells and exhibiting a positive reaction to Pan- CK. In the co-cultures without fibroblasts and in the inducing medium (Figures 8B. D, F and H), morphological changes were observed where the cells maintained a transition state between rounded and polygonal, showing a positive reaction to Pan-CK (Table 1).
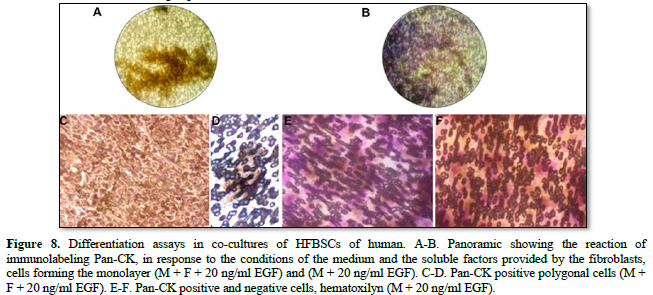
DISCUSSION
Our results reveal that HFBSCs showed differentiation towards the epidermal lineage influenced by microenvironment changes. Consistently, SCs undergo an adaptive differentiation process when cultivated in a specific medium, which induces the modification of the expression of certain genes and generates a transition from an undifferentiated phenotype (SCS) towards a defined lineage. Terminal differentiation of these cells is achieved when they express phenotypic properties characteristic of a functional mature cell in vivo.
Various studies have shown that HFBSCs can differentiate into neurons, glial cells, keratinocytes, muscle cells, and melanocytes in vitro [5,19-28]. As an advantage, the hair follicle contains different types of progenitor cells derived from the ectoderm (keratinocytes, stem cells), mesoderm (fibroblasts of the dermal papilla) and neural crest (progenitor melanocytes), which makes it an excellent source of stem cells for study.
In our study, a comparison of two three-dimensional models was established: a dermal equivalent and a co-culture model, which revealed that fibroblasts play a fundamental role so that RPFP cells can be differentiated into epithelial cells.
In this regard, it is known that the dermal equivalent offers cell a microenvironment and a spatial organization similar to tissue in vivo by virtue of simulating the normal properties of the skin, serving as support for cells and actively influencing skin processes such as adhesion, migration, differentiation and cell growth [29]. Likewise, as has been suggested in previous studies [30,31] also type I collagen as a source of extracellular matrix is the most abundant protein in the dermis, where fibroblasts are capable of synthesizing multiple growth factors similar to those they secrete in vivo. Also, it is known that hepatocyte growth factor (HGF) and keratinocyte growth factor (KGF) are produced only by fibroblasts, which bind to receptors found mainly in epidermal cells with mitogenic capacity and are classic examples of paracrine growth factors [32] that contribute to dermal-epidermal interactions. In addition, fibroblasts produce insulin-like growth factor (IGF-1), which stimulates the synthesis of sulfated proteoglycans, collagen, keratinocyte migration, and fibroblast proliferation [33]. For this reason, dermal fibroblasts play an important role in the regeneration process of skin tissues, and the presence of human fibroblasts allows the differentiation, expansion, and rapid clonal growth of keratinocytes [34].
This fact is demonstrated by the histological and immunocytochemical studies carried out, since the development of an epithelium with partial stratification was evidenced. Although a complete differentiation of all the layers of the epithelium was not achieved, there is a marked morphological change to the polygonal and spindle. Among the possible causes could be the brief time of induction and maturation of the system, because in other works during the third week, a development in the stratification of the epithelium has been evidenced [34].
CKs constitute the main component of the cytoskeleton of epithelial cells, where they form a large group of intermediate filaments. Intermediate filaments correspond to a large multigenic family of fibrillar proteins that include cytokeratins, characteristic of epithelia [35]. Together with E-cadherins, are part of a superfamily of transmembrane glycoproteins that are involved in cell-cell adhesion. Likewise, the expression and function of E-cadherin are essential for many processes during embryonic development, such as cell polarization and epithelial differentiation [36]. That is why the analysis of our results was based mainly on the morphological changes and the expression of intermediate filaments of cytokeratin and E-cadherin, finding expression levels of the Pan-CK proteins (complex of cytokeratins 4, 5, 6, 8, 10, 13, and 18) present in the cytoplasm of the cells and that of E-cadherin present in the desmosomes, being used as indicators of the differentiation towards epidermal cells and confirming that the cells maintain characteristics associated with highly proliferative epithelial cells. Showing correlation with the structural characteristics of the different layers of the epidermis in mice and humans.
In the dermal equivalents, the presence of collagen I and fibroblasts they stimulated cell differentiation and proliferation, forming a monolayer of cuboidal cells with a characteristic morphology typical of epithelial cells. Jointly, the complexity of the extracellular matrix is a significant component in the phenotypic expression of the cells attached to it [32]. For this reason, an attempt was made to simulate normal physiological conditions by keeping the cells in liquid medium for 10 days and for 4 days in the air-liquid interface to promote cell differentiation, stimulating the stratification of cells located on the stromal substitute.
Similarly, in the experimental models, the difference lay in time to reach confluence, and this process could be more accelerated when double concentration of EGF was added, as evidenced by greater cell proliferation. It is known that epidermal growth factor (EGF) is a polypeptide that exerts a wide variety of biological effects, including the promotion of cell proliferation and differentiation, due to its role in RNA transport and protein synthesis [5], contributing to the differentiation and activating the expression of genes related to the epithelial phenotype. Therefore, the growth time with colony formation and the time to cell confluence are linked to the intrinsic capacity of the cell, in addition to substrate conditions, nutrients, and hormonal factors such as EGF [37-40]. In the control models, the culture system in the absence of fibroblasts made it possible to obtain monolayers of cells, mainly keratinocytes, but this system was not so evident since its degree of stratification was limited.
In the present work, we have used a co-culture system of adult cells from the region of the prominence of hair follicles and the fibroblasts, separating both cell types by a polycarbonate membrane with pores of a size that prevents any type of contact between the cells. The polycarbonate membrane is a synthetic polymer used in the differentiation of epithelial cells in tissue engineering and has been used in the study of cell-cell and cell-extracellular matrix interactions, in the differentiation, polarity, and trans epithelial permeability [32], allows the exchange of soluble factors synthesized by the cells present in the co-culture. Furthermore, can induce polarity in the cell by simulating the basement membrane, such polarity may be vital for the full functional expression of secretory epithelia and many other cell types. Additionally, it has been reported that polycarbonate membranes favor the synthesis of keratinocytes and promote interactions between the two cell types that constitute the co-culture, being able to simulate the epithelial-mesenchymal interaction that occurs during the embryonic development of the epithelia [32,41].
In co-culture model, the ICQ determination allowed showing a moderate positive expression and a wide distribution of Pan-CK in the cells induced to differentiation, both in mice and in humans. However, when making the photographic records, it was difficult to obtain a better clarity of the cells due to the presence of the pores in the membrane, so it is recommended to consider this situation in future work.
CONCLUSION
Our data indicated that the hair follicle bulge stem cells in humans and mice present similar behavior in vitro in the analysis of the morphological aspects in different culture, conditions using two three-dimensional models such as the dermal equivalent and the co-culture. These cells showed promising plasticity in vitro, as confirmed by phenotypic changes and by the expression levels of Pan-cytokeratin and E-cadherin as indicators of differentiation into epithelial lineages. Dermal equivalent assays reveal the importance of the combined action of type I collagen and fibroblasts as substitutes for an extracellular matrix, allow a more evident differentiation pattern compared to the co-culture system. In addition, EGF supplementation had notable effects on the differentiation potentials. The results suggest that human and mouse hair follicle cells may be presented as an alternative source of easily accessible autologous stem cells for tissue engineering and regenerative medicine.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the support provided by Dr. Gerald Schatten of the University of Pittsburgh for reviewing the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest regarding the publication of this paper.
- Clarke DL, Frisén J (2001) Differentiation potential of adult stem cells. Curr Opin Genet Dev 11(5): 575-580.
- Amoh Y, Hoffman RM (2017) Hair follicle-associated-pluripotent (HAP) stem cells. Cell Cycle 16: 2169-2175.
- Molina BJ, Giansante E, Finol HJ (2018) Identification of bulge stem cells in mouse and human hair Microsc Res 6: 19-29.
- Cheng X, Yu Z, Song Y, Zhang Y, Du J, et al. (2020) Hair follicle bulge-derived stem cells promote tissue regeneration during skin expansion. Biomed Pharmacother 132: 110805.
- Molina BJ, Finol HJ (2020) Isolation, Cultivation, and Morphological Characteristics of Hair Follicle Adult Stem Cells in the Bulge Region in Mouse and Human. Microsc Res 8: 2.
- Morrison SJ, Spradling AC (2008) Stem Cells and Niches: Mechanisms That Promote Stem Cell Maintenance throughout Life Cell 132: 598-611.
- Mitsiadis TA, Barrandon O, Rochat A, Barrandon Y, De Bari C (2007) Stem cell niches in mammals. Exp Cell Res 313(16): 3377-85.
- Hsu Y, Pasolli HA, Fuchs E (2011) Dynamics between Stem Cells, Niche, and Progeny in the Hair Follicle. Cell 144: 92-105.
- Voog JC, Jones DL (2010) Stem cells and the niche: A dynamic Stem cell 6(2): 103-115.
- Blanpain C, Fuchs E (2009) Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 10: 207-217.
- Fuchs E, Tumbar T, Guasch G (2004) Socializing with the Neighbors Stem Cells and Their Niche. Cell 116: 769-778.
- Tiede S, Kloepper JE, Bodó E, Tiwari S, Kruse C, et al. (2007) Hair follicle stem cells: walking the maze. Eur J Cell Biol 86 (7): 355-376.
- Waters JM, Richardson GD, Jahoda CA (2007) Hair follicle stem Sem Cell Dev Biol 18(2): 245-254.
- Fuchs E (2008) Skin stem cells: Rising to the J Cell Biol 180: 273-284.
- Fuchs E, Horsley V (2008) More than one way to Genes Dev 22(8): 976-985.
- Plikus MV, Mayer JA, Cruz DD, Baker RE, Maini PK, et al. (2008) Cyclic dermal BMP signaling regulates stem cell activation during hair Nature 451: 340-344.
- Rendl M, Polak L, Fuchs E (2008) BMP signaling in dermal papilla cells is required for their hair follicle- inductive Genes Dev 22(4): 543-557.
- Bockxmeer FM, Martin CE (1982) Measurement of cell proliferation and cell mediated contraction in 3-dimensional hydrated collagen matrices. J Tissue Culture Methods 7: 163-167.
- Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM (2005) Multipotent nestin-positive, keratin- negative hair-follicle bulge stem cells can form neurons. Proceed Natl Acad Sci U S Aa 102 (15): 5530-5534.
- Amoh Y, Li L, Katsuoka K, Hoffman RM (2009) Multipotent nestin‐expressing hair follicle stem J Dermatol 36: 1-9.
- Amoh Y, Hamada Y, Aki R, Kawahara K, Hoffman RM, et al. (2010) Direct transplantation of uncultured hair‐follicle pluripotent stem (hfPS) cells promotes the recovery of peripheral nerve injury. J Cell Biochemistry 110: 272-277.
- Nobakht M, Najafzadeh N, Safari M, Roshandel NR, Delaviz H, et al. (2010) Bulge cells of rat hair follicles: isolation, cultivation, morphological and biological features. Yakhteh Med J 1: 51-
- Amoh Y, Mii S, Aki R, Hamada Y, Kawahara K, et al. (2012) Multipotent nestin-expressing stem cells capable of forming neurons are located in the upper, middle and lower part of the vibrissa hair follicle. Cell Cycle 11: 3513-3517.
- Mii S, Duong J, Tome Y, Uchugonova A, Liu F, et al. (2013) The role of hair follicle nestin‐expressing stem cells during whisker sensory‐nerve growth in long‐term 3D culture. J Cell Biochem 114: 1674-1684.
- Mii S, Amoh Y, Katsuoka K, Hoffman RM (2014) Comparison of Nestin‐Expressing Multipotent Stem Cells in the Tongue Fungiform Papilla and Vibrissa Hair J Cellular Biochemistry 115: 1070-1076.
- Chen P, Miao Y, Zhang F, Huang J, Chen Y, et al. (2020) Nanoscale microenvironment engineering based on layer-by-layer self- assembly to regulate hair follicle stem cell fate for regenerative medicine. Theranostics 10: 11673.
- Babakhani A, Nobakht M, Torodi HP, Dahmardehei M, Hashemi P, et (2020) Effects of hair follicle stem cells on partial thickness burn wound healing and tensile strength. Iran Biomed J 24: 99.
- Sumathy B, Nair PD (2020) Keratinocytes-hair follicle bulge stem cells-fibroblasts co-cultures on a tri-layer skin equivalent derived from gelatin/PEG methacrulate J Biomater Sci Polym Ed 31: 869.
- Arvelo F, Pérez P, Cotte CA (2004) Obtention of human skin sheets by means of tissue Acta cientifica venezolana 1: 74-82.
- Qi S, Liu P, Xie J, Shu B, Xu Y, et al. (2008) Experimental study on repairing of nude mice skin defects with composite skin consisting of xenogeneic dermis and epidermal stem cells and hair follicle dermal papilla Burns J Int Soc Burn Inj 34(3): 385-392.
- Yoo B, Shin Y, Yoon HH, Seo Y, Park JK (2010) Hair follicular cell/organ culture in tissue engineering and regenerative medicine. Biochem Eng J 48: 323-331.
- Freshney RI (2010) Comprar Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications | Ian Freshney | 9780470528129 | Wiley. Fresh, R.I (2005) Culture of animal cells: A manual of basic technique.
- Robbins SL, Cotran RS (1974) Pathologic basis of
- Arango Chamorro CI, Restrepo LM, Correa LA, Henao JN (2009) Carácterísticas histológicas de piel cultivada in vitro. Anais Brasileiros De Dermatologia 90: 00-00.
- Moll R, Divo M, Langbein L (2008) The human keratins: Biology and Histochem Cell Biol 129: 705-733.
- Leckband DE, Prakasam A (2006) Mechanism and dynamics of cadherin adhesion. Ann Rev Biomed Eng 8: 259-287.
- Noël-Hudson M, Dusser I, Collober I, Muriel MP, Bonte FJ, et al. (1995) Human epidermis reconstructed on synthetic membrane: Influence of experimental conditions on terminal differentiation. In Vitro Cell Dev Biol-Animal 31: 508-
- Zhang X, Wang Y, Gao Y, Liu X, Bai T, et al. (2013) Maintenance of high proliferation and multipotent potential of human hair follicle-derived mesenchymal stem cells by growth Int J Mol Med 31(4): 913-921.
- Xu ZC, Zhang Q, Li H (2014) Differentiation of human hair follicle stem cells into endothelial cells induced by vascular endothelial and basic fibroblast growth Mol Med Rep 9(1): 204-210.
- Shin J, Lee Y, Kim KM, Won KS, Suh S, et al. (2022) The potential role of fibroblast‐derived multi‐peptide factors in activation of growth factors and β‐Catenin in hair follicle cells. J Cosmetic Dermatol 21: 6184-6190.
- Black AF, Bouez C, Perrier E, Schlotmann K, Chapuis FR, Damour O (2005) Optimization and characterization of an engineered human skin Tissue Eng 11(5-6): 723-733.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Journal of Allergy Research (ISSN:2642-326X)
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Pathology and Toxicology Research
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)










