Research Article
Claudin-1 Expression at the Invasive Front of Human Colorectal Adenocarcinoma
4406
Views & Citations3406
Likes & Shares
Cell adhesion includes tight junction, adherens junction and desmosome. The claudin family has been identified as the major protein of the tight junction, while the cadherin family has been revealed to be adherence junction proteins. We examined the expression of claudin-1 (CL-1), claudin-4 (CL-4) and E-cadherin (E-cad) in 92 cases of human colorectal adenocarcinoma, and analyzed their clinicopathological significance. Immunohistochemically, CL-1 exhibited low-grade expression in 39.1% (36/92) and high-grade expression in 60.9% (56/92), while E-cad revealed low-grade expression in 62.0% (57/92) and high-grade expression in 38.0% (35/92). Cases with low-grade CL-1 expression, i.e., decreased CL-1 expression, frequently showed lymph node metastasis (63.6%, p<0.05) and liver metastasis (27.3%, p<0.05). Digital quantitative evaluation demonstrated that CL-1 expression levels at the invasive front (98.3 ± 14.1 arbitrary unit, p<0.05) were significantly decreased in colorectal cancer cases with recurrence/metastasis, compared with cases without recurrence/metastasis (107 ± 18.3). In conclusion, decreased CL-1 expression at the invasive front is thought to be an important prognosis prediction factor.
Keywords: Colorectal cancer, Adenocarcinoma, Claudin, Cadherin, Metastasis
INTRODUCTION
Individual cells in epithelial sheets are attached to each other by cell adhesion factors, i.e., tight junction, adherens junction and desmosome [1-3]. The tight junction is located to the apical side of cells, and plays several roles, such as cell-to-cell adhesion, cell polarity and a physical barrier preventing solutes and water from passing freely through the paracellular space. Recent studies have identified a number of integral membrane proteins associated with the tight junction, including occludin, junctional adhesion molecule and claudin. The claudin family consists of 27 four-trans membrane domain proteins. On the other hand, the cadherin family has been discovered to be adherence junction proteins, including E-cadherin (E-cad) (epithelium origin), N-cadherin (nerve origin), P-cadherin (placenta origin), and VE-cadherin (vascular endothelial cell origin).
Colorectal cancer is the third most common cancer in the United States, as well as in Japan [4,5]. When it is discovered in its early stages, colon cancer is treated with surgery and is often cured; however, many patients with colon cancer have no remarkable symptoms until the disease reaches an advanced stage, such as metastasizing to other organs. Colorectal cancer is the second leading cause of cancer deaths in the United States. Recent advances in molecular biology have clarified multi-step carcinogenesis of colorectal cancer, i.e., adenoma-carcinoma sequence [6-8].
In a recent study, claudin-1 (CL-1) was shown to be involved in the β-catenin-Tcf/LEF signaling pathway, and to regulate cellular transformation and metastatic behavior in colon cancer. In addition, the expression of CL-1, claudin-3, and claudin-4 (CL-4) was up regulated in colorectal tumor tissues; however, the immune histochemical localization of CL-1, CL4 and E-cad have not yet been extensively analyzed. In this study, we examined the expression of CL-1, CL-4, and E-cad in human colorectal adenocarcinoma, and discuss their clinicopathological significance.
MATERIALS AND METHODS
Colorectal tissue specimens
Tissue samples were obtained from 92 patients with colorectal cancer lesions (59 men and 33 women; mean age 67.2, age: 41 to 88. The cases were surgically resected at Hirosaki University Hospital. The stages of colorectal cancer were classified according to the TNM classification [9]. Tumor budding (sprouting) was defined as a clump of cancer cells having less than five nuclei at the invasive front [10-12].
Histological Examination
Colorectal tissue specimens were rapidly fixed in 10% buffered formalin for 24-48 h for histological and immunohistochemical analyses, and routinely embedded in paraffin. Tumor invasion was examined in sections 4 μm thick stained with hematoxylin and eosin as described previously [13].
Immunohistochemical staining
Sections for immunostaining were routinely deparaffinized in xylene, dehydrated in graded ethanol solutions and washed with phosphate buffered saline (PBS) [14].
Immunostaining of CL-1, CL-4, and E-cad were preceded by digestion in citric acid 0.01 M for 10 min using an autoclave at 121oC. After rinse in PBS, endogenous peroxidase activity was quenched by dipping the sections into 0.3% hydrogen peroxide and 100% methanol for 20 min. The sections were then incubated with normal goat serum for 15 min at room temperature. The sections were subsequently incubated with (a) the first antibodies of CL-1 (claudin-1 rabbit polyclonal antibody diluted 1:100 (Zymed, South San Francisco, CA)), CL-4 (claudin-4 mouse monoclonal antibody diluted 1:400 (Zymed)), and E-cad (E-cadherin mouse monoclonal antibody diluted 1:50 (Takara, Tokyo, Japan)) overnight at 4oC; (b) secondary antibodies (goat-anti-rabbit antibody, SAB-PO (R) kit or rabbit-anti-mouse antibody, SAB-PO (M) kit; Nichirei, Tokyo, Japan) followed by incubation with for 20 min at room temperature; (c) peroxidase-conjugated avidin for 20 min at room temperature; and (d) a mixture of 0.1% hydrogen peroxidase and 0.05% diaminobenzidine for 30-60 sec. The sections were counterstained with hematoxylin.
Histological semiquantitative evaluation
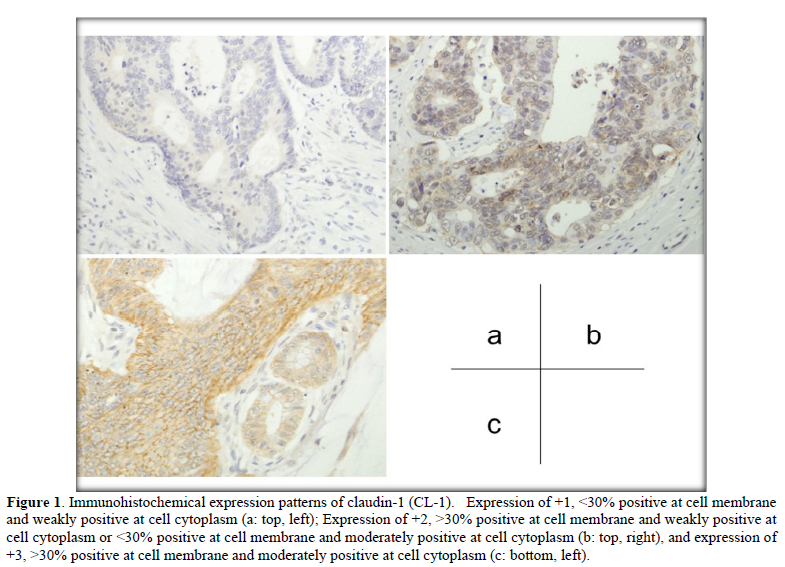
Immuno reactivity was evaluated at the invasive front (vertical advanced margin), lateral front (lateral advanced margin) and central part of each colorectal cancer tissue. Immunohistochemical expression patterns of CL-1, CL-4, and E-cad were divided into four groups as follows: -, negative; +1, <30% positive at the cell membrane and weakly positive at the cell cytoplasm; +2, >30% positive at the cell membrane and weakly positive at the cell cytoplasm or <30% positive at the cell membrane and moderately positive at the cell cytoplasm; +3, >30% positive at the cell membrane and moderately positive at the cell cytoplasm.
Digital quantitative evaluation
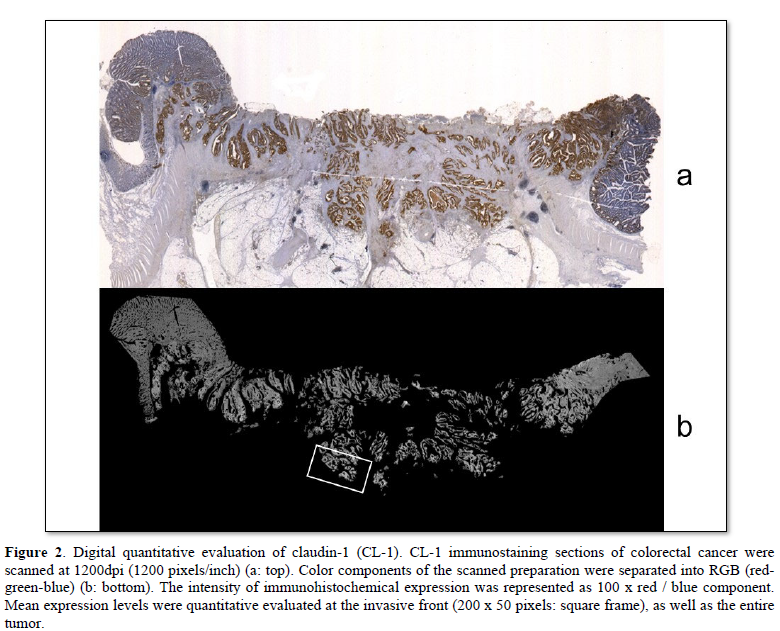
Computer digital scanning was performed. Slice preparations of colorectal cancers were scanned at 1200 dpi (1200 pixels/inch). The color component of scanned preparation was divided for RGB (red-green-blue). The red component was (r), the green component was (g), and the blue component was (b), defined respectively. The intensity of the immunohistochemical expression was represented as 100 x r/b. These mean expression levels were quantitatively evaluated at the invasive front (200 x 50 pixels), and in the entire tumor. The expression level in non-neoplastic glands was defined as 100.
Statistical analyses
The expressions of CL-1, CL-4 and E-cad for histological evaluation and clinicopathological parameters were examined using Fisher's exact test and the Chi-squared test (software; SPSS for Windows, version 12.0 [Chicago, IL, USA]). The expression of CL-1 for digital evaluation and clinicopathological parameters were examined using Student's T test (software; SPSS version 12.0). P values were two-tailed, and a P value < 0.05 was statistically significant.
RESULTS
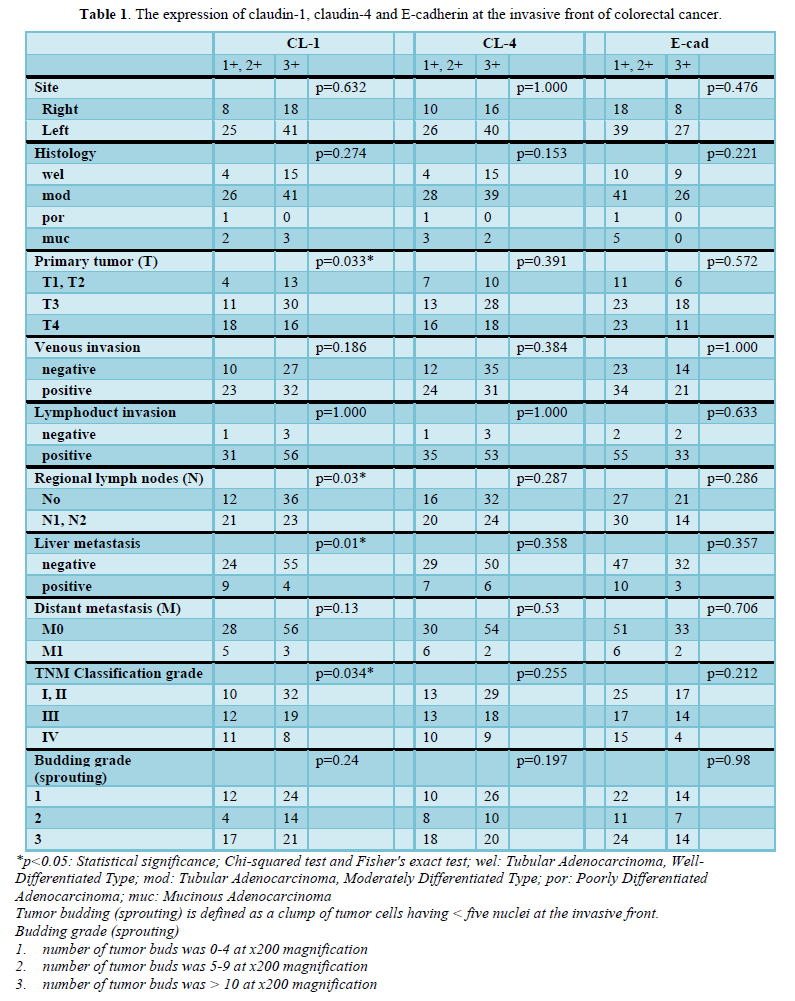
The invasive front of colorectal cancer cases ubiquitously expressed CL-1, i.e., +1 or +2 (low-grade expression) in 35.9% (33/92) and +3 (high-grade expression) in 64.1% (59/92) of the colorectal adenocarcinoma tissues examined (Table 1 & Figure 1). CL-1 exhibited low-grade expression in 39.1% (36/92) and high-grade expression in 60.9% (56/92), while E-cad revealed low-grade expression in 62.0% (57/92) and high-grade expression in 38.0% (35/92). Low-grade CL-1 expression cases, i.e., decreased CL-1 expression, frequently showed lymph node metastasis (63.6%, p<0.05) and liver metastasis (27.3%, p<0.05), compared with CL-1 high-grade expression (40.0% and 6.8%, respectively). At the lateral front and central part of colorectal cancer cases, there were no significant differences between CL-1 expression and clinicopathological factors, while the colorectal cancers ubiquitously expressed CL-1. In addition, there were no significant differences between CL-4/E-cad expression and the clinicopathological factors (Table 1).


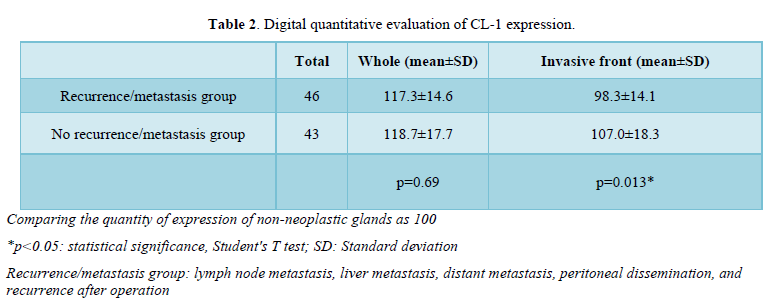
Digital quantitative evaluation of CL-1 expression demonstrated 117.3 ± 14.6 in colorectal cancer cases with recurrence/metastasis and 118.7 ± 17.7 in cases without recurrence/metastasis (mean ± standard deviation) (Table 2 & Figure 2). CL-1 expression levels at the invasive front (98.3 ± 14.1, p<0.05) were significantly decreased in colorectal cancer cases with recurrence/metastasis, compared with cases without recurrence/metastasis (107.0 ± 18.3).
DISCUSSION
We examined the immune histochemical expression of CL1, CL4 and E-cad in human colorectal adenocarcinomas and analyzed their clinicopathological significance. This study examined the relationship between low-grade CL-1 expression at the invasive front and metastatic potentials (lymph node and liver metastases) in colorectal cancer patients. In addition, this is the first study demonstrating decreased CL-1 expression in colorectal cancers with metastasis/recurrence using digital quantitative evaluation. There were no apparent statistical correlations between clinicopathological features and the expressions of CL4 and E-cad.
Cell adhesion is crucial for the assembly of individual cells into three-dimensional tissues [1-3]. The functional units of cell adhesion are typically multi protein complexes consisting of three general classes, i.e., cell adhesion molecules/adhesion receptors, extracellular matrix proteins and cytoplasmic plaque/peripheral membrane proteins. Recent advances in molecular biology have clarified the structures and functional regulations of cell adhesion including the tight junction, adherens junction and desmosome. The claudin family and occludin have been identified as the major proteins of the tight junction, while the cadherin family has been discovered as adherence junction proteins [2].
ACKNOWLEDGMENTS
This study was supported by JSPS KAKENHI, Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
-
Tsukita S, Tanaka H, Tamura A (2019) The claudins: From tight junctions to biological systems. Trends Biochem Sci 44: 141-152.
-
Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2: 285-293.
-
Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, et al. (2006) Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Renal Physiol 291: F1288-1299.
-
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, et al. (2012) GLOBOCAN v1.1, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer.
-
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, et al. (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66: 683-691.
-
Yao T, Tsutsumi S, Akaiwa Y, Takata M, Nishiyama K, et al. (2001) Phenotypic expression of colorectal adenocarcinomas with reference to tumor development and biological behavior. Jpn J Cancer Res 92: 755-761.
-
Ho SB, Niehans GA, Lyftogt C, Yan PS, Cherwitz DL, et al. (1993) Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res 53: 641-651.
-
Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, et al. (1988) Genetic alterations during colorectal tumor development. N Engl J Med 319: 525-532.
-
Brierley JD, Gospodarowicz MK, Wittekind C (2017) eds: Colon and Retum. In: TNM classification of malignant tumors, 8th John Wiley & Sons, Ltd West Sussex, UK, pp: 73-76.
-
Hase K, Shatney C, Johnson D, Trollope M, Vierra M (1993) Prognostic value of tumor "budding" in patients with colorectal cancer. Dis Colon Rectum 36: 627-635.
-
Tanaka M, Hashiguchi Y, Ueno H, Hase K, Mochizuki H (2003) Tumor budding at the invasive margin can predict patients at high risk of recurrence after curative surgery for stage II, T3 colon cancer. Dis Colon Rectum 46: 1054-1059.
-
Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, et al. (2004) Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 127: 385-394.
-
Nagata J, Yoshizawa T, Goto S, Kubota S, Ogasawara H, et al. (2022) Desmoplasia and angiogenesis of submucosa invasive carcinoma of the stomach. BioMed Res J 6: 520-524.
-
Aisawa H, Yoshizawa T, Goto S, Chiba H, Morohashi S, et al. (2022) Mucin Expression Patterns of Human Colorectal Adenocarcinoma. BioMed Res J 6: 531-535.
-
Miwa N, Furuse M, Tsukita S, Niikawa N, Nakamura Y, et al. (2001) Involvement of Claudin-1 in the β-Catenin/Tcf Signaling Pathway and its Frequent Upregulation in Human Colorectal Cancers. Oncol Res 12: 469-476.
-
De Oliveira SS, de Oliveira IM, De Souza W, Morgado-Diaz JA (2005) Claudin upregulation in human colorectal cancer. FEBS Lett 579: 6179-6185.
-
Suren D, Yildirim M, Kaya V, Alikanoǧlu AS, Bülbüller N, et al. (2014) Loss of tight junction proteins (Claudin 1, 4, and 7) correlates with aggressive behavior in colorectal carcinoma. Med Sci Monit 20: 1255-1262.
-
Shibutani M, Noda E, Maeda K, Nagahara H, Ohtani H, et al. (2013) Low expression of claudin-1 and presence of poorly differentiated tumor clusters correlate with poor prognosis in colorectal cancer. Anticancer Res 33: 3301-3306.
-
Ouban A (2018) Claudin-1 role in colon cancer: An update and a review. Histol Histopathol 33: 1013-1019.
-
Kokura S, Yoshida N, Imamoto E, Ueda M, Ishikawa T, et al. (2004) Anoxia/reoxygenation down-regulates the expression of E-cadherin in human colon cancer cell lines. Cancer Lett 211: 79-87.
-
Kapitanovic S, Cacev T, Antica M, Kralj M, Cavric G, et al. (2006) Effect of indomethacin on E-cadherin and beta-catenin expression in HT-29 colon cancer cells. Exp Mol Pathol 80: 91-96.
-
Bravou V, Klironomos G, Papadaki E, Taraviras S, Varakis J (2006) ILK over-expression in human colon cancer progression correlates with activation of beta-catenin, down-regulation of E-cadherin and activation of the Akt-FKHR pathway. J Pathol 208: 91-99.
-
Foran E, McWilliam P, Kelleher D, Croke DT, Long A (2006) The leukocyte protein L-plastin induces proliferation, invasion and loss of E-cadherin expression in colon cancer cells. Int J Cancer 118: 2098-2104.
-
Reinacher-Schick A, Baldus SE, Romdhana B, Landsberg S, Zapatka M, et al. (2004) Loss of Smad4 correlates with loss of the invasion suppressor E-cadherin in advanced colorectal carcinomas. J Pathol 202: 412-420.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- Journal of Pathology and Toxicology Research
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)




