Research Article
Desmoplasia and Angiogenesis of Submucosa Invasive Carcinoma of the Stomach
5003
Views & Citations4003
Likes & Shares
The prognosis of stomach cancer (gastric cancer) in advanced stage is generally poor, because the tumor has often metastasized. Desmoplasia (reactive fibrosis) and angiogenesis in the cancer microenvironment are important processes of cancer invasion/metastasis. We focused on the desmoplasia and angiogenesis using surgically/endoscopically resected submucosa-invasive carcinomas of the stomach. Desmoplasia is recognized as increase of cancer-associated fibroblasts, and irregular collagen bundles. Desmoplasia-positive cases significantly showed high-grade lymphatic/venous angiogenesis, compared with desmoplasia-negative cases (angiogenesis score: lymphatic 2.34 vs 1.27, p<0.001; venous 2.21 vs 1.69, p<0.005). Lymphatic/venous invasion of cancer cells was frequently found in the desmoplasia-positive cases, compared with desmoplasia-negative cases (lymphatic 89.5% vs 7.7%, p<0.001; venous 76.3% vs 34.6%, p<0.001). In conclusion, the desmoplasia is thought to play important roles of angiogenesis, and lymphatic/venous invasion, i.e., metastatic potentials of stomach cancer.
Keywords: Gastric cancer, Desmoplasia, Angiogenesis, Cancer invasion
INTRODUCTION
Worldwide, stomach cancer (gastric cancer) is the fifth most-common cancer, and becomes the third-leading cause of cancer-related death [1,2]. The most cases of stomach cancers are adenocarcinoma, and one of the most common causes is persistent infection of Helicobacter pylori. The patient’s prognosis in advanced stage is generally poor, because the tumor has often metastasized. Presence of lymphatic/venous invasion of cancer cells becomes risk factors for lymph node/distant metastasis of the stomach cancer. The analysis of lymphatic/venous angiogenesis is important to determine the invasive progression and metastasis in patients.
In this study, we focus on the desmoplasia (reactive fibrosis) in the cancer stroma, and describe lymphatic/venous invasion and angiogenesis, using endoscopically/surgically resected submucosa-invasive carcinomas of the stomach.
MATERIALS AND METHODS
Stomach cancer specimens
This study performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of Hirosaki University Graduate School of Medicine (organization number #1149). The pathological features of each cancer lesion were evaluated using paraffin-embedded tissue specimens from surgically/endoscopically resected 64 cases with submucosa-invasive carcinoma (pT1b tumor by TNM classification) [3]. The 64 cases included 17 well differentiated adenocarcinomas, 34 moderately differentiated adenocarcinomas, 13 poorly differentiated adenocarcinomas with/without signet-ring cell carcinoma components.
The area of deepest invasion was selected as a representative histological specimen of each cancer lesion. The selected sections were stained with hematoxylin & eosin (H&E), Masson trichrome (collagen fibers, blue), Verhoeff-van Gieson (elastic fibers, black), and immunohistochemical staining of D2-40 (podoplanin: marker of lymphatic endothelial cells), and α-smooth muscle actin (marker of cancer-associated fibroblast) [4,5].
Histological evaluation of desmoplasia, angiogenesis, and lymphatic/venous invasion of cancer cells
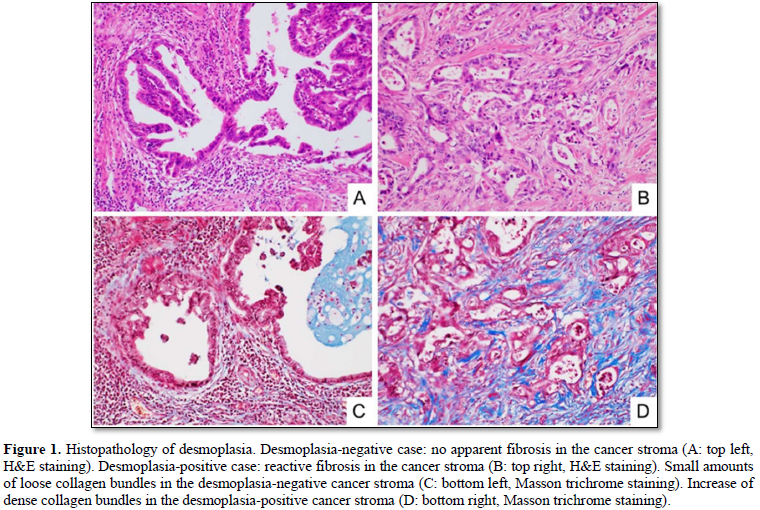
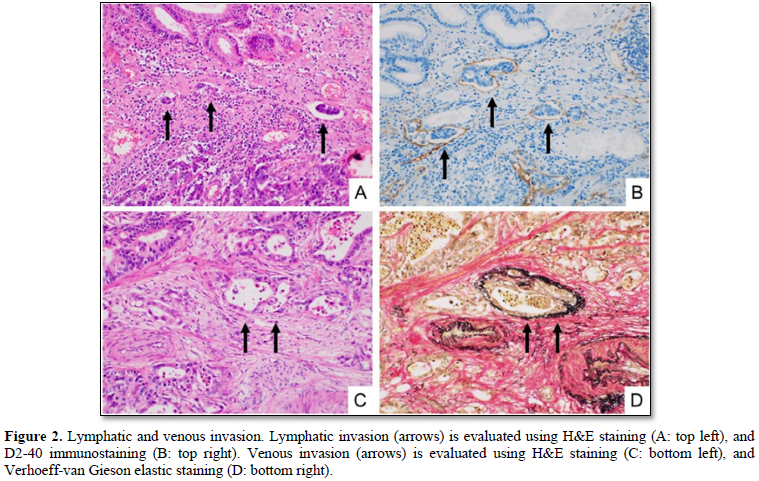
We divided the 64 cases into the two groups: desmoplasia-positive, and desmoplasia-negative (Figures 1A & 1B). The desmoplasia-positive cases were defined as cancers in which more than 10% of area showed stromal desmoplastic reaction (H&E stain), increased collagen bundles (Masson trichrome stain) (Figures 1C & 1D) and presence of cancer-associated fibroblasts (α-smooth muscle immunostaining). Degrees of lymphatic/venous angiogenesis were classified into four groups: score 0 (no apparent angiogenesis in cancer area), score 1 (mild angiogenesis: one or two vessels over 200 µm in diameter), score 2 (moderate angiogenesis: three or four vessels), and score 3 (severe angiogenesis: more than five vessels). We divided the cases into the two groups: lymphatic/venous invasion-positive, and lymphatic/venous invasion-negative. Lymphatic and venous invasions were evaluated using D2-40 immunostaining (Figures 2A & 2B) and Verhoeff-van Gieson elastic staining (Figures 2C & 2D) respectively.

Statistical analysis
Statistical comparisons between two groups were analyzed using the Student’s t-test (angiogenesis score) or the Pearson’s chi-square test (lymphatic/venous invasion) for categorical data. Differences were considered to be statistically significant if the p-value was <0.05.
RESULTS
Desmoplasia of sub mucosa-invasive carcinomas
Thirty-eight of the 64 submucosa-invasive carcinomas (59.4%) were desmoplasia-positive cases, in which more than 10% of area showed stromal desmoplastic reaction with increased collagen bundles and cancer-associated fibroblasts, while 26 (40.6%) were desmoplasia-negative.
Lymphatic/venous angiogenesis of sub mucosa-invasive carcinomas
According to the scoring of lymphatic angiogenesis (lymphangiogenesis), we classified the 64 cases into four groups as follows: one case (1.6%) of score 0, 24 cases (37.5%) of score 1, 19 cases (29.7%) of score 2, and 20 cases (31.3%) of score 3.
According to the scoring of venous angiogenesis, we classified the 64 cases into four groups as follows: none of score 0, 18 cases (28.1%) of score 1, 28 cases (43.8%) of score 2, and 18 cases (28.1%) of score 3.

Correlation between desmoplasia and angiogenesis of submucosa-invasive carcinomas
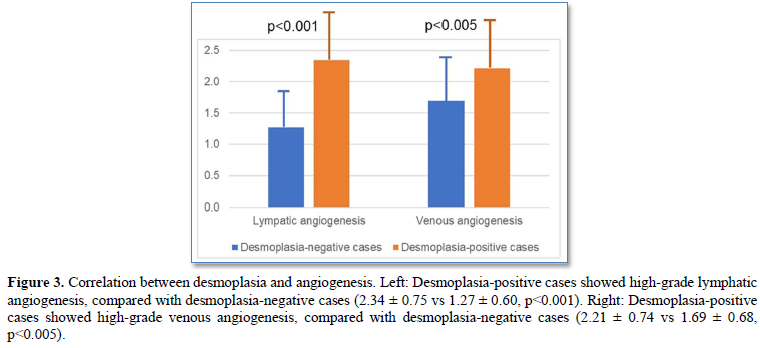
Desmoplasia-positive cases significantly showed high-grade lymphatic/venous angiogenesis, compared with desmoplasia-negative cases (lymphatic angiogenesis score: 2.34 ± 0.75 vs 1.27 ± 0.60, p<0.001; venous angiogenesis score: 2.21 ± 0.74 vs 1.69 ± 0.68, p<0.005) (Figures 3).

Correlation between desmoplasia and lymphatic/venous invasion of sub mucosa-invasive carcinomas
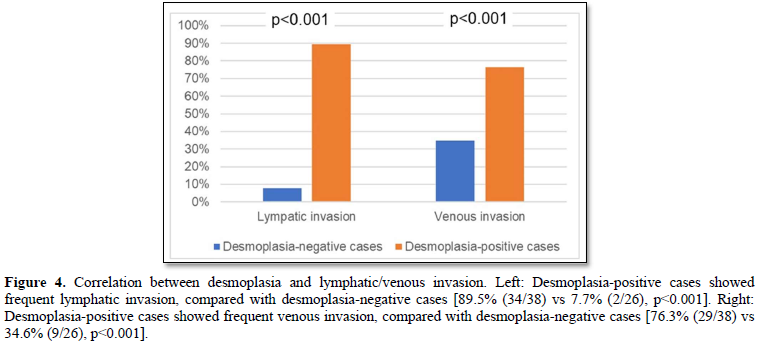
Lymphatic/venous invasion of cancer cells was frequently found in the desmoplasia-positive cases, compared with desmoplasia-negative cases (lymphatic invasion: 89.5% (34/38) vs 7.7% (2/26), p<0.001; venous 76.3% (29/38) vs 34.6% (9/26), p<0.001) (Figures 4).

DISCUSSION
In this study, we examined desmoplasia (reactive fibrosis) in the cancer stroma, using submucosa-invasive carcinomas of the stomach. The desmoplasia was statistically correlated with angiogenesis, and lymphatic/venous invasion, i.e., metastatic potentials stomach cancer.
Stomach cancer (gastric cancer) is the fifth most-common cancer, and becomes the third-leading cause of cancer-related death [1,2]. More than one million new cases were estimated worldwide in 2018 [6]. The stomach cancer is a multifactorial disease, and about 90% of cases are sporadic. The patient’s prognosis in advanced stage is generally poor, because the tumor has often metastasized. Presence of lymphatic/venous invasion of cancer cells becomes risk factors for lymph node/distant metastasis of the stomach cancer [7].
Cancer microenvironment is important for the cancer cells growth in their native tissues/organs [8]. Invasion and metastasis are recognized as malignant phenotypes, and closely associated with interaction between cancer cells and non-cancerous stroma [9,10]. The non-cancerous stroma includes several cancer-associated cells, such as inflammatory cells, fibroblasts, and endothelial cells. The cancer-associated fibroblasts induce desmoplasia (reactive fibrosis with irregular collagen bundles) in the cancer stroma, and are associated with invasive growth and malignant potentials [11-16]. In addition, the cancer cells stimulate endothelial cells, and induce the angiogenesis, closely associated with cancer metastasis.
Several studies have reported that the intratumoral vascularization is correlated with metastatic potentials in the stomach cancer cases [17,18]. Our histopathological study defined the desmoplasia-positive cases, in which more than 10% of area showed stromal desmoplastic reaction with increased collagen bundles and cancer-associated fibroblasts. Desmoplasia-positive cases significantly showed high-grade lymphatic/venous angiogenesis, and frequent lymphatic/venous invasion of cancer cells.
In conclusion, the desmoplasia is thought to play important roles of angiogenesis, and lymphatic/venous invasion, i.e., metastatic potentials stomach cancer.
ACKNOWLEDGEMENTS
This study was supported by JSPS KAKENHI, Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
- Carneiro F, Fukayama M, Grabsch H, Yasui W (2019) Gastric adenocarcinoma. In: WHO classification of tumours editorial board. Digestive system tumor. WHO classification of tumors, 5th IARC Press: Lyon, pp: 85-95.
- Odze RD, Montgomery E, Wang HH, Lauwers GY, Greenson JK, et al. (2019) Tumors of the esophagus and stomach. Atlas of tumor pathology. 4th series, fascicle 28. Armed Forces Institute of Pathology (AFIP): Washington DC, pp: 199-280.
- Brierley JD, Gospodarowicz M, Witterkind C (2017) TNM classification of malignant tumors, 8th John Wiley & Sons: Chichester, pp: 63-66.
- Hirai H, Yoshizawa T, Morohashi S, Haga T, Wu Y, et al. (2016) Clinicopathological significance of gastric poorly differentiated medullary carcinoma. Biomed Res 37: 77-84.
- Japanese Society for Cancer of the Colon and Rectum (2019) Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: the 3rd English Edition [Secondary Publication] J Anus Rectum Colon 3: 175-195.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. (2020) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68: 394-424.
- Sasaki H, Morohashi S, Toba T, Seino H, Yoshizawa T, et al. (2018) Neoangiogenesis of gastric submucosa-invasive adenocarcinoma. Oncol Lett 16: 3895-3900.
- Strayer DS, Saffitz JE (2020) Rubin’s Pathology: Mechanisms of human disease. Walters Kluwer: Philadelphia, pp: 195-202.
- Goto S, Ishido K, Yoshizawa T, Haga T, Morohashi S, et al. (2019) Histopathological Characteristics of Pancreatic Cancer Stroma Induced by Neoadjuvant Chemotherapy. BioMed Res J 3: 61-64.
- Ogasawara H, Yoshizawa T, Kubota S, Goto S, Morohashi S, et al. (2021) Phenotypic Changes and Cell Migration of Bile Duct Cancer Cells Induced by Mesenchymal Cells. BioMed Res J 5: 485-490.
- Zeltz C, Primac I, Erusappan P, Alam J, Noel A, et al. (2020) Cancer-associated fibroblasts in desmoplastic tumors: Emerging role of integrins. Semin Cancer Biol 62: 166-181.
- Yamaguchi H, Sakai R (2015) Direct interaction between carcinoma cells and cancer associated fibroblasts for the regulation of cancer invasion. Cancers (Basel) 7: 2054-2062.
- Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, et al. (2012) Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res 10: 1403-1418.
- Takatsuna M, Morohashi S, Yoshizawa T, Hirai H, Haga T, et al. (2016) Myofibroblast distribution is associated with invasive growth types of colorectal cancer. Oncol Rep 36: 3154-3160.
- Takatsuna M, Morohashi S, Yoshizawa T, Hirai H, Haga T, et al. (2016) Myofibroblasts of the muscle layer stimulates the malignant potential of colorectal cancer. Oncol Rep 36: 1251-1257.
- Nishishita R, Morohashi S, Seino H, Wu Y, Yoshizawa T, et al. (2018) Expression of cancer-associated fibroblast markers in advanced colorectal cancer. Oncol Lett 15: 6195-6202.
- Tomoda M, Maehara Y, Kakeji Y, Ohno S, Ichiyoshi Y, et al. (1999) Intratumoral neovascularization and growth pattern in early gastric carcinoma. Cancer 85: 2340-2346.
- Lee K, do Park J, Choe G, Kim HH, Kim WH, et al. (2010) Increased intratumoral lymphatic vessel density correlates with lymph node metastasis in early gastric carcinoma. Ann Surg Oncol 17: 73-80.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Pathology and Toxicology Research






