Research Article
Phenotypic Changes and Cell Migration of Bile Duct Cancer Cells Induced by Mesenchymal Cells
1044
Views & Citations44
Likes & Shares
Bile duct cancer (biliary tract cancer) is one of the most high-grade malignancies because of its invasiveness and metastasis. Local aggressive invasiveness of the cancer is thought to depend on microenvironments with cancer cells and stromal cells. We analyzed phenotypic changes and cell migration of the TFK-1 human extra hepatic bile duct cancer cells, co-cultured with MSC-43 human mesenchymal stem cells. When co-cultured with MSC-43 cells, the TFK-1 cells morphologically showed epithelial mesenchymal transition (EMT), and immunohistochemically decreased E-cadherin expression. Migration assay demonstrated that the TFK-1 cells co-cultured with MSC-43 cells showed significantly higher migration rate than the TFK-1 cells only (2.48 vs 1.82, p<0.001). Transwell migration assay revealed that the TFK-1 cells indirectly co-cultured with MSC-43 cells exhibited higher migration rate than the TFK-1 cells only (3.27 vs 1.67, p<0.005). In conclusion, the stromal mesenchymal cells are thought to play an important role of phenotypic changes and cell migration of human bile duct cancer.
Keywords: Bile Duct Cancer, Stromal Mesenchymal Cells, Cell Migration, Morphology
INTRODUCTION
Extra hepatic bile duct cancer (biliary tract cancer) is recognized as one of the most aggressive malignancies because of its frequent metastasis and recurrence [1-3]. Most of the patients are usually treated at an advanced stage, and the prognosis remains poor despite the development of recent diagnosis and treatment. Therefore, the bile duct cancer is the second most lethal malignancy, following the pancreatic cancer. Local invasiveness of the extra hepatic bile duct cancer has not yet been analyzed morphologically, while status of the histopathology of lymph node metastasis has been reported previously [4].
Here, we focus on the co-culture of human bile duct cancer cells with human mesenchymal stem cells, and describe phenotypic changes and cell migration of the bile duct cancer.
MATERIALS AND METHODS
Cells
TFK-1 is a human cell line derived from extrahepatic bile duct carcinoma, obtained from the RIKEN BRC through the National Bio-Resource Project of MEXT (Ministry for Education, Culture Sports, Science and Technology), Japan [5]. The TFK-1 cells cultured in RPMI 1640 medium with 10% FBS and 1% penicillin-streptomycin. MSC-43 is derived from human mesenchymal stem cells, obtained from the RIKEN BRC through the National Bio-Resource Project of MEXT, Japan. The MSC-43 cells cultured in DMEM with 10% FBS and 3ng/mL basic fibroblast growth factor (bFGF).
Direct co-culture of TFK-1 cells and MSC-43 cells
When co-cultured with MSC-43 cells, the TFK-1 cells were cultured in DMEM, and grew up well on the monolayer plate (Figures 1A and 1B). Immunostainings of E-cadherin (epithelial marker) and vimentin (mesenchymal marker) were performed using Leica BOND-MAX (Leica Biosystems, Nußloch, Germany).
Indirect co-culture of TFK-1 cells and MSC-43 cells
TFK-1 cells were cultured on the monolayer plate, while MSC-43 cells were cultured on the upper transwell insert (Figure 1C).
Migration assay
The cultured cells on the monolayer plate were scratched with a cell scraper, and no cellular area on the plate was made (Figure 1D). Co-culture was maintained for 6 days. The experiments were performed at least triplicated. Migration rate [μm/h] is calculated by the formula: Width (Day 0) - Width (Day N) x 0.5 / 24 x N.
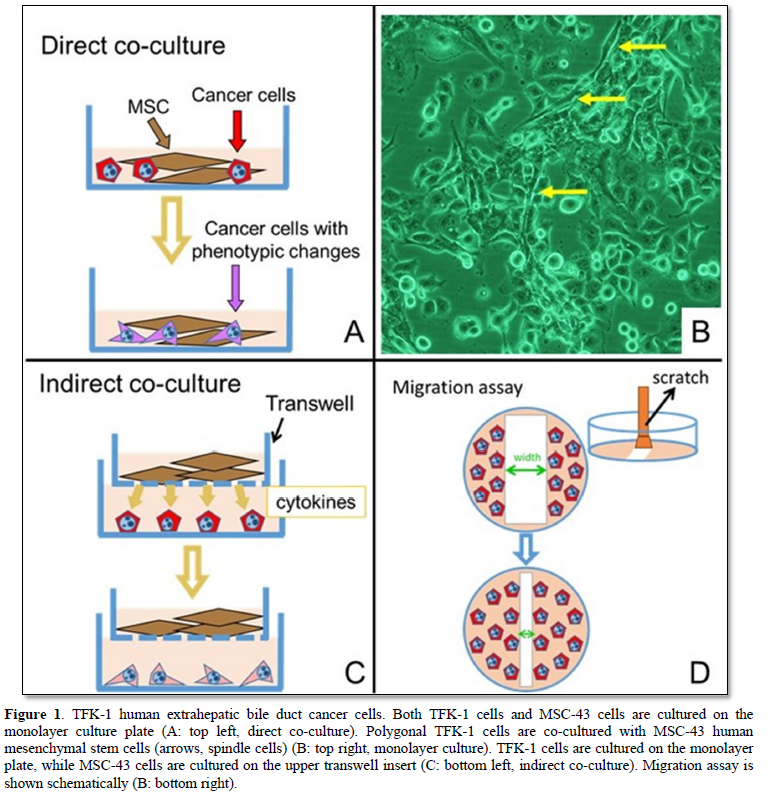
RESULTS
Direct co-culture of TFK-1 cells and MSC-43 cells
When co-cultured with MSC-43 cells, the TFK-1 cells grew up well on the monolayer plate (Figure 2). Morphologically, the polygonal TFK-1 cells formed monolayer nests, while some of the cells become spindle in shape. Immunohistochemically, the cell membrane of polygonal TFK-1 cells expressed E-cadherin, an epithelial marker, while the cytoplasm of MSC-43 cells showed vimentin, a mesenchymal marker. The spindle TFK-1 cells decreased E-cadherin expression, and exhibited weak vimentin expression (Figure 2D), indicating epithelial mesenchymal transition (EMT).
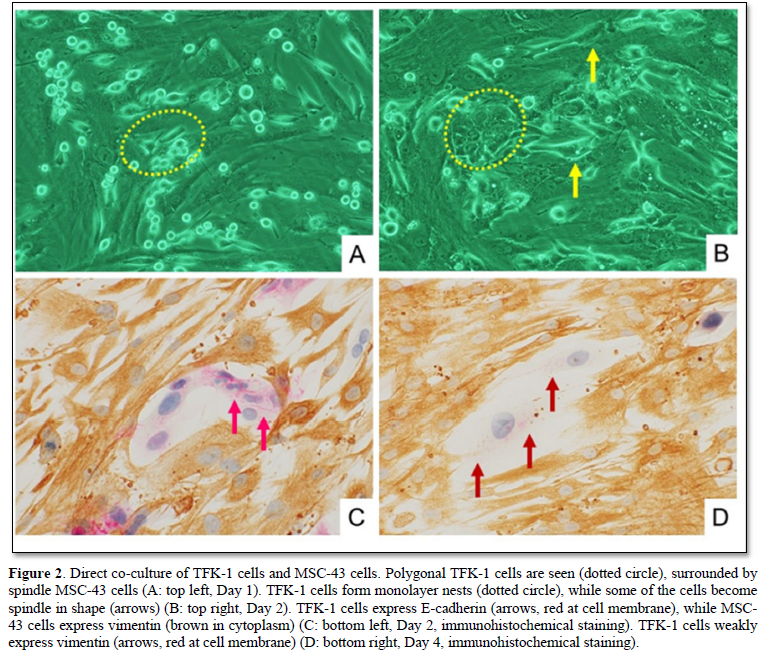
Cell migration of TFK-1 cells directly co-cultured with MSC-43 cells
The TFK-1 cells were co-cultured with MSC-43 cells on the monolayer plate. Migration assay demonstrated cell migration of both TFK-1 cells and MSC-43 cells on the monolayer plate (Figures 3A, 3B, and 4A). TFK-1 cells co-cultured with MSC-43 cells showed significantly higher migration rate than the TFK-1 cells only (2.48 ± 0.32 vs 1.82 ± 0.32, p<0.001).
Cell migration of TFK-1 cells indirectly co-cultured with MSC-43 cells
TFK-1 cells were cultured on the monolayer plate, while MSC-43 cells were cultured on the upper transwell insert. Migration assay demonstrated that TFK-1 cells indirectly co-cultured with MSC-43 cells showed significantly higher migration rate than the TFK-1 cells only (3.27 ± 0.47 vs 1.67 ± 0.68, p<0.05) (Figures 3C, 3D, and 4B).
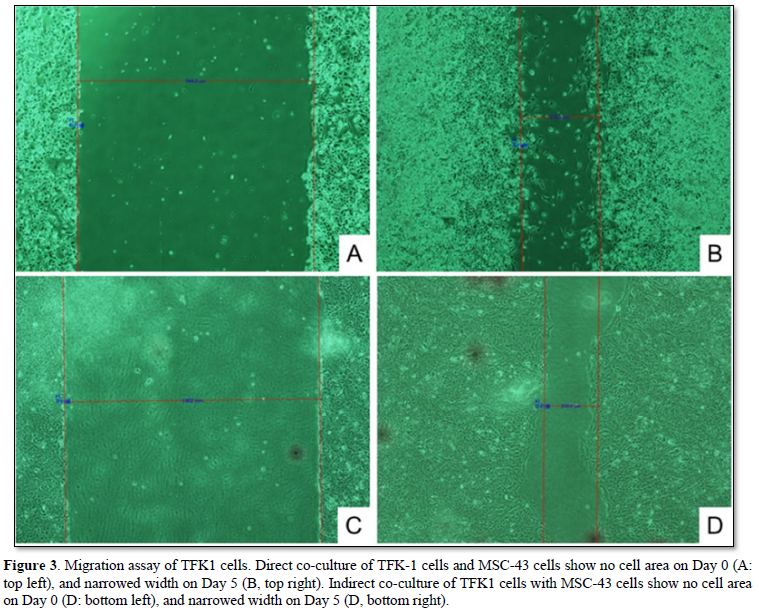
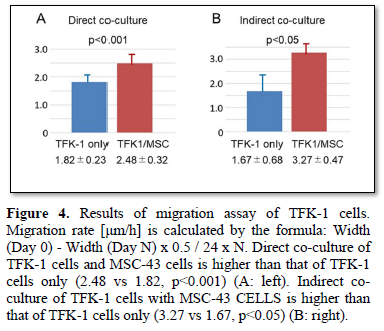


DISCUSSION
We describe morphological and cell biological characteristics of biliary cancer. When co-cultured with MSC-43 cells, the TFK-1 human bile duct cancer cells showed morphological changes indicating EMT, and high cell migration rate.
The most extra hepatic bile duct cancers are malignancies arising from the bile duct epithelium, and exhibit one of the highly aggressive malignancies [6-8]. We demonstrated that invasive growth with high-grade tumor budding was thought to be associated with aggressiveness of the pancreato-biliary cancer [9]. However, histopathological mechanisms of local invasiveness in the cancer microenvironment have not yet clarified, while the molecular/genetic profiles of cancer cells have been shown recently.
Microenvironment is important when the cancer cells grow up in their native tissues/organs. Malignancy in cancers is characterized by invasion and metastasis that are closely associated with interaction between cancer cells and non-cancerous stroma [10,11]. The cancer cells interact with adjacent epithelial cells, tumor-associated matrix constituents, and stromal/inflammatory cells [12,13]. However, mechanisms of cancer cell migration/invasion have not well understood. Our study is the first to demonstrate histopathological findings of cancer cell migration using direct/indirect co-culture with stromal mesenchymal cells.
EMT is one of important steps in cancer invasion and metastasis, and involve related behavioral patterns that lead cells to remove intercellular bonds digest basement membranes, rearrange extracellular matrix, and navigate through that matrix [14]. The cancer cells in EMT processes are characterized by the loss of epithelial factors, including cytokeratin and E-cadherin, and the up regulated expression of mesenchymal factors, including vimentin and N-cadherin [15-18]. Our morphological/immune histochemical study revealed that the TFK-1 human bile duct cancer cells showed EMT when co-cultured with MSC-43 cells. Moreover, TFK-1 cells co-cultured with MSC-43 cells directly/indirectly showed the significantly higher cell migration. The results indicated that indirect interactions such as cytokines were thought to associate with the migration and local invasion of the cancer cells.
In conclusion, we demonstrate histopathological characteristics of the extra hepatic bile duct cancer cells co-cultured with mesenchymal cells. The stromal mesenchymal cells are thought to play an important role of phenotypic changes and cell migration of human bile duct cancer.
ACKNOWLEDGEMENTS
This study was supported by JSPS KAKENHI, Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
- Albores-Saavedra J, Henson DE, Klimstra DS (2015) Tumors of the gallbladder, extrahepatic bile ducts and ampulla of Vater. Atlas of tumor pathology. 4th series, fascicle 23. Armed Forces Institute of Pathology (AFIP), Washington DC, pp: 303-374.
- WHO classification of tumors editorial board (2019). Digestive system tumor. WHO classification of tumors, 5th edition. Lyon: IARC Press pp: 289-291.
- Kijima H, Wu Y, Yoshizawa T, Suzuki T, Tsugeno Y, et al. (2014) Pathological characteristics of early to advanced gallbladder carcinoma and extra hepatic cholangio carcinoma. J Hepatobiliary Pancreat Sci 21: 453-458.
- Yoshizawa T, Ishido K, Saito K, Haga T, Seino H, et al. (2017) Prognostic impact of extra capsular lymph node invasion and myofibroblastic activity in extra hepatic bile duct cancer. Clin Med Insights Pathol 10: 1179555717729652.
- Saijyo S, Kudo T, Suzuki M, Katayose Y, Shinoda M, et al. (1995) Establishment of a new extrahepatic bile duct carcinoma cell line, TFK-1. Tohoku J Exp Med 177: 61-71.
- Yoshizawa T, Toyoki Y, Hirai H, Haga T, Toba T, et al. (2014) Invasive micro papillary carcinoma of the extra hepatic bile duct and its malignant potential. Oncol Rep 32: 1355-1361.
- Ohashi M, Kusumi T, Sato F, Kudo Y, Jin H, et al. (2009) Expression of syndecan-1 and E-cadherin is inversely correlated with poor patient's prognosis and recurrent status of extra hepatic bile duct carcinoma. Biomed Res 30: 79-86.
- Okano K, Yoshizawa T, Miura T, Ishido K, Kudo D, et al. (2018) Impact of the histological phenotype of extrahepatic bile duct carcinoma. Mol Clin Oncol 8: 54-60.
- Yoshizawa T, Hong SM, Jung D, Noë M, Kiemen A, et al. (2020) Three-dimensional analysis of extrahepatic cholangiocarcinoma and tumor budding. J Pathol 251: 400-410.
- Miura T, Yoshizawa T, Hirai H, Seino H, Morohashi S, et al. (2017) Prognostic Impact of CD163+ Macrophages in Tumor Stroma and CD8+ T-Cells in Cancer Cell Nests in Invasive Extra hepatic Bile Duct Cancer. Anticancer Res 37: 183-190.
- Goto S, Ishido K, Yoshizawa T, Haga T, Morohashi S, et al. (2019) Histopathological Characteristics of Pancreatic Cancer Stroma Induced by Neoadjuvant Chemotherapy. Bomed Res J 3: 61-64.
- Weinberg RA (2013) The Biology of Cancer, 2nd New York, NY: Garland Science pp: 643-651.
- Kumar V, Abbas AK, Aster JC (2021) Robbins and Cotran Pathologic Basis of Disease, 10th Edition. Philadelphia, PA: Elsevier Saunders pp: 306-309.
- Strayer DS, Saffitz JE (2020) Rubin’s Pathology, Mechanisms of human disease, 8th Philadelphia, PA: Wolters Kluwer. pp: 195-203.
- Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 7: 131-142.
- Jing Yang J, Weinberg RA (2008) Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev Cell 14: 818-829.
- Wu Y, Sato F, Yamada T, Bhawal UK, Kawamoto T, et al. (2012) The BHLH transcription factor DEC1 plays an important role in the epithelial-mesenchymal transition of pancreatic cancer. Int J Oncol 41: 1337-1346.
- Tsugeno Y, Yoshizawa T, Wu Y, Goto S, Haga T, et al. (2019) Type I Collagen Induces DEC Expression and Epithelial-Mesenchymal Transition (EMT) in Human Breast Cancer MCF-7 Cells. BioMed Res J 3: 125-131.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Allergy Research (ISSN:2642-326X)





