Research Article
Evaluation of Epidermal Growth Factor Receptor Expression in Triple Negative Breast Carcinoma and Its Correlation with the Clinicopathological Parameters
940
Views & Citations10
Likes & Shares
Introduction: Triple negative breast carcinomas (TNBC) constitute about 10-17% of all the invasive breast cancers worldwide. The epidermal growth factor receptor (EGFR) is a tyrosine kinase receptor in the Human epidermal growth factor receptor (HER) family and its expression in TNBC is an independent poor prognostic marker associated with higher histological grades, larger tumor size and poor clinical outcomes.
Methods: In the present prospective study, 40 histologically confirmed cases of TNBCs during the period of May 2018 to July 2020 were included in the study. The cases were subjected to EGFR immunostaining and its expression was evaluated and correlated with the various clinico-pathological parameters. Obtained results were analyzed using Chi- square test (Statistical Package for Social Sciences (SPSS) version 23), value of P
Results: EGFR expression was observed in 70% of TNBCs and a statistically significant result was observed with histological grade (P=0.006) and the tumor size (P=0.030) while no significant correlation was seen with the age group, histological type, lymph node status or lymphovascular (LVI) and perineural invasion (PNI).
Conclusion: EGFR is a poor prognostic marker in TNBC and its expression could serve as a biomarker for identifying TNBC patients with poor survival. Studies like these can open doors in the research field for the development of more novel anti EGFR targeted therapies.
Keywords: Epidermal growth factor receptor, Triple negative breast carcinoma, Histological grade, Aggressive
INTRODUCTION
Invasive breast cancer is the most common cancer in women worldwide, with over 2 million new cases diagnosed in 2018 and accounts for 23% of all cancers in women globally [1]. According to estimates, by the year 2020, around 17,97,900 women in India may have breast cancer [2]. The risk of breast cancer doubles each decade until menopause, after which the increase slows. In many countries having advanced medical care facilities, the five-year survival rate of breast carcinoma is 80-90 per cent, falling to 24 per cent for breast cancers which are diagnosed at a more advanced stage [3].
TNBC is a diagnosis of exclusion, which is based on lack of estrogen (ER) and progesterone receptor (PR) expression by immunohistochemistry (IHC), and lack of Her2 neu overexpression or gene amplification by IHC and in situ hybridization. These carcinomas constitute about 10 - 17% of all invasive breast cancers and are known to be morphologically aggressive than other types of breast cancer. They are known to have worse prognosis and higher risk of metastases [4].
The EGFR is a tyrosine kinase receptor in the HER family and it is widely expressed in a number of epithelial tumors [5].EGFR activation results in cell proliferation, survival, angiogenesis, invasion and metastasis. The expression of EGFR can also be seen in many of the human epithelial cancers such as breast cancer, head and neck squamous cell carcinoma, non-small cell lung, colorectal, pancreatic and brain cancers [6]. Its expression is seen in 15-45% of breast carcinomas and upto 72% in TNBCs and Basal like breast carcinoma (BLBC) cases [7]. EGFR receptor plays a crucial role not only in molecular diagnosis of breast cancer, but its expression also induces resistance to chemotherapy and radiation treatment, and therefore it is a marker of poor prognosis and survival. Hence, EGFR targeted therapies may have a promising role in triple negative breast cancers, although further studies are necessary to better understand the best ways to use EGFR targeted therapy [8].
The present study aims to analyze EGFR expression in TNBCs and its correlation with the age, type of histology, histological grade, pathological stage, lymph node status, LVI and PNI.
MATERIALS AND METHODS
The present prospective study was conducted in the Department of Pathology at SRMS Medical College and Hospital, Bareilly, a tertiary health care center from the period of May 2018 to July 2020. 40 cases of histologically and immune histochemically confirmed triple negative invasive breast carcinoma was included in the study. Resection specimens were received in the Department of Pathology in 10% neutral buffered formalin. After adequate fixation for 24 hours, the biopsy specimen was submitted for routine processing, followed by paraffin embedding and stained with Hematoxylin and Eosin. The histologically confirmed cases of invasive carcinoma breast were subjected to immune histochemical staining for ER, PR, and HER2neu. The triple negative cases were then subjected to immunohistochemical staining for EGFR.
Histological Grade
Grading was done according to the Modified Bloom Richardson (Nottingham) grading system and the tumors were graded into Grade-1 (well differentiated), Grade-2 (Moderately differentiated) and Grade-3 (Poorly differentiated).
Immunohistochemical staining method (Thermo Scientific method)
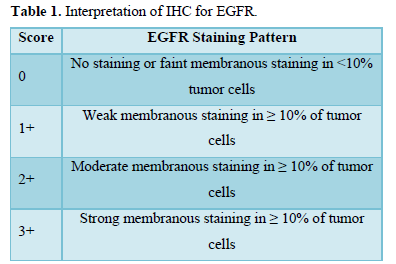
IHC profile of the tumor was assessed by submitting one representive section from tumor block to EGFR. IHC was performed on 4 µm thick sections according to streptoavidin-biotin immunoperoxidase technique. The intensity of EGFR expression and percentage were assessed and scored on a scale of 0 to 3+. Only membrane staining (dark golden-brown precipitate) of malignant cells was evaluated and cases with only cytoplasmic staining were considered negative, irrespective of staining intensity (Table 1).
For statistical analysis of EGFR expression, the cases were dichotomized as:
- Negative - for a score of 0, 1+
- Positive - for score of 2+, 3+ [9]
Statistical analysis
Data was analyzed using Statistical package for social sciences (SPSS), version 23. Results for continuous variables are presented as mean ± standard deviation, whereas results for categorical variables are presented as number (percentage). Correlation of EGFR expression with independent variables such as histological type and grades, LVI, PNI, Lymph node status was analyzed by applying Chi Square test. The level P < 0.005 was considered value of significance.
RESULTS
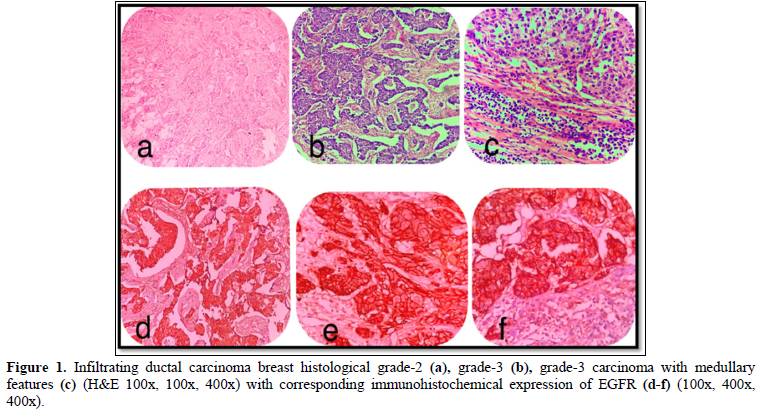
The age range observed in our study was 21 to 76 years with a mean of 48.76 ± 24.602 years. Maximum belonged to the age group 41-50 years (37%) and minimum cases were seen in the age group > 70 years (5%). Among the 40 cases studied, 25 (62.5%) cases were Grade-2 (Figure 1a) followed by 10 (25%) cases, Grade-3 (Figure 1b) and only 5 (12%) cases were Grade -1. Invasive carcinoma of no special type accounted for maximum number of cases i.e 34 (85%), and it was observed that only 6 (15%) cases were of Carcinoma with medullary features. (Figure 1c).
Out of the 40 cases, in 21 (52.5%) cases the tumor size was >2 cm but
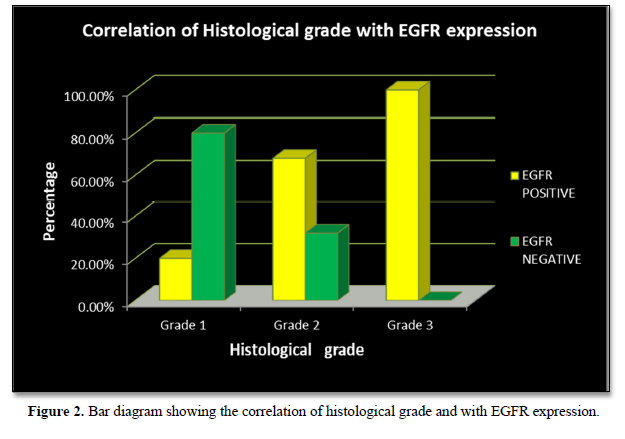
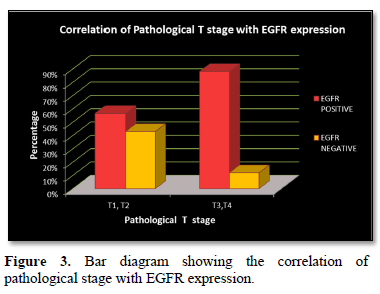
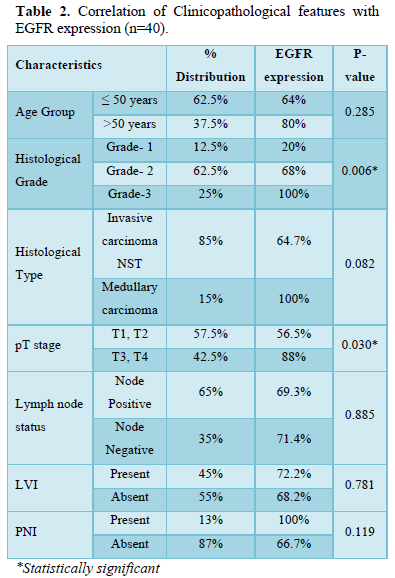
EGFR expression was observed in 28 (70%) cases with a score of 2+ in 10 (25%) cases and a score of 3+ in 18 (45%) cases, while it was negative in 12 (30%) cases which included score 0 (6 cases- 15%) and score 1 (6 cases- 15%). EGFR expression was highest in Grade 3 tumors (Figure 2) in age group >50 years and in those belonging to the histological type carcinoma with medullary features. (Figures 1d-1f) The highest frequency of EGFR positive cases belonged to the stage T3, T4 (Figure 3). It was also noted that expression was slightly higher in cases that showed negative nodes. All the cases which had LVI and PNI showed positive EGFR expression although results were not statistically significant (Table 2).




DISCUSSION
TNBCs are usually high-grade tumors and EGFR expression in TNBC is an independent poor prognostic marker associated with higher histological grades, larger tumor size, poor clinical outcomes, and plays an important role in resistance to chemotherapy and radiotherapy [8].
In our study, a total of 40 histologically and immunohistochemically confirmed TNBC cases were analyzed to observe the expression of EGFR and its correlation with various clinicopathological parameters.
TNBC affects relatively younger women with majority of cases occurring in the age group less than 50 years. The age range observed in our study was 21 to 76 years with a mean of 48.76 ± 24.602 years which is in concordance with studies by Zhang [10], Changavi [11], Rakha [12], Sabatier [13] and Sutton [14].
In our study majority of the triple negative tumors belonged to Modified Bloom Richardson Nottingham histological grade 2 (62.5%) followed by grade 3 (25%), similar to the study done by Abdelrahman [15]. In contrast to our study, studies by Livasy [16], Rakha [12], Zhang [10] and Tan [17] reported maximum cases of triple negative breast carcinoma in grade 3 comprising 100%, 90.7%, 52.9% and 96.8% cases respectively which could be due to the different geographical and sociocultural factors.
In our study, out of 40 cases most of them belonged to the invasive carcinoma of no special type (85%) consistent with the studies done by Changavi [11], Tan [17], Abdelrahman [15], Zhang [10] and Rakha [12].
Tumor size is one of the important prognostic factors in invasive breast carcinomas. The single greatest dimension of the largest invasive carcinoma is used to determine the T classification. The distribution of pathological T stage of tumor in the present study showed that maximum cases were in stage pT2 (52.5%) which is comparable to the studies by Abdelrahman [15], Zhang [10]. In contrast Tan [17] and Rehman [18] reported majority cases in stage pT1.
In the present prospective study, overall node positive cases were 26 out of 40 (65%) and node negative cases were 14 (35%) which was in concordance to studies by Zhang [10] and Abdelrahman [15] while Tan et al observed more node negative cases associated with TNBC and Rakha [12] also found no association between TNBC and lymph node status [19].
In invasive breast malignancies the presence of LVI is considered an important prognostic factor and it is associated with nodal metastasis, high locoregional spread and recurrence. In our study LVI was observed in only 18 (45%) cases and was comparable to studies by Zhang [10] and Ahn [20].
PNI is considered positive when the tumor cells invade the perineurium or the neural fascicles. In the present study, PNI was observed only in 5 (13%) cases. Similar findings are reported by Rehman [18], Ahn [20], Menbari [21] and Cernea [22]. PNI has no prognostic value in breast carcinoma as no difference is seen in the disease-free survival between the PNI-positive and PNI-negative patients [15].
The EGFR is a membrane bound tyrosine kinase protein of the HER family which plays an important role in the cell survival, proliferation, angiogenesis and tissue invasion. Expression of EGFR is usually seen in around 15-45% of breast carcinoma. Its expression in triple negative breast cancers has a wide range. In our study among 40 TNBC cases, EGFR expression was observed in 28 (70%) cases while it was negative in 12 (30%) cases which is in concordance to studies by Abdelrahman [15], Livasy [16] and Changavi [11] who also reported an EGFR expression of 71.4%, 72.2% and 89.47% respectively.
EGFR expression was significantly correlated with increasing histological grades of TNBC in the present study. The p value was 0.006 which was in concordance with the studies by Abdelrahman [15], Changavi [11] and Zhang [10] who also observed a statistically significant correlation with P values of 0.006, 0.034 and 0.005. Fox [23] showed a statistically insignificant result (P = 0.085).
A significant correlation (P = 0.030) was observed with larger tumor size and EGFR expression which was comparable to the study done by Zhang [10] who also showed a significant correlation (P = 0.018) [10]. In contrast studies by Fox [23] and Abdelrahman [15] showed a statistically insignificant result (P = 0.198 and 0.161).
In this study no correlation was observed between the expression of EGFR and age group (P = 0.589) which was in concordance with the study by Abdelrahman [15] (P = 0.589). While studies by Fox [23] and Changavi [11] showed a significant correlation between EGFR expression and younger age group (P = 0.0006 and P = 0.003).
No correlation was found between nodal metastases and EGFR expression in our study. The result was statistically insignificant (P = 0.885). These findings were consistent with studies done by Fox [23] and Sutton [14] (P = 0.11 and 0.45). Lymphovascular invasion and perineural invasion was also correlated with EGFR expression in the present study. The result was statistically insignificant with P values of 0.781 and 0.119. Comparable studies were not available as there is very limited literature regarding correlation of EGFR expression with lymphovascular and perineural invasion. Although if present EGFR expression correlates with positive lymphovascular and perineural invasion, increased rate of nodal metastasis and poorer outcome [24].
CONCLUSION
Our study concluded that there is a statistically significant correlation between EGFR expression with higher histological grade and with larger tumor size. No statistically significant correlation was observed between EGFR expression and age group, histological type, lymph node status, LVI and PNI. Further studies of larger number of TNBC cases are needed to confirm our results.
FUTURE IMPLICATIONS
EGFR is a poor prognostic marker in triple negative breast cancer and its expression could serve as biomarkers for identifying TNBC patients with poor survival that are unlikely to benefit from neoadjuvant chemotherapy. Studies like these can open doors in the research field for the development of more novel anti EGFR targeted therapies. Authors suggest role might be relevant from clinical and therapeutic point of view and EGFR inhibitors should be investigated in this context.
CONFLICTS OF INTERESTS
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FINANCIAL SUPPORT
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ETHICAL APPROVAL
The study was ethically approved by institutional committee.
- Park K (2015) Epidemiology of chronic non-communicable diseases and conditions. Preventive and Social Medicine. 23rd ed, pp: 389-390.
- Malvia S, Bagadi SA, Dubey US, Saxena S (2017) Epidemiology of breast cancer in Indian women. Asia-Pac J Clin Oncol 13: 289-295.
- Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, Vijver VMJ (2012) (Eds.) WHO Classification of Tumors of the Breast. IARC: Lyon.
- Carey L, Winer E, Viale G, Cameron D, Gianni L (2010) Triple negative breast cancer: Disease entity or title of convenience. Nat Rev Clin Oncol 7: 683-692.
- Mohamed AS, Al-Tamimi DM, Ahmed A (2011) Very low prevalence of epidermal growth factor receptor (EGFR) protein expression and gene amplification and gene amplification in Saudi breast cancer patients. Diagn Pathol 6: 57-62.
- Brand TM, Iida M, Li C, Wheeler DL (2011) The nuclear epidermal growth factor receptor signaling network and its role in cancer. Discov Med 12: 419-432.
- Baselga J (2002) Why the epidermal growth factor receptor? The rationale for cancer therapy. Oncologist 7(4): 2-8.
- Masuda H, Zhang D, Bartholomeusz C, Doihara H, Hortobagyi GN, et al. (2012) Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res Treat 136: 331-345.
- Kim A, Jang MH, Lee SJ, Bae KY (2017) Mutations of the Epidermal Growth Factor Receptor Gene in Triple Negative Breast Cancer. J Breast Cancer 20(2): 150-159.
- Zhang M, Zhang X, Zhao S, Wang Y, Di W, et al. (2014) Prognostic value of surviving and EGFR protein expression in triple-negative breast cancer (TNBC) patients. Targ Oncol 9(4): 349-357.
- Changavi AA, Shashikala A, Ramji AS (2015) Epidermal growth factor receptor expression in triple negative and nontriple negative breast carcinomas. J Lab Physicians 7(2): 79-83.
- Rakha EA, El‐Sayed ME, Green AR, Lee AH, Robertson JF, et al. (2007) Prognostic markers in triple‐negative breast cancer. Cancer 109(1): 25-32.
- Sabatier R, Jacquemier J, Bertucci F, Esterni B, Finetti P, et al. (2011) Peritumoral vascular invasion a major determinant of triple negative breast cancer outcome. Eur J Cancer 47(10): 1537-1545.
- Sutton L M, Han J S, Molberg K H, Sarode V R, Cao D, et al. (2010) Intratumoral expression level of epidermal growth factor receptor and cytokeratin 5/6 is significantly associated with nodal and distant metastases in patients with basal-like triple-negative breast carcinoma. Am J Clin Pathol 134(5): 782-787.
- Abdelrahman AE, Rashed HE, Abdelgawad M, Abdelhamid MI (2017) Prognostic impact of EGFR and cytokeratin 5/6 immunohistochemical expression in triple-negative breast cancer. Ann Diagn Patho 28: 43-53.
- Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, et al. (2006) Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol 19(2): 264-271.
- Tan DS, Marchio C, Jones RL, Savage K, Smith IE, et al. (2008) Triple negative breast cancer: Molecular profiling and prognostic impact in adjuvant anthracycline-treated patients. Breast Cancer Res Treat 111(1): 27-44.
- Rehman A, Kim Y, Kim H, Sim J, Ahn H, et al. (2018) FOXO3a expression is associated with lymph node metastasis and poor disease-free survival in triple-negative breast cancer. J Clin Pathol 71(9): 806-813.
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, et al. (2007) Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res 13(15): 4429-4434.
- Ahn KJ, Park J, Choi Y (2017) Lymphovascular invasion as a negative prognostic factor for triple-negative breast cancer after surgery. Radiat Oncol J 35(4): 332.
- Menbari MN, Rahimi K, Ahmadi A, Mohammadi-Yeganeh S, Elyasi A, et al. (2020) Association of HDAC8 Expression with Pathological Findings in Triple Negative and Non-Triple Negative Breast Cancer: Implications for Diagnosis. Iran Biomed J 24(5): 283-289.
- Cernea A, Fernández-Martínez JL, deAndrés-Galiana EJ, Galván Hernández JA, García Pravia C, et al. (2018) Analysis of clinical prognostic variables for triple negative breast cancer histological grading and lymph node metastasis. J Med Inf Decis Making 1: 14-36.
- Fox SB, Smith K, Hollyer J, Greenall M, Hastrich D, et al. (1994) The epidermal growth factor receptor as a prognostic marker: results of 370 patients and review of 3009 patients. Breast Cancer Res 29(1): 41-49.
- Duraker N, Caynak ZC, Turkoz K (2006) Perineural invasion has no prognostic value in patients with invasive breast carcinoma. Breast 15(5): 629-634.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Pathology and Toxicology Research
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- International Journal of Diabetes (ISSN: 2644-3031)




