Research Article
Occurrence and Antibiotic Resistant Pattern of Microbes Isolated from Local Herbal Product (AGBO) Sold at Oja-Bisi in Ado-Ekiti
7550
Views & Citations6550
Likes & Shares
The use of herbal medicine to treat various illness as means of primary health care has become so common worldwide. However, during preparations herbal plants may be exposed to contamination by bacteria agents that may lead to infections. This study is therefore aim at assessing the occurrence and antibiotic resistant pattern of microbes isolated from herbal products sold in Oja Bisi market in Ado-Ekiti, Ekiti State, Nigeria. Seven herbal preparations were randomly bought from different sellers for this cross-sectional study that took place between the month of February to July 2020. Aliquots of the various serially diluted herbal products were cultured on various media plates; Nutrient agar, EMB and MacConkey agar and incubated at 37℃ for 24 h. Total bacteria count was done by pour plate method and isolates were further identified by their full morphological and biochemical characteristics with their various antibiotic resistance profiles determined by disc diffusion method. The seven herbal medicine preparations all had bacteria contamination with a minimum bacterium count of 2.0 x102 and a maximum count of 8.0 x102. Additionally, a total number of Nine isolates like Staphylococcus aureus, Staphylococcus saprophyticus, Staphylococcus hemolyticus, Klebsiella pneumonia, Klebsiella ozaenae, Klebsiella oxytoca, Pseudomonas aeruginosa, Enterobacter cloacae and Enterobacter aerogenes were isolated. Susceptibility testing shows that CIP(Ciprofloxacin) and CRO(Cefriaxone) were the most susceptible antibiotic, while AM(Ampicillin) and OX(Oxacillin) were the most resistance antibiotics with most of the isolates having multiple drug resistance. The herbal medicine sold in Oja Bisi market were mildly contaminated with bacteria count within WHO limit. This may facilitate transmission of communicable diseases in the population and therefore pose a public health concern. Herbal product sellers should have a well-coordinated periodic training, enlightment on these herbal products and collaboration on hygienic practices and safety stand.
Keywords: Antibiotics, Resistance, Microbes, Susceptible, Isolates
Abbreviations: EMB: Eosin Methylene Blue Agar; WHO: World Health Organization
INTRODUCTION
The use of herbal medicine is generally increasing worldwide [1]. In many developed countries, 70-80% of the population still depends upon herbal drugs for their health care [2]. Similarly, in developing countries, up to 80% of the population relies on herbal medicine as a major source of therapy [3]. Although the World Health Organization (WHO) has advocated the integration of herbal medicinal products (HMPs) into the primary health care system of developing countries, safety issues related to herbal drug preparations continue to be ignored [4]. Thus, the safety of herbal products became a major concern in public health [5]. This is because microorganisms of various kinds are normally adherent to leaves, stem, flowers, seeds, and roots from which herbal medicine can be prepared and potential pathogens may also be introduced during harvesting, handling [6], open-air drying, preserving, manufacturing [5], and use of contaminated materials for storage [7]. Different studies that have been performed on herbal medicinal products revealed the presence of bacterial pathogens with multiple drug resistance [8]. Antibiotic resistance is a serious and growing phenomenon in contemporary medicine and has emerged as one of the pre-eminent public health concerns in 21st century.
Antimicrobial drug resistance (ADR) hampers the control of infectious diseases and has potential to threaten health security, damage trade and economies but it is difficult to think of “the world without antibiotics”. It may be a deadly situation because the routine surgery, cancer treatments, organ transplants etc. become just impossible without antibiotics. So, we need to save antibiotics for certain therapeutic interventions. It is also important to take urgent and coordinated action to save the world from entering a post-antibiotic era, in which common infections and minor injuries can become life threatening. Development of ADR is a natural phenomenon [9]. All attempts are in progress to promote optimal use of antibiotics both in humans and animals to address problem of growing AMR. Most of the pathogenic bacteria have developed resistance to modern antibiotics as a result of which we are evidencing multi drug resistance among bacteria. We are running out of antibiotics and could not add any new group of antibiotics since last three decades.
At the same time, there is no potential antibiotic in pipeline for release in near future. As a result, research in alternative medicine has begun and one such alternative is use of herbal drugs to treat infections. Since ancient times, herbs and their essential oils are known for their varying degrees of antimicrobial activity. Medicinal plants are densely distributed in the tropical rainforest zones of the world of which Nigeria fall into [10]. The medical systems in developing Countries involve both traditional herbal systems and orthodox medicines.
In Africa, particularly Nigeria, new drugs are not often affordable thus up to 80 % of the population use medicinal plants as remedies. It was reported by WHO that in Nigeria, the ratio of Traditional Health Practitioners to the population was 1:110, while the ratio of Medical Doctors to the population was 1:16, 400. This gives credence to the fact that people patronize Traditional Medicine Practitioners (TMPs) for their primary health needs more than orthodox medical doctors [11,12]. This condition and the fact that international commercial orthodox medicines are becoming increasingly out of reach for most Nigerians contributed to the dependence of a large percentage of the Nigeria people on local herbal medicine. It is however worth noting that Africa, North and South America together with Asia are the areas containing the worlds’ greatest number of plants species that are not found elsewhere. From this immense reservoir of plants, herbalists in Nigeria source different herbal medicines which are widely used today and surprisingly gaining recognition by government as can be seen by the establishment of the Herbal Medicine Board. The growing and unhygienic use of herbal medicinal products has become a major concern in public health [13]. However, in Nigeria, particularly in the study area, Ado-Ekiti; data which addresses the bacteriological quality of herbal medicinal products is scarce. Hence the aim of this study is to assess the occurrence and antibiotic resistant pattern of bacteria isolated from herbal products precisely AGBO as it is locally called, sold in Oja Bisi market and to check for pathogenic organisms in herbal product bought from Oja Bisi market in in Ado-Ekiti, Ekiti State, Nigeria.
Study Area, Study Design, and Period
A cross- sectional study was conducted from February 2020 to July 2020, at Ado- Ekiti, a city in the Southwestern part of Nigeria. It is the State Capital and Headquarters of Ekiti state. Ado Ekiti is located between latitude 7° 34' and 7° 44' north of the equator and longitude 5° 11' and 5° 18' east of the Greenwich Meridian on total land area of 36.7km2 [14], having a total population of 424,340 [15]. Oja Bisi market, a major market that attracts sellers from all over the smaller districts and villages around Ado Ekiti was used as the major area of the study. Herbal products for the study were bought from the Oja Bisi market hence sampling represented all rural markets in the districts. These products were bought from Oja Bisi market every other market day to capture various sellers. Isolation and identification of microorganisms, and Antimicrobial resistance was carried out in the Microbiology Laboratory, Afe Babalola University Ado-Ekiti (ABUAD) with the use of Inoculating loops, straight wire, Bunsen burner, Incubators at 370C, petri dishes, test tubes, Media (TSI agar, EMB agar, Mac Conkey agar, nutrient agar), conical flask, autoclave, water bath, bijou bottles, sensitivity disc, universal bottles sterile polythene Zip lock bags etc.
Sample Collection and Processing
For this study a total of 7 different mixtures were bought on each market day. All orally and locally consumed powder form preparations and all liquid samples were purchased and included in this study. Each of the sample was collected into a sterile polythene Zip lock bag and transported to the laboratory at Afe Babalola University immediately for analysis. Six of the samples were in liquid form and ready for use, while the seventh sample was powdered and needed to be dissolved in water, a bottled water was used to dissolved the powder and all samples labeled A, B, C, D, E, F& G were stored in the refrigerator while awaiting preparation of culture media. The seven samples collected were described specifically according to their functions as follows:
- For baby convulsion
- For typhoid
- For typhoid
- For malaria
- For malaria
- For blood cleansing
- For allergic reaction
Preparation of Culture Media
All culture media used for this study were prepared following the manufacturer’s instruction. The different media used were weighed appropriately and they were all dissolved using distilled water and then they were heated in a water bath for proper dissolving before sterilizing was done at 1210C using an autoclave, swirling to distribute the media evenly when poured into a Petri dish. The media which were prepared for culturing include; peptones broth, Nutrient agar, Mannitol salt agar, salmonella/shigella agar, Mueller-Hinton agar, Eosin methylene blue agar, Simon citrate agar, MacConkey agar, Motility broth and blood agar. Quality control was carried out on all prepared media before usage, this was done by assessing their sterility and potency following standard practice.
Inoculation/Isolation of Organisms
Weighed samples were inoculated into peptone broth and incubated at 370C for 24 h. After incubation was done for 24 h, a loop-full of the peptone broth was transferred and streaked separately onto the surface of the following agars; Nutrient agar, Mannitol salt agar, salmonella/shigella agar, Mueller-Hinton agar, Eosin methylene blue agar, Simon citrate agar and MacConkey agar. The plates were incubated at 370C for 24 h and growth of typical colonies was observed and subcultures to get pure isolates. A loop full of pure isolates were transferred into nutrient agar, EMB, Mac Conkey slants and stored in the refrigerator at 40C for further analysis.
Bacteria Count
At the end of the incubation period, the number of colony-forming units per gram (CFU/g) was calculated by multiplying the average number of colonies by the dilution factor. The obtained CFU/g of sample was compared with WHO standards. Samples that presented bacterial growth greater than 105 CFU in 1 g of herbal medicine were considered unsatisfactory or inadequate according to WHO guidelines for aerobic bacteria.
Biochemical Tests
Biochemical test description for the identification of microorganisms based on microscopic appearance and characterization was carried out using The Becton Dickinson bbl Crystal Identification Systems Enteric/Non-Fermenter Machine.
- Oxidase Test: A piece of clean filter paper was placed on a clean plate and 2-3 drops of oxidase reagent dropped on the filter paper and a loop full of organism of each isolates was streamed across the surface of the droplet. A positive reaction was indicated by the appearance of a purple color within 10-15 s while a colorless appearance indicated a negative reaction. This test was carried out on 24 h isolates.
- Catalase Test: This test was done on a clean grease free slide whereby 2-3 drops of 3% hydrogen peroxide were placed on the slide and a loop full of an inoculum placed and mixed properly with hydrogen peroxide. A positive result is indicated by effervescence; a negative reaction showed absence of effervescence.
- Indole Test: One ml (1ml) of Kovacs reagent was dispensed into a 48-h broth culture of isolates and appearance of red ring on the surface of the broth indicated indole positive while yellow-green color indicated indole negative.
- Motility Test: This was done by stabbing the test isolates into the motility broth and incubating at 370C for 48 h. Motile organisms were shown by cloudy appearance of the organism around the broth (diffuse growth) while region stabbed that is clearly seen or limited to the line of inoculum was termed to be non-motile.
- Citrate Test: This test was carried out by inoculating the prepared agar slant with the test isolates and incubated at 370c for 24 h. If the isolates utilize citrate as its carbon source, a green to blue color change is indicated but there if there is no change, it shows that the organisms were not able to utilize citrate as its only carbon source.
- Triple Sugar Iron: Tubes containing TSI medium slant was inoculated with test isolates by stabbing the agar and striking the surface of the slant, then closing aseptically with a loose fitted cap and incubated at 370 The cultured medium was observed for gas production: indicated by blackening of the agar, glucose, lactose and sucrose production. Presence of red color on the slope (alkaline) and presence of yellow color on the butt (acid).
- Methyl Red Test: The tube of MR medium (glucose phosphate medium) was incubated with the test organism and incubate at 370c for 48 h. One quarter of the culture was decanted into a clean test tube and 2 drops of methyl red solution was added and shaken vigorously. A positive result showed a red color at the surface while a negative result showed yellow color at the surface.
- Antimicrobial Susceptibility Testing: The antibiotic susceptibility test was performed by using disc diffusion method recommended by the Clinical and Laboratory Standards Institute (CLSI) guidelines on Muller- Hinton agar plate. The antibiotic discs and their concentration were Septrin (SXT), Azithromycin (AZM), Oxacillin, Gentamycin, Vancomycin, Ciprofloxacin, Ceftriaxone, Ampicillin, Doxycycline, Cefuroxime, Erythromycin, and Augmentin. A sterile swab was placed into the broth culture of a specific organism and the excess water was removed gently by pressing or rotating the swab inside the tube. Before placing the antimicrobial disc, the swab with the bacterial suspension was distributed evenly over the entire surface of Mueller- Hinton plates. The plates were incubated at 37℃ for 18-24 h. The diameter of the zone of inhibition was measured and interpreted using standard chart as sensitive and resistant.
- Statistical Analysis: Data was checked for completeness, cleaned manually, entered, and analyzed using SPSS version 20 statistical package. Analysis was made using frequency tables. Pearson’s test and odds ratio with 95% CI were used for measures of association and P values less than 0.05 was considered as statistically significant.
RESULTS
Bacteria Count
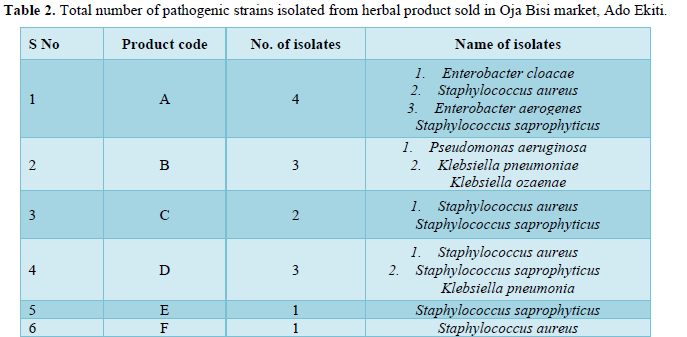
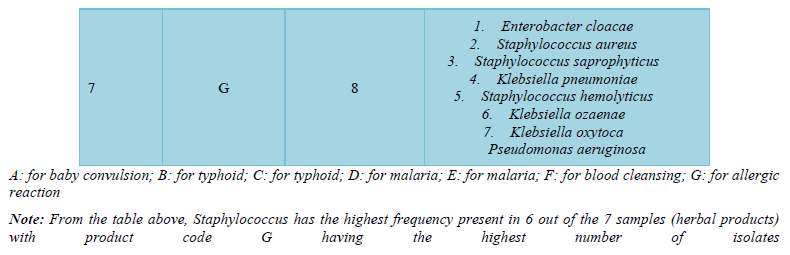
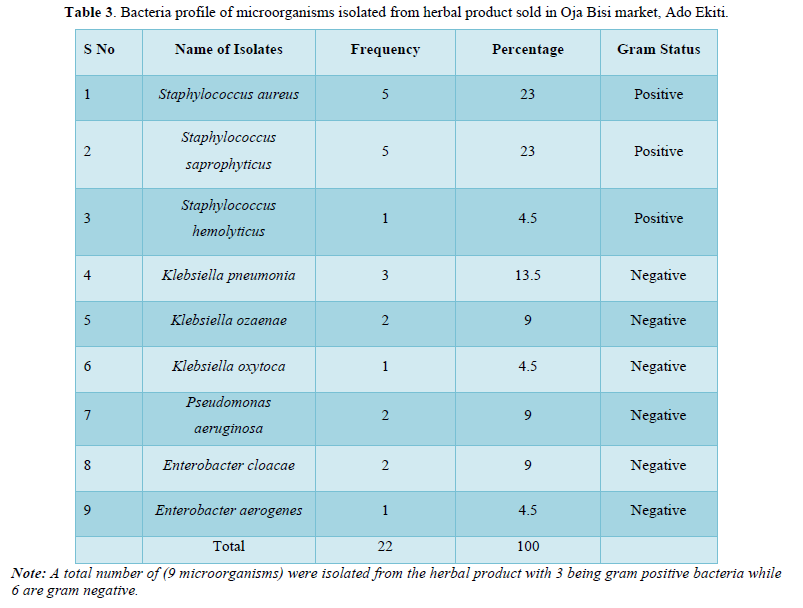
The bacteria count obtained from the seven herbal medicine preparations bought from Oja Bisi market had a minimum count of 2.0 x102 and a maximum count of 9.0 x102, which were all in the WHO standard limit. Also, no E. coli pathogen was isolated (Table 1). A total number of 9 pathogenic microorganisms with 3 being gram positive and 6-gram negative were isolated with sample G having the highest number of pathogenic isolates as shown in Table 2 and in Table 3, Staphylococcus aureus and Staphylococcus saprophyticus were having the highest frequency compared to other organisms. Also, the standard biochemical full identification of the microorganisms is shown in Table 4.
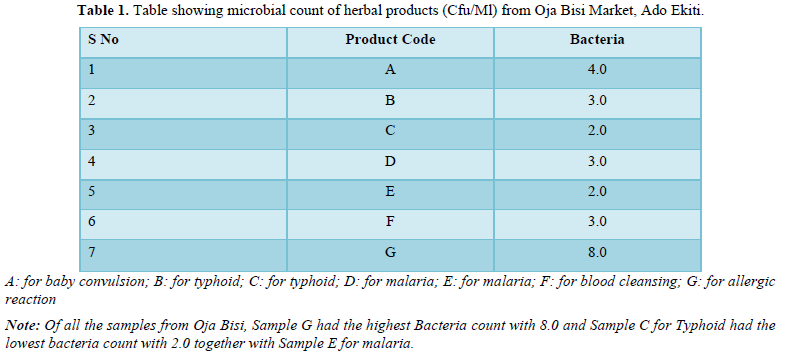
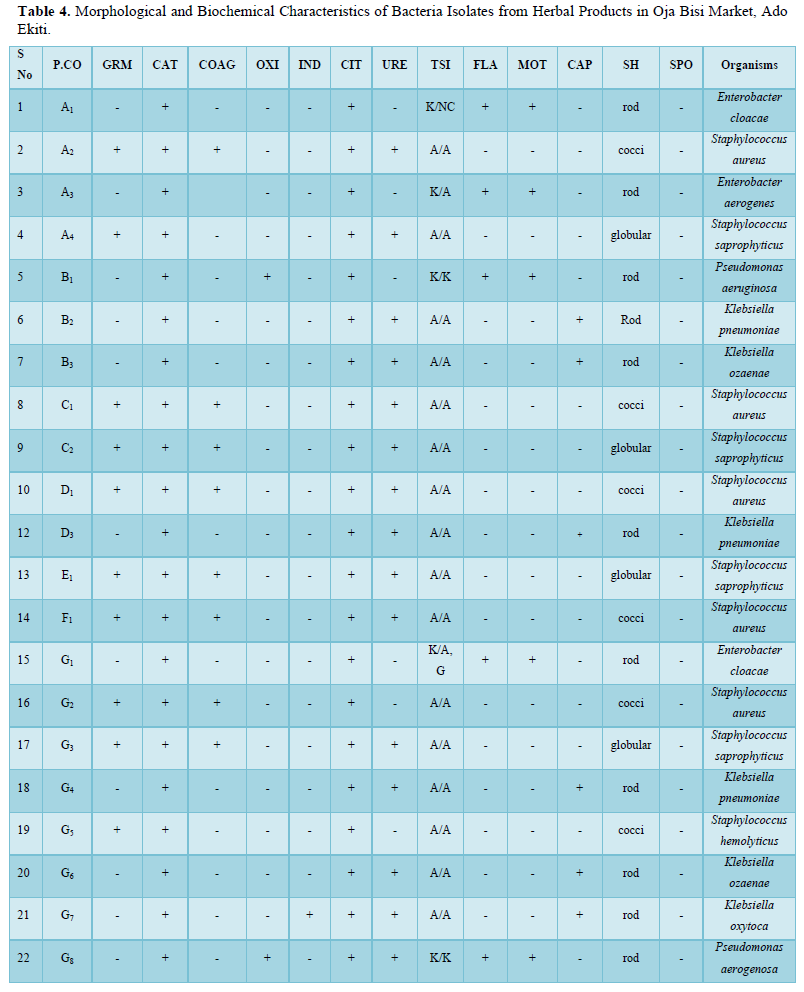





P.CO: Product Code; GRM: Gram Reaction; CAT: Catalase; COAG: Coagulase; OXI: Oxidase; IND: Indole; CIT: Citrate; URE: Urease; TSI: Triple Sugar Iron; FLA: Flagella; MOT: Motility; CAP: Capsule; SH: Shape; SPO: Spore; K/N.C: Alkaline Slant/No Change in Butt; A/A: Acidic Slant/Acidic Butt; K/A: Alkaline Slant/Acidic Butt; K/K: Alkaline Slant/ Alkaline Butt
Also, the standard biochemical full identification of the microorganisms is shown in Table 4 and the antibiotics susceptibility pattern of gram-positive and negative isolated from these products were determined in Figures 1 and 2, it was observed that Staphylococcus spp were having the highest percentage of (23%) followed by K. pneumoniae with about 13%, Klebsiella oxytoca and Enterobacter aerogenes had the lowest percentage of 5%.
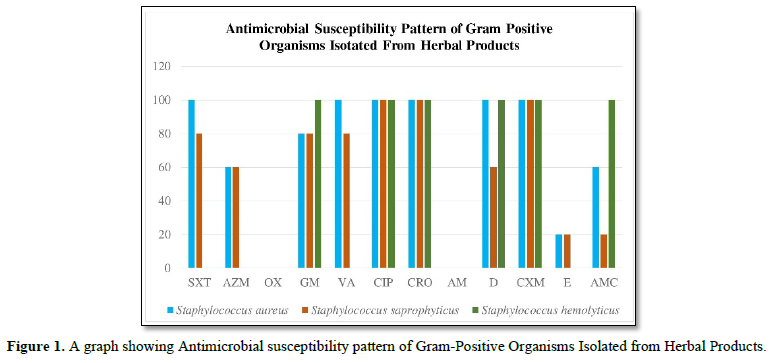


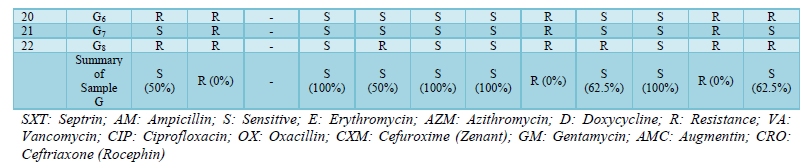
SXT: Septrin; AM: Ampicillin; S: Sensitive; E: Erythromycin; AZM: Azithromycin; D: Doxycycline; R: Resistance; VA: Vancomycin; CIP: Ciprofloxacin; OX: Oxacillin; CXM: Cefuroxime (Zenant); GM: Gentamycin; AMC: Augmentin; CRO: Ceftriaxone (Rocephin)
From the graph, Staphylococcus aureus was 100 percent susceptible to Septrin, Vancomycin, Ciproflaxin, Ceftriaxone, Doxycycline and Cefuroxine unlike Azithromycin, Gentamycin, Erythromycin and Augumetin which have susceptibility level of 60%,80%, 20% and 60% respectively. Also, on the one hand Staphylococcus saprophyticus is 100% susceptible to Ciproflaxin, Ceftriaxone and Cefuroxine while on the other hand Septrin, Azithromycin, Gentamycin, Vancomycin, Doxycycline, Erythromycin and Augumetin’s susceptibility were 80%,60%,80%,80%,60%,20% and 20%. Finally, on the graph Staphylococcus hemolyticus was 100% susceptible to Gentamycin, Ciproflaxin, Ceftriaxone, Doxycycline, Cefuroxine and Augumentin. All three gram-positive isolates were resistant to oxacillin and ampicillin.
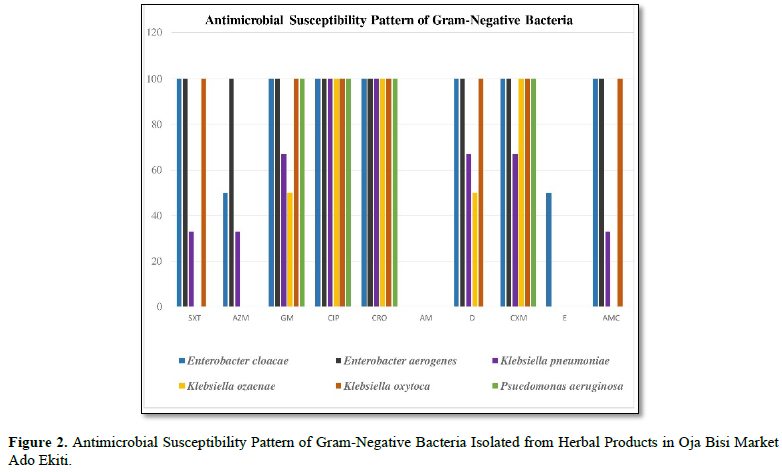
SXT: Septrin; AM: Ampicillin; E: Erythromycin; AZM: Azithromycin; D: Doxycycline; CIP: Ciprofloxacin; CXM: Cefuroxine (Zenant); GM: Gentamycin; CRO: Ceftriaxone (Rocephin); AMC: Augumentin
From the graph Enterobacter cloacae was 100% susceptible to Septrin, Gentamycin, Ciproflaxin, Cefriaxone, Doxycycline, Cefuroxine and Augumentin meanwhile the susceptibility for Azithromycin, and Eryhromycin is less than 100%. In the same vein, Enterobacter Aerogenes is 100% susceptible to Septrim, Gentamycin, Azithromycin, Doxycycline, Ciproflaxin, Ceftriaxone, Cefuroxine and Augumentin. Also, Klebsiella pneumonia was only 100% susceptible to Ciproflaxin and Ceftriaxone while the same organism was less than 100% susceptible to other antibiotics.
Antibiotics Susceptibility Testing
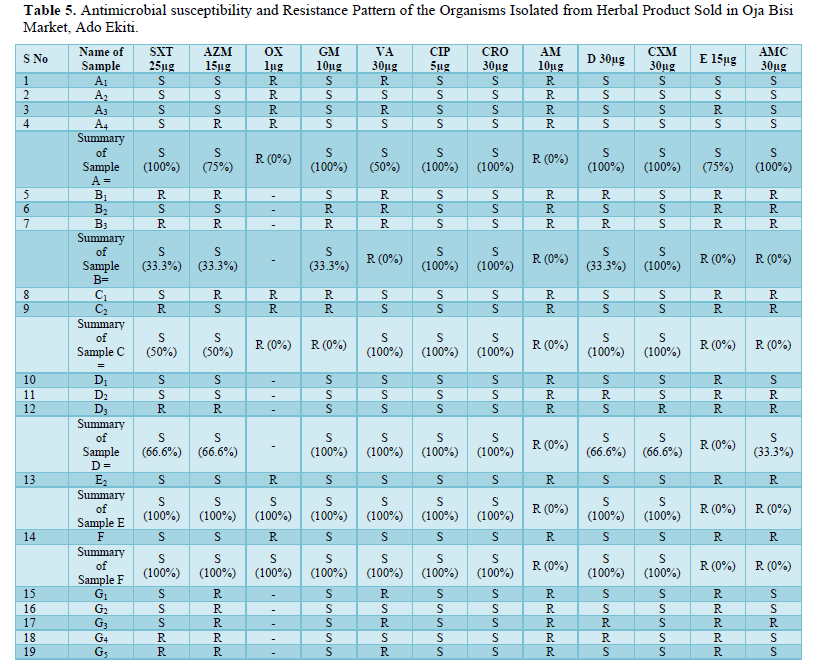
Antibiotics susceptibility and resistance pattern of the organisms isolated from all the samples were shown in Table 5 and it was observed that out of all the sample, sample C had the highest level of antibiotics resistivity. As said earlier, the isolates were resistant to Oxacillin and Ampicillin, fewer organisms were susceptible to SXT (Septrin), AZM (Azithromycin) and E (Erythromycin).
DISCUSSION
Medicinal plant preparation is expected to be carried out in an aseptic condition ideally and products duly approved by standard organizations before getting to the markets and eventually to the consumers. However, what we find in our society today, is on the contrary a lot of sharp practices. The report of our study shows that the seven herbal medicine preparations bought from Oja Bisi market all had bacteria contamination with a minimum bacterium count of 2.0 x102 and a maximum count of 8.0 x102. Although none of them exceeded the safety limits (CFU/g ≤ 105) according to WHO guidelines [4], it is still worrisome as most of the isolate were enteric pathogens that have public health importance. Our findings of contaminated herbal product in this study agrees with Onyambu [16] who observed in their study that although regulated herbal drug was contaminated but contamination was not beyond WHO limits. Our findings however disagree with a study carried out in Ethiopia by Yesuf [13] whose bacteria count values were all above WHO limit. Yesuf [13] explained that the high coliform count in their study may be due to unhygienic practices and the fact that most of the herbalist work within their living house which tend to increase human drug contact. On the other hand, the tolerable limit observe in our study may be attributed to the fact that most of the customers that patronize Oja Bisi market are the educated population of the international accredited University that is in the community hence it would have impacted on the awareness of best hygienic practices to the sellers.
However, samples collected from Oja-Bisi market showed a total number of 9 pathogenic microorganisms isolated with sample G having the highest number of pathogenic microorganisms (36%). The high number of pathogens in sample G may be connected to the fact that sample G was the only powdered sample that required dissolving into water, and this may likely be contaminated from grinding processes. The present study shows bacteria isolates like Staphylococcus aureus, Staphylococcus saprophyticus, Staphylococcus hemolyticus, Klebsiella pneumoniae, Klebsiella ozaenae, Klebsiella oxytoca, Pseudomonas aeruginosa, Enterobacter cloacae and Enterobacter aerogenes. The finding of the fecal indicator organisms like Enterobacter, pseudomonas and Klebsiella reflects poor hygienic practices while the finding of the organisms of contamination by contact such as Staphylococcus species which are normal flora of the skin and external surfaces would have arisen from poor handling techniques. This agreed with similar studies conducted in Nigeria and Kaduna Metropolis by Esimone [17] and by Abba [6] who explained that the presence of fecal indicator organisms in herbs was due to unsafe collection, transportation, drying, preparing, storing and dispensing processes. The only slight difference is that E. coli which was one of their major fecal indicator organisms was not isolated in this study.
The organisms isolated in this study were most susceptible to ciprofloxacin (CIP) and ceftriaxone (CRO) also known as Rocephin which are fourth generation antibiotic, very expensive and not readily available hence may be beyond the reach of the populace in the area of study, explaining why they are not as abused. On the other hand, the most resistance antibiotics are ampicillin (AM) and oxacillin (OX), which are household antibiotics and usually over prescribed. These findings are also in agreement with Yesuf [13]. Furthermore, multiple drug resistance was common in this present study. It was found that isolates were resistance to two or more antibiotics tested. This was also similar to a study done by Adeleye [18] who reported that most of the isolates were resistant to Ampicillin, Penicillin, Cotrimoxazole, and Gentamicin. In all antibiotic susceptibility studies on the bacteria contamination of the liquid herbal products, the isolates were susceptible to some of the antibiotics tested and resistant to others.
The study showed that herbal medicine sold in Oja Bisi market were mildly contaminated with bacteria count within WHO limit. (CFU/g ≤ 105). Bacteria of health importance were isolated and most of them were resistant to first- and second-generation antibiotics with multiple drug resistant as isolates were resistant to 2 or more antibiotics tested. The contaminated herbal products may facilitate transmission of communicable diseases in the population and therefore present a public health problem especially with the fact that most of the herbal medicine analyzed in this study may be consumed at room temperature and do not get heated to above 60℃ before consumption. This will call for regulation of herbal products to ensure their processing, preparation and transportation complies with good manufacturing practices, reducing the risk to consumers and patients. Also, good advocacy and awareness should be given to the herbal products manufacturers and sellers in form of training to encourage them to abide by the regulation.
CONFLICT OF INTEREST
The Authors hereby declare there is no conflict of interest associated with this study or any of the procedures and materials used for the purpose of this study.
- Olisa NS, Oyelola FT (2009) Evaluation of Use of Herbal Medicines Among Ambulatory Hypertensive Patients Attending a Secondary Health Care Facility in Nigeria. Int J Pharm Pract 17: 101-105.
- Oreagba IA, Oshikoya KA, Amachree M (2011) Herbal Medicine Use Among Urban Residents in Lagos, Nigeria. BMC Complement Altern Med 11: 117.
- Qi Z (2013) WHO Traditional Medicine Strategy 2014-2023. Geneva: World Health Organization. Available online at: https://apps.who.int/iris/bitstream/handle/10665/92455/9789241506090_eng.pdf?sequence=1
- World Health Organization (2007) Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues, WHO Press, Geneva, Switzerland. Available online at: https://apps.who.int/iris/bitstream/handle/10665/43510/9789241594448_eng.pdf?sequence=1&isAllowed=y
- Kosalec I, Cvek J, Tomić S (2009) Contaminants of Medicinal Herbs and Herbal Products. Arh Hig Rada Toksikol 60: 485-501.
- Abba D, Inabo HI Yakubu SE, Olonitola SO (2009) Contamination of Herbal Medicinal Products Marketed in Kaduna Metropolis with Selected Pathogenic Bacteria. Afr J Tradit Complement Altern Med 6(1): 70-77.
- Khattak KF (2012) Microbiological Quality Assessment of Commercially Available Medicinal Plants in Peshawar City, Pakistan. Pak J Bot 44: 1203-1208.
- Oluyege JO, Adelabu DM (2010) Microbial Contamination of Some Hawked Herbal Products in Ado-Ekiti, Nigeria. Cont J Microbiol 4: 8-14.
- Vadhana P, Singh BR, Bharadwaj M, Singh SV (2015) Emergence of Herbal Antimicrobial Drug Resistance in Clinical bacterial isolates. Pharm Anal Acta 6: 434.
- Falodun A, Imieje V (2013) Herbal medicine in Nigeria: Holistic Overview. Niger J Sci Environ 12: 1-13.
- World Health Organization (2002a) WHO Traditional Medicine Strategy 2002 - 2005. Geneva. Available online at: https://apps.who.int/iris/bitstream/handle/10665/67163/WHO_EDM_TRM_2002.1_eng.pdf?sequence=1&isAllowed=y
- World Health Organization (2002b) Traditional Medicine- Growing Needs and Potential. Geneva. Available online at: https://apps.who.int/iris/bitstream/handle/10665/67294/WHO_EDM_2002.4_eng.pdf?sequence=1&isAllowed=y
- Yesuf A, Wondimeneh Y, Gebrecherkos T, Moges F (2016) Occurrence of Potential Bacterial Pathogens and Their Antimicrobial Susceptibility Patterns Isolated from Herbal Medicinal Products Sold in Different Markets of Gondar Town, Northwest Ethiopia. Int J Bacteriol 8: 4-10.
- Olusegun O (2013) Urban Expansion and Urban Land Use in Ado- Ekiti, Nigeria. Am J Res Commun 1: 128- 139.
- World Population Review (2018) Population of Cities in Nigeria. Accessed on: June 6, 2019. Available online at: http://worldpopulationreview.com/countries/nigeria-population/cities/
- Onyambu MO, Chepkwony HK, Thoithi GN, Ouya GO, Osanjo GO (2013) Microbial Quality of Unregulated Herbal Medicinal Products in Kenya. Afr J Pharmacol Ther 2: 70-77.
- Esimone CO, Oleghe PO, Ibezim EC, Okeh CO, Iroha IR (2007) Susceptibility-Resistance Profile of Microorganisms Isolated from Herbal Medicine Products Sold in Nigeria. Afr J Biotechnol 6: 24-25.
- Adeleye IA, Okogi G, Ojo EO (2005) Microbial Contamination of Herbal Preparations in Lagos, Nigeria. J Health Popul Nutr 23: 296-297.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Rheumatology Research (ISSN:2641-6999)
- Journal of Pathology and Toxicology Research
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)




