3877
Views & Citations2877
Likes & Shares
Extrahepatic bile duct cancer represents one of the highly aggressive malignancy because of its frequent metastasis and recurrence. Lymphatic invasion/lymph node metastasis is thought to be one of the prognostic factors. We morphologically analyzed lymphatic invasion of the TFK-1 extrahepatic bile duct cancer cells, using 3D human stromal tissues with lymphatic vessels. The TFK-1 cells showed epithelial mesenchymal transition (EMT) at the start of stromal invasion. Light microscopy and transmission electron microscopy demonstrated that TFK-1 cells became spindle in shape, i.e., EMT at the intravasation and extravasation. The 3D human stromal tissues with lymphatic vessels are thought to become a useful model analyzing the lymphatic invasion mechanisms of extrahepatic bile duct cancer.
Keywords: Epithelial mesenchymal transition, Extrahepatic bile duct cancer, Transmission electron microscopy, Histopathology
INTRODUCTION
Biliary tract cancer, i.e., cancer of extrahepatic bile duct/gallbladder, is the most common malignancy of the biliary tract, and is recognized as one of the high-grade malignancies because of its frequent metastasis and recurrence [1-3]. Most patients with biliary tract cancer are usually treated at an advanced stage, and the prognosis remains poor despite the development of recent diagnostic methods. Biliary tract cancer frequently shows the lymphatic invasion leading to lymph node metastasis, but its morphological analyses have not yet been examined.
Here, we focus on the extrahepatic bile duct cancer, and describe morphological characteristics of the lymphatic invasion using 3D vascularized human stromal tissues.
MATERIALS AND METHODS
Cells
TFK-1 is a human cell line derived from extrahepatic bile duct carcinoma, obtained from the RIKEN BRC through the National Bio-Resource Project of MEXT, Japan [4]. The TFK-1 cells cultured in RPMI 1640 medium with 10% FBS and 1% penicillin-streptomycin.
3D human stromal tissue models with lymphatic vessels 1x106 of normal human dermal fibroblasts (NHDF) coated with fibronectin-gelatin (FN-G) nanofilms were seeded onto each 24-well trans-well insert and incubated for 24 h to form 4-layered NHDF tissues [5,6]. Then, one layered of FN-G nanofilms coated lymphatic endothelial cells (LECs) were then seeded to obtain 4-layered/one-layered tissues. Additionally, the same amount of NHDFs were accumulated on to form a sandwich. After 1-2 days, the 3D lymph-capillary models were constructed. To mimic the tumor invasion models, the TFK-1 cells were seeded on the tissue and cultured for 24, 48, 96, and 144 h.
Histopathology
The 3D tissues were routinely fixed with 10% solution of buffered formalin and embedded in paraffin. Four-μm-thick sections of the 3D tissues were stained with hematoxylin- eosin (H&E) (Figure 1A). Immunostaining of D2-40 were performed using Ventana Bench Mark GX slide staining system (Roche Diagnostics K.K., Tokyo, Japan). Lymphatic vessel formation in the tissue was confirmed with D2-40 immunostaining (Figure 1B).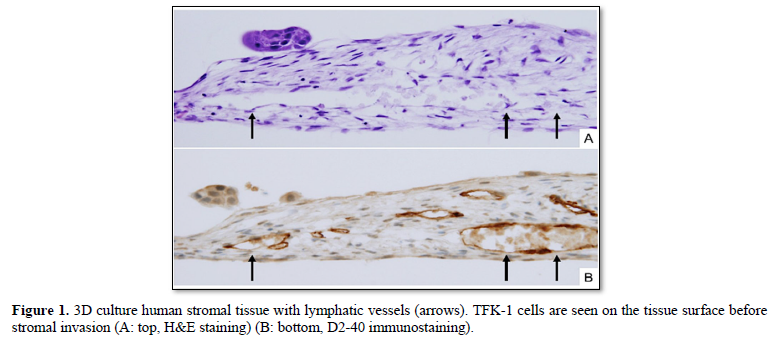
Transmission electron microscopy: The tissues were fixed with 2% paraformaldehyde, 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) [6]. The tissues were cut into one-millimeter pieces and post-fixed in 1% osmium tetroxide in a 0.1 M phosphate buffer. The tissues were then dehydrated and embedded in Epon 812 (Nisshin EM, Tokyo, Japan), followed by section preparation using an ultramicrotome (REICHERT ULTRACUT S, Leica, Wetzlar, Germany). Sections with a thickness of 70 nm were then stained with a 4% uranyl acetate and lead staining solution (Sigma Aldrich, St. Louis, MO) using conventional methods and observed using a transmission electron microscope (JEM-1230, JEOL, Tokyo, Japan).
RESULTS
Early invasion of TFK-1 cells
TFK-1 cells with many microvilli showed stromal invasion on the 3D stromal tissue at 24 h after seeding (Figure 2). Some of the TFK-1 cells attached to the fibroblasts, and changed morphologically. The TFK-1 cell became spindle in shape, and inserted its cellular part into the gap of fibroblasts, i.e., epithelial mesenchymal transition (EMT) at the start of stromal invasion.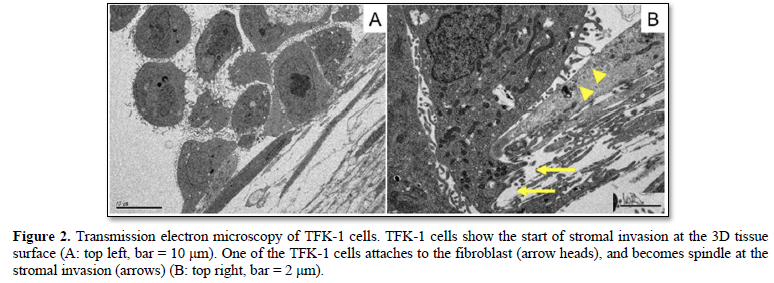
Lymphatic invasion of TFK-1 cells
TFK-1 cells frequently showed lymphatic invasion at 48, 96, 144 h after seeding. TFK-1 cells morphologically became spindle in shape, i.e., EMT when they entered the lymphatic vessels (intravasation). Then, the TFK-1 cells formed spherical nests in the lymphatic vessels (Figures 3A & 3B). The TFK-1 reached the other side of lympho-vascular wall (extravasation), and also showed EMT morphologically at 96 and 144 h after seeding (Figure 3C). Some of the spherical nests in the lymphatic vessels became large in size, mimicking a tumor thrombus (Figure 3D). Transmission electron microscopy of lymphatic invasion showed TFK-1 cells attaching the endothelial cells, and becoming spindle in shape, i.e., EMT morphologically (Figure 4).
Transmission electron microscopy of lymphatic invasion showed TFK-1 cells attaching the endothelial cells, and becoming spindle in shape, i.e., EMT morphologically (Figure 4).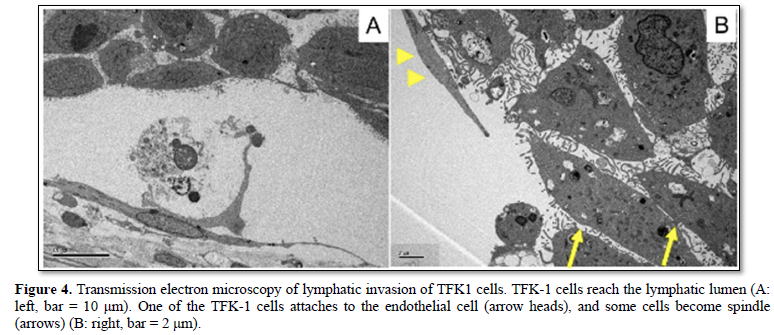
DISCUSSION
We describe histological and electron-microscopical characteristics of lymphatic invasion of the bile duct cancer using 3D human stromal tissue model. TFK-1 human bile duct cancer cells morphologically became spindle in shape, i.e., EMT when they entered the lymphatic vessels. The TFK-1 cells formed spherical nests in the lymphatic vessels.
The most cases of extrahepatic bile duct cancer are epithelial tumors that originate from the bile ducts, and represent one of the highly aggressive malignancies [7-10]. In a recent study, the median disease-specific survival rate after surgery in patients with lymph node metastasis was lower than that of patients without lymph node metastasis [11]. It is an important issue to analyze the mechanisms of the lymphatic invasion leading to lymph node metastasis. However, molecular/histological mechanisms of lymphatic invasion have not yet clarified, while the metastatic cascade via blood vessel have been demonstrated recently.
Malignancy in cancers is characterized by invasion and metastasis that are closely associated with interaction between cancer cells and non-cancerous stroma [12,13]. Recent studies have demonstrated invasion-metastasis cascade such as invasion of basement membrane, passage through extracellular matrix, intravasation, vascular dissemination, extravasation, and formation of metastatic foci [14,15]. However, these sequential steps of the cascade are associated with blood vessels, but not lymphatic vessels. Mechanisms of lymphatic spread have not yet understood. Our study is the first to demonstrate histological/electron-microscopical findings of lymphatic invasion using human extrahepatic bile duct cancer cells.
EMT is an important process that occurs during the cancer progression. It is characterized by the loss of epithelial factors, including E-cadherin, claudins, and cytokeratin’s, and the upregulated expression of mesenchymal markers, including N-cadherin, vimentin and fibronectin. Cancer cells that have undergone EMT are thought to acquire a fibroblast-like motile and an invasive phenotype [16-19]. TFK-1 human bile duct cancer cells morphologically showed EMT at the start of stromal invasion. Moreover, TFK-1 cells significantly exhibited EMT at the lymphatic intravasation/extravasation, and formed spherical nests in the lymphatic vessels. These processes are extremely consistent with the invasion-metastasis cascade of blood vessel.
In conclusion, we demonstrate morphological characteristics of lymphatic invasion of the extrahepatic bile duct cancer, using 3D human stromal tissues with lymphatic vessels. The 3D tissues with lymphatic vessels are thought to become a useful model analyzing the lymphatic invasion mechanisms.
ACKNOWLEDGEMENTS
This study was supported by JSPS KAKENHI, Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
-
Kijima H, Wu Y, Yoshizawa T, Suzuki T, Tsugeno Y, et al. (2014) Pathological characteristics of early to advanced gallbladder carcinoma and extrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci 21: 453-458.
-
Albores-Saavedra J, Henson DE, Klimstra DS (2015) Tumors of the gallbladder, extrahepatic bile ducts and ampulla of Vater. Atlas of tumor pathology. 4th series, fascicle 23. Armed Forces Institute of Pathology (AFIP), Washington DC. pp: 303-374.
-
WHO classification of tumors editorial board (2019) Digestive system tumor. WHO classification of tumors, 5th ed. Lyon: IARC Press. pp: 289-291.
-
Saijyo S, Kudo T, Suzuki M, Katayose Y, Shinoda M, et al. (1995) Establishment of a new extrahepatic bile duct carcinoma cell line, TFK-1. Tohoku J Exp Med 177: 61-71.
-
Nishiguchi A, Matsusaki M, Kano MR, Nishihara H, Okano D, et al. (2018) In vitro 3D blood/lymph-vascularized human stromal tissues for preclinical assays of cancer metastasis. Biomaterials. 179: 144-155.
-
Oikiri H, Asano Y, Matsusaki M, Akashi M, Shimoda H, et al. (2019) Inhibitory effect of carbonyl reductase 1 against peritoneal progression of ovarian cancer: evaluation by ex vivo 3D-human peritoneal model. Mol Biol Rep. 46: 4685-4697.
-
Yoshizawa T, Ishido K, Saito K, Haga T, Seino H, et al. (2017) Prognostic impact of extracapsular lymph node invasion and myofibroblastic activity in extrahepatic bile duct cancer. Clin Med Insights Pathol 10: 1179555717729652.
-
Yoshizawa T, Toyoki Y, Hirai H, Haga T, Toba T, et al. (2014) Invasive micropapillary carcinoma of the extrahepatic bile duct and its malignant potential. Oncol Rep 32: 1355-1361.
-
Ohashi M, Kusumi T, Sato F, Kudo Y, Jin H, et al. (2009) Expression of syndecan-1 and E-cadherin is inversely correlated with poor patient's prognosis and recurrent status of extrahepatic bile duct carcinoma. Biomed Res 30: 79-86.
-
Okano K, Yoshizawa T, Miura T, Ishido K, Kudo D, et al. (2018) Impact of the histological phenotype of extrahepatic bile duct carcinoma. Mol Clin Oncol 8: 54-60.
-
Ito K, Ito H, Allen PJ, Gonen M, Klimstra D, et al. (2010) Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg 251: 675-681.
-
Miura T, Yoshizawa T, Hirai H, Seino H, Morohashi S, et al. (2017) Prognostic Impact of CD163+ Macrophages in Tumor Stroma and CD8+ T-Cells in Cancer Cell Nests in Invasive Extrahepatic Bile Duct Cancer. Anticancer Res 37: 183-190.
-
Goto S, Ishido K, Yoshizawa T, Haga T, Morohashi S, et al. (2019) Histopathological Characteristics of Pancreatic Cancer Stroma Induced by Neoadjuvant Chemotherapy. Bomed Res J 3: 61-64.
-
Weinberg RA (2013) The Biology of Cancer, 2nd ed. New York, NY: Garland Science. pp: 643-651.
-
Kumar V, Abbas AK, Aster JC (2015) Robbins and Cotran Pathologic Basis of Disease, 9th ed. Philadelphia, PA: Elsevier Saunders. pp: 306-310.
-
Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 7: 131-142.
-
Jing Yang J, Weinberg RA (2008) Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 14: 818-829.
-
Wu Y, Sato F, Yamada T, Bhawal UK, Kawamoto T, et al. (2012) The BHLH transcription factor DEC1 plays an important role in the epithelial-mesenchymal transition of pancreatic cancer. Int J Oncol 41: 1337-1346.
-
Tsugeno Y, Yoshizawa T, Wu Y, Goto S, Haga T, et al. (2019) Type I Collagen Induces DEC Expression and Epithelial-Mesenchymal Transition (EMT) in Human Breast Cancer MCF-7 Cells. BioMed Res J 3: 125-131.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)






