Research Article
Prevalence and Associated Factors of Anemia among Lactating Women in Ethiopia from 2010 to 2020: A Systematic Review and Meta-Analysis
5871
Views & Citations4871
Likes & Shares
Background: Anemia is one of the major nutritional public health problems in developing countries. This systematic review
and meta-analysis aimed to indicate the magnitude and determinants of anemia among lactating women in Ethiopia.
Objective: The study aimed to synthesize the existing literature on the prevalence and determinants of anemia among lactating women in Ethiopia, 2010 to 2020.
Methods: Studies searched through the search engine of Google Scholar, Hinari, MEDLINE/ PubMed, Cochrane Library, and Africa Wide Information. Searching was made using Keywords/ MeSH terms of anemia, low hemoglobin, postpartum, lactating women and Ethiopia. Data were analyzed and compared with the WHO criteria to declare a public health problem. Heterogeneity was assessed using Cochran Q test and I2 statistics. A random-effects model with a 95% confidence interval was used for prevalence and odds ratio estimations.
Results: Eleven studies were included in this systematic review and meta-analysis. The overall prevalence of anemia among lactating women in Ethiopia was 28.9% (95% CI: 22.7%-35.1%). It was a severe public health problem in the Somali (58.7%) and the Afar (44.9%) regions, a mild problem in Addis Ababa (11.6%) and a moderate health problem in there maining 8 regions ranged from 20.9% in Tigray to 35.9% in Dire Dawa. Lactating women who lacked formal education (OR=1.7; 95%CI: 1.1, 2.5), rural residents (OR=1.7; 95%CI: 1.4, 1.9), higher parity (OR=1.3; 95% CI: 1.1, 1.4), lower antenatal care visits (OR=1.6; 95%CI: 1.2, 2.3), lacked family planning utilization (OR=1.5;95%CI: 1.4,1.7), underweight (OR=1.3;95% CI: 1.1, 1.6), lower dietary diversity (OR=1.9; 95% CI: 1.5, 2.5), food insecurity (OR= 3.0; 95% CI: 1.5, 6.2) and malaria infection (OR=3.7; 95%CI: 2.8,4.9) were factors associated with higher odds of developing anemia. Moreover, taking iron supplementation (OR=0.55; 95% CI: 0.49, 0.63), being employed women (OR= 0.73; 95% CI: 0.66, 0.80) and rich wealth quintile (OR= 0.79; 95% CI: 0.68, 0.91) were significantly associated with lower risk of anemia.
Conclusions: Anemia among lactating women is still a moderate public health problem in Ethiopia and a severe health problem in Somali and Afar regions. Hence; the government needs to develop integrated multi-sectoral policies and strategies to address the significant variations a cross regions attributed to various interrelated factors like poor socio-economic status, lower maternal health services utilization, inadequate dietary diversity, and food insecurity in order to reduce anemia among lactating women in Ethiopia.
Keywords: Anemia, Hemoglobin, Lactating women, Ethiopia
BACKGROUND
Anemia is one of the common public health problems that have still remained affecting populations across the world [1,2]. Children, pregnant women, and lactating mothers are vulnerable population groups highly affected by anemia in the world [3]. A systematic analysis of 257 population- representative data from 1995 to 2011 showed that anemia prevalence decreased from 33% to 29 % in non-pregnant women, from 43% to 38% in pregnant women, and from 47% to 43% in children [3]. However, the prevalence of anemia varied a cross regions and countries [3-5]. The highest rates of anemia among reproductive-age women were observed in Sub-Saharan Africa (48%) and South Asia (47%), while the lowest rate was found in high-income regions (16%) [3]. Moreover, according to a 2018 global nutrition report, anemia among childbearing women was slightly increased to 33% since 2012[6].
Anemia among lactating women is also a common nutritional problem throughout the world, especially in developing countries [7, 8]. The prevalence of anemia,
particularly two days after delivery was about 50% in developed countries whereas, in developing countries, it ranged from 50% to 80% [9]. Studies have identified that presence of multiple interlinked factors like poor quality of health services, inadequate micronutrient intake, low iron supplementation, higher infectious diseases, nutritional deficiencies, poverty, and ineffective implementation of interventions in developing countries attributed to the presence of higher rates of anemia in these countries compared with developed countries [10-13].
Ethiopia is one of the countries with the highest micronutrient deficiencies and related morbidity and mortality [14, 15]. The prevalence of dietary iron deficiency in Ethiopia declined from 1990 to 2010 and then increased in 2017 [15]. A recent systemic review showed that about 1/3 of pregnant women were anemic (32%) in Ethiopia [14]. Based on the Ethiopian health and demographic surveys (EDHS) reports, the prevalence of anemia among lactating women in the country increased from 18.5% in 2011 [29] to 28.6% in 2016 [16].
Food fortification, food diversification, iron supplementation, nutrition education, and quality maternal health service provision are the main effective interventions recommended to prevent micronutrient deficiencies and associated mortality among pregnant and lactating women in low- and middle-income countries [17, 18]. However, in Ethiopia, the nutrition programs are mainly facility-based interventions like nutrition counseling and iron supplementation and it did not address the community-based approaches [19, 20]. Studies also revealed that interventions that depend on strong health systems or behavioral changes seem to be stuck that require-evaluation to find more effective approaches to reaching the majority of women and children [21].
In Ethiopia, a number of studies are conducted to assess the prevalence and determinants of anemia during the postpartum period [22, 23]. These separated studies reported the prevalence of anemia among lactating women in Ethiopian ranged from 7.3% in Addis Ababa [22] to 68.3% in the Somali region [23]. There were a considerable variation and uncertainty related to the prevalence of anemia and its associated factors among lactating women across the nation from the findings of these studies. Therefore, this study was conducted to determine the overall prevalence and associated factors of anemia among lactating women in Ethiopia in order to provide evidence for policymakers and stakeholders to design and implement evidence-based interventions to combat anemia morbidity and associated mortality in lactating women in Ethiopia.
METHODS
Study design and search strategy
A systematic review and meta-analysis of published and unpublished studies were used to determine the prevalence of anemia and its determinant factors among lactating women in Ethiopia. Searching was made from the first January to the end of March 2020. A review of all published studies was done in the following major databases; Google Scholar, Hinari, MEDLINE/PubMed, Cochrane Library, Africa Wide Information, African Index Medicus, and other sources like contacting experts and researchers to retrieve new articles and by manually searching to identify unpublished studies. Searching was made using Keywords/ MeSH terms of “Anemia”, “low hemoglobin”, “Postpartum”, “Lactating women”, “breastfeeding” “women”, and “Ethiopia”. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was strictly followed when conducting this review.
Study selection and eligibility criteria
This review included studies that were conducted in Ethiopia with cross-section a land cohort study designs were considered eligible for this systematic and meta-analysis. Studies that were excluded if they were not primary studies which included review articles, conference abstracts, editorials. Other eligibility criteria were studies that measured the prevalence of anemia among lactating women from 2010 to 2020. Selections of the studies were conducted based on the Preferred. Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guideline [24]. The studies were screened using eligibility criteria by two authors independently and cross-checked for consistency. Moreover, discrepancies between authors were resolved through discussion and consensus. This systematic review and meta-analysis were not registered with PROSPERO.
Data extraction process
An agreed abstraction format was developed to extract data from the selected studies. The data extraction format has the following structure: author details (name & year of publication), study year, study setting, study design, sample size, study population, sampling procedures, data collection procedures, and findings. The data were extracted independently by two authors and checked for consistency. In case of inconsistency, the articles were reviewed again and disagreements were resolved by verification and further discussion.
Outcome of interest
The outcome variable for this systematic and meta-analysis was anemia among lactating women. The World Health Organization (WHO) defines anemia as, a hemoglobin concentration below 12.0 g/dl was considered as anemic; with its severity of mild, moderate, and severe anemia determined using cutoff points of 10.0-11.9 g/dl, 7.0-9.9 g/dl, and
Risk of bias and quality of evidence assessments
Risk of bias assessment of the studies was carried out by two authors independently using Hoy 2012 tool with ten criteria; Representation of the population, sampling frame, methods of participants’ selected, non-response bias, data collection directly from subjects, acceptability of case definition, reliability and validity of study tools, mode of data collection, length of prevalence period and appropriateness of numerator and denominator. Four items assess selection and non- response bias, five items evaluate measurement bias and one item assesses bias related to analysis and results reporting. Each item was assessed as either low or high risk of bias and the overall risk of bias was determined based on the score of high-risk items to bias per study: low (≤2), moderate (3–4), and high (≥ 5) [26]. An assessment of the degree of certainty of the evidence for the outcome was performed using the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) tool. With the GRADE quality evaluation tool, observational studies start from low quality of evidence, but downgraded to a very low quality based on the five factors; risk of bias, inconsistency, indirectness, imprecision, and publication bias and could be upgraded provided no other limitations have been found based on these five factors. Evaluations were performed for the five main domains (risk of bias, consistency, directness, precision and publication bias), as well as for the overall quality of the evidence. Based on the GRADE recommendation, the study design was used as a starting point, and for each domain that was not met one step was downgraded. [27].
Statistical analysis and synthesis
Extracted data were entered and analyzed using Review Manager (Rev Man) version 5.3 statistical software. The variance of postnatal anemia prevalence for each article was calculated based on the binomial distribution formula by extracting the frequency of outcome and sample size [28]. The pooled odds ratios (OR) with a 95% CI were calculated using the random effect model to determine factors associated with anemia among lactating women in Ethiopia Heterogeneity among studies was assessed using a Cochran Q test (P-value2statistics (at least 50% considered for significant) [29].
A random-effects model with a 95% confidence interval (CI) was used for estimation because significant variations were shown between the study findings. The random-effect model is more conservative than the fixed-effect model and helps to account heterogeneity inherent in meta- analysis. Subgroup analysis was performed based on the region, quality of studies, survey year, and study setting. Funnel plot analysis, Egger weighted regression, and Begg rank correlation tests were used to detect publication bias, and P-value
RESULTS
Characteristics of the studies
A total of 17,431 published and 16 unpublished studies were retrieved through searching from different databases. Out of 17, 447 studies, 6, 119 studies were excluded due to duplication, and 11,302 studies were excluded after reading the title and abstract using inclusion and exclusion criteria since they did not relate to the aim of this study. The remaining 26 full- articles were assessed for eligibility. Finally, 11 studies were included in the systematic review and meta- analysis (Figure 1).
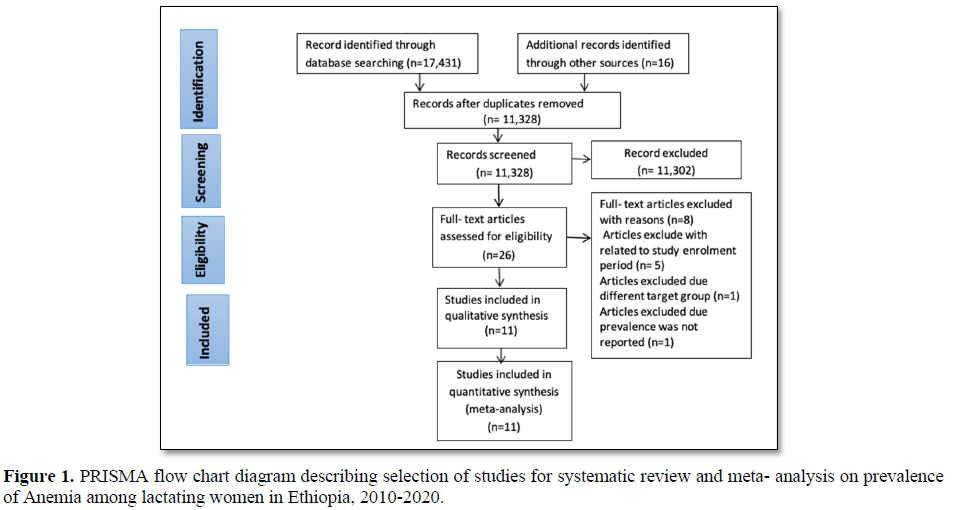

Among the eleven studies included in a systematic review and meta-analysis, 10 studies were cross-sectional studies and one study was a longitudinal study conducted during pre-harvest and post-harvest seasons [30]. Out of 11 articles, 9 were conducted in the community setting [22,23,30-36] and the remaining 2 articles were done in a health facility setting [37,38]. Study population varied from 162 [34] to 5,047 lactating women [22]. About 13,495 lactating women were included in the analysis. The earliest study was conducted in 2010 [22], and the recent study conducted in 2017 [38] (Table 1).

Risk of bias and heterogeneity
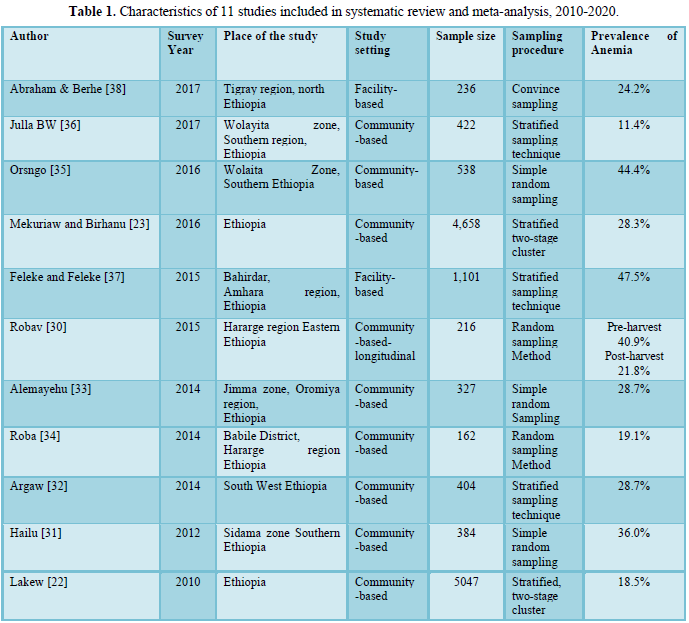
The risk of bias assessment of the eleven individual articles included in the systematic review and meta-analysis was carried out using Hoy 2012 tool with ten criteria described in the method section [26]. Out of 11 included studies eight studies (72.3%) had a low risk of bias [22,23,31-33,35-37] and three studies (27.7%) had a moderate risk of bias [30,34,38]. The main cause of study bias was selection bias. A high risk of selection bias was observed in five studies. Bias related to analysis was also observed in four studies. Measurement bias was observed in two studies [32,38] (Table S1).
Table S1 showed risk of bias assessment of 11 included studies using the Hoy [26] tool with ten criteria’s.
Table S1 showed risk of bias assessment of 11 included studies using the Hoy [26] tool with ten criteria’s.
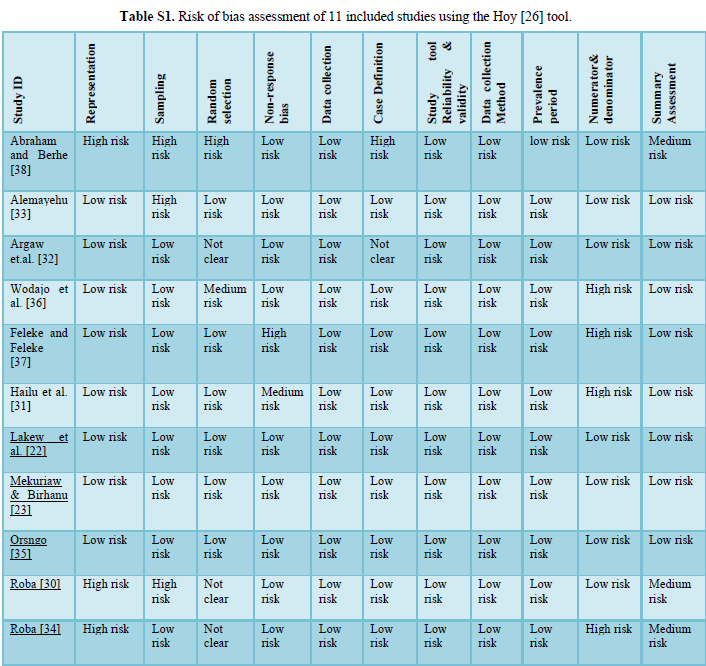
Risk of bias assessment Hoy [26] tool: Yes (low risk); No (high risk)
- Representation: Was the study population a close representation of the national population?
- Sampling: Was the sampling frame a true or close representation of the target population?
- Random selection: Was some form of random selection used to select the sample OR was a census undertaken?
- Non-response bias: Was the likelihood of non-response bias minimal?
- Data collection: Were data collected directly from the subjects?
- Case definition: Was an acceptable case definition used in the study?
- Reliability and validity of study tool: Was the study instrument that measured the parameter of interest show to have reliability and validity?
- Data collection: Was the same mode of data collection used for all subjects?
- Prevalence period: Was the length of the prevalence period for the parameter of interest appropriate?
10. Numerators and denominators: Were the numerator(s)
and denominator(s) for the parameter of interest appropriate?
The overall risk of bias was then scored according to the number of high risks of bias per study: Low (≤2), moderate (3-4) and high (≥5).
Out of 11 included studies eight studies (72.3%) had low risk of bias, and three studies (27.7%) had a moderate risk of bias.
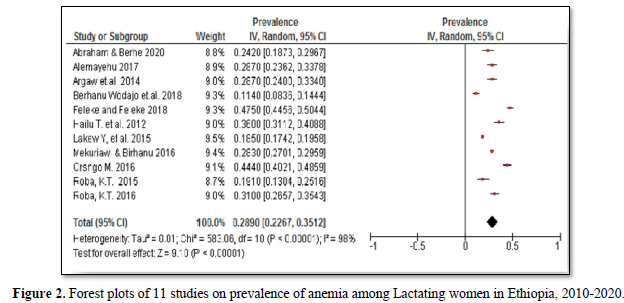
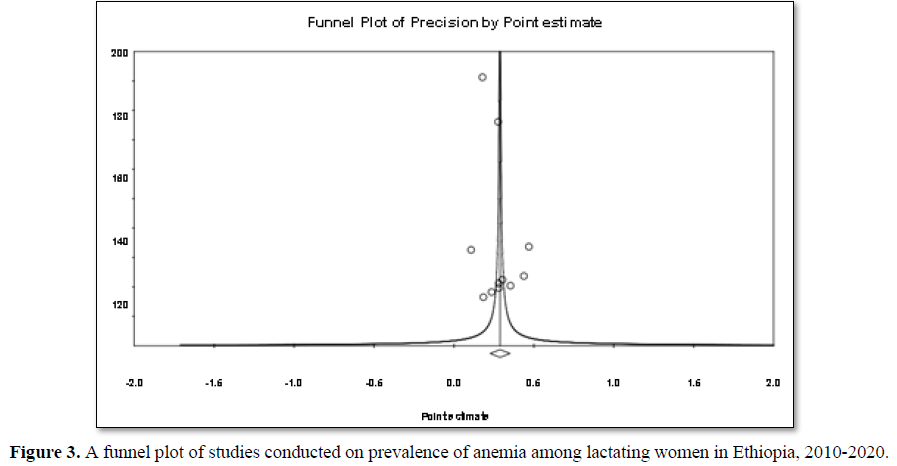
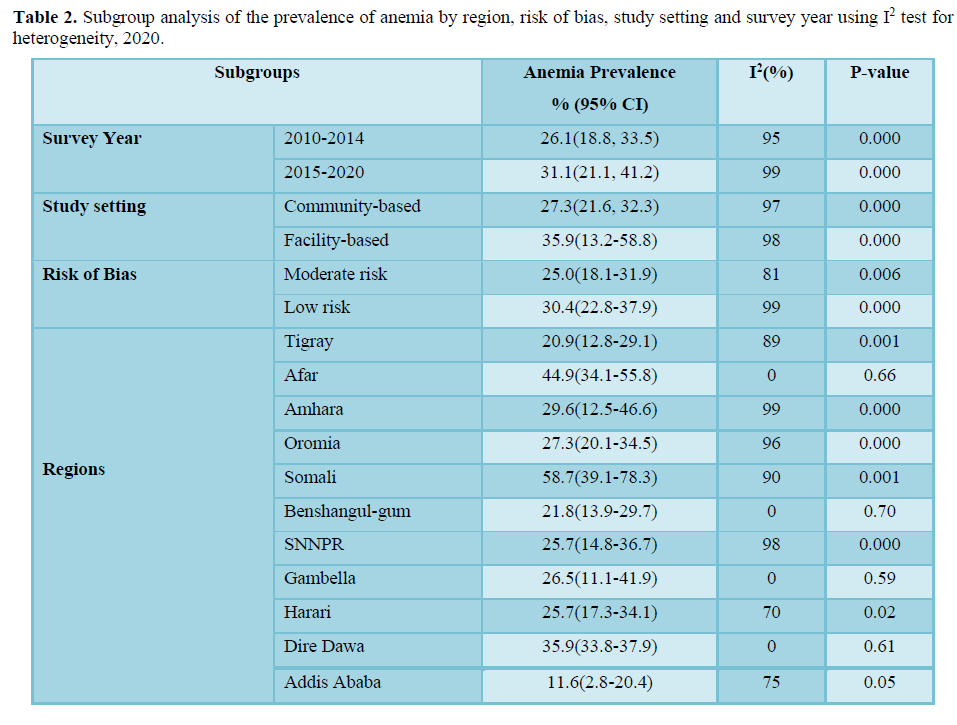
The 11 studies included assessing prevalence of anemia in lactating women exhibited high heterogeneity (Cochrane Q test p= 0.00001, I2 test (98%) which is indicative to use the random-effects model (Figure 2). Nevertheless, the Egger weighted regression statistics of studies conducted on the prevalence of anemia (P= 0.2) and Begg rank correlation statistics (p = 0.3) indicated no evidence of publication bias. There was no sign of publication bias and symmetry in the funnel plot (Figure 3). To decrease the heterogeneity, subgroup analysis was performed based on the region, quality of studies, survey year and study setting. However, the heterogeneity in all subgroups was considerable (Table 2). Based on the GRADE evidence tool assessment the overall quality of evidence for the prevalence of anemia among lactating women was found to be very low due to the risk of bias and inconsistency (Table S2).
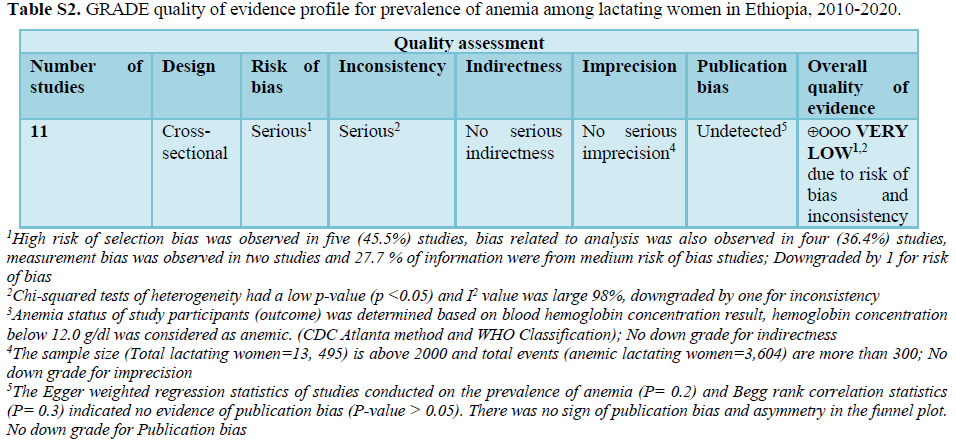




An evaluation of the degree of certainty of the evidence for the outcome (prevalence of anemia) was performed using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) tool. In GRADE approach observational studies start from low quality evidence but downgraded to very low based on the five factors; risk of bias, inconsistency, indirectness, imprecision and publication bias. Evidence from observational studies can be upgraded provided no other limitations have been identified based on the five factors. Assessments were made for the five main domains (risk of bias, consistency, directness, precision and publication bias), as well as overall quality of evidence. We used study design as our starting point and downgraded by one step for each domain that was not met; based on the GRADE recommendation.
Risk of bias: Is limitations in the study design and implementation may bias the estimates of an intervention effect. Most information is from studies at low risk of bias: No serious limitations, No downgrade. The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results: Serious limitations, downgraded one level. High risk of selection bias was observed in five studies. Bias related to analysis was also observed in four studies, Measurement bias was observed in two studies and 27.7 % of information were from medium risk of bias; Downgraded by 1 for risk of bias.
Inconsistency: Refers to an unexplained heterogeneity of results. Criteria to determine inconsistency can be applied when results are from more than one study and include: statistical criteria including tests of heterogeneity (e.g. chi-squared or Chi2) have a low p-value (p <0.05), I2 value is large (> 25%). The outcome was downgraded by one due to inconsistency (tests of heterogeneity p 2 is large).
Indirectness: The question being addressed by the authors of a systematic review is different from the available evidence regarding the population, intervention, comparator, or an outcome. The quality of the evidence may be downgraded when substitute measurements or surrogate endpoints are measured instead of patient-important outcomes. Anemia status of study participants (outcome) was determined based on blood hemoglobin concentration result, hemoglobin concentration below 12.0 g/dl was considered as anemic. (CDC Atlanta method and WHO Classification); No down grade for indirectness.
Imprecision: Results are imprecise when studies include relatively few patients and few events and thus have wide confidence intervals around the estimate of the effect. For dichotomous outcomes you should consider downgrading the quality of evidence because of imprecision for either of the following two reasons: The total sample size is lower than 2000 and/or total number of events is less than 300 (a threshold rule-of-thumb value). Since the sample size (Total lactating women=13,495) is above 2000 and total events (anemic lactating women=3,604) are more than 300; No down grade for imprecision.
Publication bias: Is a systematic under-estimate or an over-estimate of the underlying beneficial or harmful effect due to the selective publication of studies. Funnel plot analysis, Egger weighted regression and Begg rank correlation tests were used to detect publication bias and P-value0.05). There was no sign of publication bias and asymmetry in the funnel plot. No down grade for publication bias (Table 3).

Prevalence of anemia among lactating women in Ethiopia
A total of 11 studies were included in this systematic review and Meta-analysis to reveal the prevalence of anemia in lactating women in Ethiopia from 2010 to 2020. A study done in 2010 reported an 18.5% prevalence of anemia among lactating women in Ethiopia. The prevalence was lowest in Addis Ababa (7.3%) and higher (48.3%) in the Somali region [22]. Whereas in a study conducted in 2012 the prevalence of anemia in lactating women was 26.0% in southern Ethiopia [31]. Based on the three studies conducted in 2014, the prevalence of anemia was found to be 19.1% in eastern Ethiopia [34], and 28.7% in southwest Ethiopia [32,33]. In 2015, a longitudinal community-based study reported anemia prevalence of 21.8% in post-harvest and 40.9% in pre- harvest seasons in the Hararge region, Ethiopia [30]. Another study conducted in the Amhara region, documented relatively high prevalence (47.5%) of anemia in lactating women in 2015 [36]. The 2016 study reported 28.3% prevalence of anemia in lactating in Ethiopia ranged from 16.3% in Addis Ababa to 68.3% in Somali region [23]. Another study which was done in the southern Ethiopia also found a 44.4% prevalence of anemia among lactating women [35]. Moreover, two recent studies [36, 38] conducted in 2017, reported a relatively lower prevalence rate of 11.4% anemia in the southern region and 24.2% in the Tigray region (Table 1).
The pooled prevalence of anemia among lactating women in Ethiopia was 28.9% (95%CI: 22.7%- 35.1%) (Figure 2). Furthermore, subgroup analysis based on the regions showed that the prevalence of anemia in lactating women was significantly higher; 58.7% (95% CI: 39.1%-78.3%) in the Somali region, 44.9% (95% CI: 34.1%-55.8%) in the Afar region and 35.9% (95% CI: 33.8%-37.9%) in the Dire Dawa administrative city as compared to the lowest prevalence; 11.6% (95% CI: 2.8%-20.4%) in the Addis Ababa city. However, the subgroup analysis of anemia prevalence rates based on the survey year, the quality of studies and study settings indicated no significant difference. The prevalence of anemia in lactating women was 26.1% (95% CI: 18.8%, 33.5%) in studies conducted from 2010 to 2014 and 31.1% (95% CI: 21.1%, 41.2%) in studies conducted from 2015 to 2020. While the subgroup analysis based on risk of bias indicated that the prevalence of anemia in studies with a low risk of bias was 30.4% (95% CI: 22.8%-37.9%) and 25.0% (95% CI: 18.1%-31.9%) in moderate risk studies. Similarly, the prevalence of anemia in facility-based studies was 35.9% (95% CI: 13.2%-58.8%) and 27.3% (95% CI: 21.6%, 32.3%) in community-based studies (Table 2).
Factors associated with Anemia among lactating women in Ethiopia
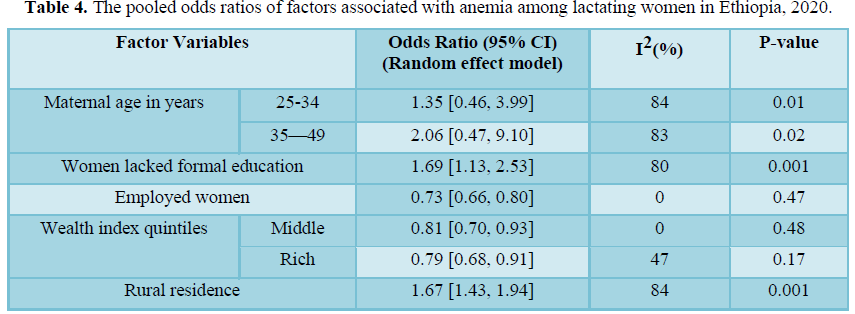
Maternal education, residence, wealth index, and employment status of lactating mothers were socioeconomic factors significantly associated with anemia in lactating women. Women who lacked formal education were 1.69 times more likely to be anemic than their educated counter parts (OR=1.69; 95% CI: 1.13, 2.53). Similarly, rural residents had 1.67 times higher odds of anemia than urban residents (OR=1.67; 95% CI: 1.43, 1.94). Employed lactating women had 27% lower odds of being anemic compared to unemployed women (OR= 0.73; 95% CI: 0.66, 0.80). In addition, women in middle and rich wealth index quintiles had about 19% and 21% lower odds of developing anemia as compared to those lactating mothers from poorer wealth quintiles, respectively.
Among women’s reproductive characteristics, parity, antenatal care visits, and utilizing family planning were also factors significantly associated with anemia in lactating mothers in Ethiopia. Lactating women with higher parity (above 4) (OR=1.24; 95% CI: 1.08, 1.42), women who had lower antenatal care visits (below 4) (OR=1.64; 95% CI:1.18, 2.28), and mothers who don’t utilize family planning (OR=1.51; 95% CI: 1.35, 1.70) had significantly higher odds of developing anemia compared to their respective counterparts.
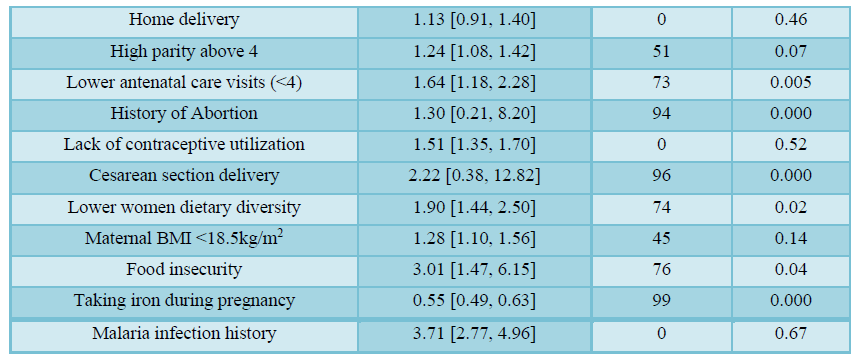
The meta-analysis also showed that underweight lactating women (BMI<18.5kg/m2) were 1.3 times more likely to be anemic than normal-weight women (OR=1.28; 95% CI: 1.10, 1.56). Lactating women with lower dietary diversity had about 2 times higher odds of anemia than those women who had adequate dietary diversity (OR=1.90; 95% CI: 1.44, 2.50). Similarly, lactating mothers from food-insecure households were 3 times more likely to be anemic than women from food secured households (OR= 3.01; 95% CI: 1.47, 6.15).
Lactating women who had a history of malaria infection were 3.7 times more likely to be anemic than their counterparts (3.71; 95% CI: 2.77, 4.9). Moreover, taking iron supplementation during pregnancy was negatively associated with anemia in lactating mothers. Women who took iron supplementation during their last pregnancy had a 45% lower risk of developing anemia compared to their counterparts (OR=0.55; 95% CI: 0.49, 0.63). However, maternal age, delivery mode, history of abortion, and place of delivery were not significantly associated with anemia in lactating mothers (Table 4).

DISCUSSION
This systemic review and meta-analysis synthesized evidence on the prevalence and associated factors of anemia in lactating women in Ethiopia from 2010 to 2020. Accordingly, the pooled prevalence of anemia among lactating women in Ethiopia was found to be 28.9%. The prevalence was relatively higher 31.1% in studies conducted from 2015 to 2020 compared with 26.1% in studies conducted from 2010 to 2014. This finding is supported by the EDHS results that the prevalence of anemia among lactating women in the country was increased from a mild public health problem (18.5%) in 2011 [39] to a moderate public health problem (28.6%) in 2016 [16]. Similarly, a recent study showed that the prevalence of dietary iron deficiency in Ethiopia declined from 1990 to 2010 and then increased in 2017 [15]. This may be attributable to the relatively higher prevalence of infectious diseases, inadequate dietary diversity, low micronutrient intake, weak health institutions and lower coverage of iron, and folate supplementation [16,40]. Moreover, inadequate maternal nutrition prioritization and lack of community-level programs could also contribute to high maternal malnutrition during pregnancy and lactation in Ethiopia [19,20]. Furthermore, the decrease in consumption of animal products due to an increase in cost and meat export rate could have an impact on the domestic meat cost and consumption [41,42]. Subgroup analysis based on regions indicated significantly higher prevalence of anemia in Somali (58.7%), Afar (44.9%) and, Dire Dawa regions (35.9%) compared with the lowest rate in Addis Ababa (11.6%). These regions with a higher rate of anemia are relatively less developed pastoralist states compared with other regions in terms of healthcare service coverage [16,43,44], socioeconomic status [16], household food security and dietary diversity [45-47]. For instance, sever food insecurity was the highest in the Somali region (39.6%) and lowest in Addis Ababa (3.3%) [47]. In addition, the pastoralist communities are also heavily dependent on animal milk as a source of their daily food, which has a low iron bio availability [48]. Moreover, in these regions, most women (85%) gave birth at home that could expose them to severe bleeding and about 87% of women did not receive postnatal care services [16]. This lower health service utilization could also result in missed opportunities for the lactating women to be benefited from nutrition counseling, iron supplementation, diagnosis of disease, and treatment services provided at health facilities [16]. Therefore, community-based intervention approaches may need to be prioritized in regions where maternal and childcare utilization rates are low. While, facility-based integrative nutritional interventions may be a better approach in regions where healthcare utilization rates are high. Based on the world health organization (WHO) classification, the overall prevalence rate revealed that anemia in lactating women is still a moderate public health problem in Ethiopia [25]. Anemia among lactating women was a severe public health problem in Afar and Somali regions (≥40%) and a mild public health problem in Addis Ababa anemia (<20%). Whereas, in the remaining 8 regions out of 11 regions in Ethiopia, it was found to be a moderate public health problem (20%-40%) [25]. The observed anemia prevalence differences a cross the regions could be attributed to the regional difference in preferences of food consumption [45-47], the rate of infectious disease occurrence [43] and variation in healthcare services accessibility [16,44]. Moreover, the unavailability of improved latrine and clean water facilities [16] could escalate the incidence of infections that could lead to anemia [49]. These variations a cross regions reflect the importance of disaggregated data for evidence-based policymaking and program design and context-specific interventions are required in order to reduce anemia in Ethiopia.
Lactating women’s educational status was significantly associated with anemia. Women who had formal education had a significantly lower likelihood of developing anemia than uneducated counter parts. This finding is consistent with other studies from developing countries [50-52]. This is because educated mothers are more likely to use health care services and eat more diversified foods than illiterate women. Education improves the nutritional status of lactating women through improving women’s knowledge on nutrition, reducing cultural food taboos and enhancing their preference of quality and quantity of diversified food groups consumed during pregnancy and lactation period. In contrast, Illiterate women had lower autonomy that restricts them from access to maternal and child health care services [40, 50]. Likewise, the risk of anemia in rural residents was higher than urban residents. This is due to the reason that the rural areas are in low socioeconomic status, have limited access to use iron-rich foods [53,54] and had a lower rate of utilizing maternal health services compared to urban regions [55].
The study also indicated that lactating women in higher wealth quintiles had a lower odd of having anemia compared with lower wealth index quintile. This finding is in line with findings that lower socioeconomic status associated with a higher risk of anemia [50,56]. This could be due to lactating women from higher wealth quintiles households had a better opportunity to access a balanced diet, higher meal frequency, and diversified foods [57,58]. Similarly, anemia was significantly lower in employed lactating women compared to unemployed women. This might be due to the extra income employed women earned enabled them to access more diversified food groups and improved their micronutrient intakes [54,59]. In addition, being employed could give them decision making power of their health to receive better health care service rather than depending on their husband’s income. Hence, improving the socioeconomic status of lactating women could contribute more to preventing and reducing anemia morbidity in Ethiopia.
Lactating mothers who had used family planning had a lower likelihood of having anemia than those who had never used family planning. Similarly, studies from Ethiopia and Timor-Leste also showed that the use of family planning was associated with a lower risk of anemia [50,60]. The importance of family planning in reducing the risk of anemia was also supported by studies done in low and middle-income countries [61,62]. This is because taking contraceptives might have a chance of reduction of monthly menstrual bleeding, reduce the risk of anemia due to hemorrhage during pregnancy, and postpartum by reducing the number of pregnancy and childbirth. Similarly, higher parity is associated with anemia. Frequent births may not provide a sufficient time period to replenish lost nutrient stores before another childbirth [63], especially among mothers with poor feeding practice [64]. In addition, women with frequent births in short periods might have both ante partum and postpartum hemorrhage with subsequent births which could result in anemia. Menstrual blood loss is lower in women using oral contraception and higher in women using intrauterine contraceptive device [65]. These findings reflected that postpartum complications should be managed effectively and family planning methods that decrease bleeding should be used in order to reduce anemia prevalence in Ethiopia.
Women who had received the recommend at least four antenatal care visits were found to be less likely to develop anemia during the postpartum period. The provision of nutrition and health education about the consumption of various sources of iron-rich diets and the provision of iron supplementation during ANC visits could be contributed to the reduction of anemia [16,63]. In Ethiopia, about 42% of women age 15-49 years took iron supplements and two-third of women (66%) received nutrition counseling during their ANC visits in 2016 [16]. They are also screened for some diseases, including anemia, and benefited from prophylactic measures against malarial infection, iron, and folic acid supplementation [14,16]. This is also supported by the finding that lactating women who had received iron supplementation during their last pregnancy had a lower risk of anemia than their counterparts. In high-risk populations where anemia prevalence greater than 20%, weekly iron and folic acid supplements are recommended for childbearing women [66]. Studies also revealed the effectiveness of weekly iron supplementation in preventing iron deficiency anemia among lactating mothers in developing countries [67,68]. Therefore, improving iron supplementation coverage in Ethiopia could minimize the harmful effects of anemia in pregnant and lactating women and in their children [18].
Maternal nutritional status was found to be significantly associated with anemia. Undernourished lactating mothers (BMI2) had higher odds of developing anemia than normal-weight women. Studies from Ethiopia and Thailand also reported BMI correlated with the rate of developing anemia [50,69]. When mothers are at risk of deficiency of macronutrients, they are also most likely at risk of other micronutrient decencies such as iron [70]. Studies indicated that anemiaamongreproductive-agewomenindevelopingcountriesismainlyduetomalnutritionand poor dietary intake [71,72]. Most women from low and middle-income countries consume diets lacking haem iron and diets adequate in non-haem iron which has a low absorption that could lead to iron deficiency anemia [73].
Mothers who consumed less diversified food groups were more likely to be anemic than their counterparts. This finding also supported by the finding that food insecurity significantly associated with higher odds of developing anemia among lactating women [54,74,75]. This is explained by the fact that inadequate dietary diversity leads to a deficiency of minerals and vitamins which in turn affects iron intake [70,72]. Consumption of various food types which include good sources of iron like fruits, vegetables, whole grains, milk products, lean meat, fish, dry beans, egg, and nuts prevents anemia during pregnancy and lactating women. However, high fiber diets, low-fat diets, and high tea and coffee consumption without vitamin C consumption inhibits iron absorption which could expose mothers to develop anemia [25,40]. However, only less than 20% of women in Ethiopia met minimum dietary diversity [47] mainly due to food taboos towards consuming certain foods, lack of knowledge about dietary diversity, high food insecurity, and economic constraints [54,75]. Hence, a multi-component strategy that will tackle the economic and socio-behavioral issues is crucial to improve the nutritional status of women in Ethiopia.
Anemia prevalence was significantly higher among women who had a history of malaria infection compared with their counterparts. This is explained by the fact that the number of red blood cells could be reduced due to the plasmodium species ingests the red blood cells of the host and the reduced appetite and nutrient consumption. Studies also confirmed that infections like malaria and hookworm cause anemia as a result of decreasing appetite, loss of nutrients, reducing the efficiency of absorption, and the use of micronutrients [14,49].
Generally, multiple interacted factors such as poor socioeconomic status, communicable diseases, household food insecurity, inadequate dietary diversity, and low health care services utilization are the main factors for the high prevalence of anemia among lactating women in Ethiopia. Therefore, integrated, and comprehensive nutrition packages should be designed and implemented in order to prevent nutrient deficiencies and associated morbidity in lactating women in Ethiopia.
LIMITATIONS OF THE STUDY
The different sources of biases like the in accurate selection of study participants, the small sample size in some studies, data collection and analysis limitations, and selective results reporting in the included studies could affect findings in the meta-analysis [76]. Since the original studies incorporated in this review were cross-sectional design, the confounding variables might affect the estimates. Extensive searches using the different searching strategies (manual and electronic) were conducted, and both published and unpolished articles were included. In order to minimize bias, data were extracted by two authors independently using a pre-determined tool. This author also conducted a quality assessment. High heterogeneity was found across included studies. Some of the included studies did not report the outcome with variables that limits us to do intensive sub- group analysis. High heterogeneity was addressed by conducting subgroup analysis and using the random-effects model to compute the pooled prevalence and odds ratio. The random-effects model considers any heterogeneity embedded in meta-analysis. Nevertheless, several unexamined confounders might contribute to the heterogeneity of anemia prevalence among reviewed studies. Hence, the overall very low quality of evidence shall be considered when interpreting the findings.
CONCLUSION
The study indicated that anemia in lactating women is still a moderate public health problem in the majority of regions in Ethiopia and has remained a severe public health problem in Afar and Somali regions. Lower socioeconomic status, maternal malnutrition, malaria infection, food insecurity, and inadequate food diversity were factors associated with higher odds of developing anemia in lactating women in Ethiopia. Moreover, maternal health services utilization like antenatal care, family planning, and iron supplementation were significantly correlated with lower odds of anemia. Hence; the government of Ethiopia needs to monitor and evaluate the implementation and effectiveness of nutrition programs in Ethiopia in order to strengthen, design, and effectively implement comprehensive multi-sectorial community and facility-based interventions like food diversification, food fortification, micronutrient supplementation, and nutrition education in order to prevent and reduce anemia morbidity among lactating women in Ethiopia.
DECLARATIONS
Ethics approval and consent to participate
This section is not applicable because this study is a systematic review and Meta-analysis.
Registration of systematic review
This systematic review has not been submitted for protocol registration elsewhere.
Availability of data and material
All data included in systematic review and Meta-analysis are available in the main manuscript and additional supporting files.
Authors’ contributions
The corresponding author Benyam Seifu contributed to the conception, design, data extraction, synthesis, and analysis, preparation of the manuscript and Delelegn Yilma contributed to the design, data extraction, synthesis and analysis, write up of the manuscript.
Competing interests
Authors declared that have no financial and non-financial competing interest.
ACKNOWLEDGMENTS
We would like to acknowledge Ambo University for creating the chance for conducting this systematic review and Meta-analysis. We also acknowledge the researchers who sent their articles for this review.
- Benoist B, McLean E, Egli I, Cogswell M (2008) Worldwide prevalence of anemia: WHO Global Database on Anemia, World Health Organization: Geneva, Switzerland.
- World Health Organization (2015) The Global Prevalence of Anemia in 2011. World Health Organization: Geneva, Switzerland.
- Stevens G, Finucane M, Regil LMD, J Paciorek C, Flaxman SR, et al. (2013) Global, regional and national trends in hemoglobin concentration and prevalence of total and severe anemia in children and pregnant and non-pregnant women for 1995-2011: A systematic analysis of population-representative data. Lancet Glob Health 1: e16-e25.
- Petry N, Olofin I, Hurrell RF, Boy E, Wirth JP, et al. (2016) The proportion of anemia associated with iron deficiency in low, medium and high human development index countries: A systematic analysis of national surveys. Nutrients 8(11): 693.
- Ayoya M, Bendech M, Zagre NM, Tchibindat FE (2012) Maternal anemia in west and central Africa: Time for urgent action. Pub Health Nutr 15(5): 916-927.
- Fanzo J, Hawkes C, Udomkesmalee E, Afshin A, Allemandi L, et al. (2018) Development Initiatives, 2018 Global Nutrition Report: Shining a light to spur action on nutrition. Bristol, UK: Development Initiatives.
- Sloan NL, Durocher J, Aldrich T, Blum J, Winikoff B (2010) What measured blood loss tells us about postpartum bleeding: A systematic review. BJOG: Inter J Obstetr Gynecol 117(7): 788-800.
- Bergmann RL, Richter R, Bergmann KE, Dudenhausen JW (2010) Prevalence and risk factors for early postpartum anemia. Eur J Obstet Gynecol Reprod Biol 150(2): 126-131.
- Milman N (2011) Postpartum anemia I: Definition, prevalence, causes and consequences. Ann Hematol 90(11): 1247-1253.
- Torheim L, Ferguson E, Penrose K, Arimond M (2010) Women in resource-poor settings are at risk of inadequate intakes of multiple micronutrients. J Nutr 140: 2051S-2058S.
- Harika R, Faber M, Samuel F, Kimiywe J, Mulugeta A, et al. (2017) Micronutrient status and dietary intake of iron, Vitamin A, Iodine, Folate and Zinc in women of reproductive age and pregnant women in Ethiopia, Kenya, Nigeria and South Africa: A Systematic Review of Data from 2005 to 2015. Nutrients 9(10): 1096.
- Dominic A, Ogundipe A, Ogundipe O (2019) Determinants of women access to healthcare services in sub-Saharan Africa. Open Pub Health J 12: 504-514.
- Djibril MB, Sentongo P, Kjerulff KH, Muzi Na, Liu G, et al. (2019) Adherence to iron supplementation in 22 sub-Saharan African countries and associated factors among pregnant women: A large population-based study. Curr Dev Nutr 3(12): nzz120.
- Kassa GM, Muche AA, Berhe AK, Fekadu GA (2017) Prevalence and determinants of anemia among pregnant women in Ethiopia: A systematic review and meta-analysis. BMC Hematology 17: 17.
- Hassen HY, Ali JH, Gebreyesus SH, Endris BH, Temesgen AM (2020) National incidence, prevalence and disability-adjusted life years (DALYs) of common micronutrient deficiencies in Ethiopia from 1990 to 2017: Estimates from the global burden of diseases study. Global Health Action 13(1): 1776507.
- Central Statistics Agency (2016) Ethiopia Demographic and Health Survey 2016. Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF.
- Keats E, Lynnette M, Greg S, Mduduzi NN, Zulfiqar A (2019) Improved micronutrient status and health outcomes in low- and middle-income countries following large-scale fortification: Evidence from a systematic review and meta-analysis. Am J Clin Nutr 109: 1696-1708.
- Salam RA, Das JK, Bhutta ZA (2014) Multiple micronutrient supplementation during pregnancy and lactation in low-to-middle-income developing country settings: Impact on pregnancy outcomes. Ann Nutr Metab 65: 4-12.
- Saldanha LS, Buback L, White JM, Mulugeta A, Mariam SG, et al. (2012) Policies and program implementation experience to improve maternal nutrition in Ethiopia. Food Nutr Bull 33(2Suppl1): S27-S50.
- Kennedy E, Tessema M, Hailu T, Zerfu D, Belay A, et al. (2015) Multisector Nutrition Program Governance and Implementation in Ethiopia: Opportunities and Challenges. Food Nutr Bull 36(4): 534-548.
- Bhutta ZA, Salam RA (2012) Global nutrition epidemiology and trends. Ann Nutr Metab 61(suppl1): 19-27.
- Lakew Y, Biadgilign S, Haile D (2015) Anemia prevalence and associated factors among lactating mothers in Ethiopia: Evidence from the 2005 and 2011 demographic and health surveys. BMJ Open 5(4): e006001.
- Liyew AM, Teshale AB (2020) Individual and community level factors associated with anemia among lactating mothers in Ethiopia using data from Ethiopian demographic and health survey, 2016: A multilevel analysis. BMC Public Health 20(1): 775.
- Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS MED6 (7): e1000097.
- WHO (2011) Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and mineral nutrition information system. Geneva: World Health Organization.
- Hoy D, Brooks P, Woolf A, Blyth F, March LYN, et al. (2012) Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 65: 934-939.
- Schunemann H, Brozek J, Oxman A (2009) GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2 [update March 2009]. The Grade Working Group.
- Altman D (1991) Practical statistics for medical research.
- Higgins J (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. Southern Gate: The Cochrane Collaboration.
- Roba KT, O’Connor TP, Belachew T, O’Brien NM (2015) Seasonal variation in nutritional status and anemia among lactating mothers in two agro-ecological zones of Rural Ethiopia: A longitudinal study. Nutrition 31: 1213-1218.
- Hailu T, Abuye C, Abebe H, Whiting JS (2016) Correlation of maternal nutritional status with breast milk content of iron, zinc and vitamin A in rural southern Ethiopia. Ethiopian J Pub Health Nutr 1(1): 49-56.
- Argaw A, Demeke S, Gebremariam A, Huybregets L, Tesfaye A, et al. (2014) Lactating mothers feeding practice and anemia: An implication for intervention, a case of Jimma district rural setting, Ethiopia.
- Alemayehu M (2017) Factors associated with anemia among lactating mothers in subsistence farming households from selected districts of jimma zone, South Western Ethiopia: A community based cross-sectional study. J Nutr Food Sci 7: 3.
- Roba KT, O’Connor TP, Belachew T, O’Brien NM (2014) Concurrent iron and zinc deficiencies in lactating mothers and their children 6-23 months of age in two agro-ecological zones of rural Ethiopia.
- Orsngo M (2016) Prevalence and factors associated with anemia among lactating mothers in Damotsorie district, Wolaita Zone, Southern Ethiopia.
- Julla BW, Haile A, Ayana G, Eshetu S, Kuche D, et al. (2018) Chronic energy deficiency and associated factors among lactating mothers (1549 years old) in offa woreda, Wolayita Zone, SNNPRs, Ethiopia. World Scientific Research 5(1): 13-23.
- Feleke B, Feleke E (2018) Pregnant mothers are more anemic than lactating mothers, a comparative cross-sectional study, Bahir Dar, Ethiopia. BMC Hematology 18: 2.
- Abraham G, Berhe Y (2020) Prevalence and associated risk factors of immediate postpartum anemia in two teaching hospitals in Mekelle Northern Ethiopia. Ethiopian J Reprod Health 12(1): 28-54.
- Central Statistics Agency (2011) Ethiopian demographic and health survey. Addis Ababa: CSA.
- Workicho A, Belachew T, Feyissa GT, Wondafrash B, Lachat C, et al. (2016) Household dietary diversity and animal source food consumption in Ethiopia: Evidence from the 2011 Welfare Monitoring Survey. BMC Pub Health 16: 1192.
- Birhanu AF (2019) A review on Ethiopian meat production trends, consumption and meat quality parameters. Int J Food Sci Agr 3(4): 267-274.
- Eshetie T, Hussien K, Teshome T, Mekonnen A (2018) Meat production, consumption and marketing tradeoffs and potentials in Ethiopia and its effect on GDP growth: A review. J Market Consum Res 42: 17-24.
- Ministry of Health of Ethiopia (2015) Health and health related indicator, Ethiopia: Addis Ababa.
- Ethiopian Public Health Institute (2018) Federal Ministry of Health. Service Availability and Readiness Assessment (SARA). Final Report. Addis Ababa, Ethiopia
- Ethiopian Public Health Institute Food Consumption Survey (2013) Ethiopian food consumption survey report. Addis Ababa, Ethiopia: Ethiopian Public Health Institute Food Consumption Survey.
- Berhane G, Paulos Z, Tafere K, Seneshaw T (2011) Food gain consumption and calorie intake patterns in Ethiopia, Ethiopia strategy support program II (ESSP II). Working Paper 23.
- Ayana (2015) National nutrition program end line survey. Ethiopian Public Health Institute, 2nd National Nutrition Research Dissemination Conference.
- Kibangou I, Bouhallab S, Henry G, Bureau F, Allouche S, et al. (2005) Milk proteins and iron absorption: Contrasting effects of different case in ophosphopeptides. Pediatr Res 58(4): 731-734.
- Tay SC, Nani EA, Walana W (2017) Parasitic infections and maternal anemia among expectant mothers in the Dangme East District of Ghana. BMC Res Notes 10: 3.
- Gebremedhin S, Enquselassie F (2011) Correlates of anemia among women of reproductive age in Ethiopia: Evidence from Ethiopian DHS 2005. Ethiop J Health Dev 25: 22-30.
- Haidar J (2010) Prevalence of anemia, deficiencies of iron and folic acid and their determinants in Ethiopian women. J Health Popul Nutr 28: 359-368.
- Okwu GN (2011) Studies on the predisposing factors of iron deficiency anemia among lactating women in Owerri, Nigeria. Int Res J Biochem Bioinform1: 304-309.
- Boke MM, Geremew AB (2018) Low dietary diversity and associated factors among lactating mothers in Angecha districts, Southern Ethiopia: Community based cross-sectional study. BMC Res Notes 11(1): 892.
- Engidaw MT, Gebremariam AD, Tiruneh SA, Asnakew DT, Abate BA (2019) Dietary diversity and associated factors among lactating mothers in Debre Tabor General Hospital, Northcentral Ethiopia. Int J Sci Rep 5(1): 17-23.
- Singh PK, Kumar C, Rai RK, Singh L (2014) Factors associated with maternal healthcare services utilization in nine high focus states in India: A multi-level analysis based on 14385 communities in 292 districts. Health Policy Plan 29(5): 542-559.
- Zhao A, Zhang Y, Li B, Wang P, Li J, et al. (2014) Prevalence of anemia and its risk factors among lactating mothers in Myanmar. Am J Trop Med Hyg 90(5): 963-967.
- Bodnar L, Cogswell M, Scanlon K (2002) Low income postpartum women are at risk of iron deficiency. J Nutr 132: 2298-2302.
- Sadeghian M, Fatourechi A, Lesanpezeshki M, Ahmadnezhad E (2013) Prevalence of anemia and correlated factors in the reproductive age women in rural areas of Tabas. J Fam Reprod Health 7(3): 139-144.
- Taruvinga A, Muchenje V, Mushunje A (2013) Determinants of rural household dietary diversity: The case of Amatole and Nyandeni districts, South Africa. Int J Dev Sustain 2: 2233-2247.
- Lovermail AA, Hartman M, Chia KS, Heymann DL (2014) Demographic and spatial predictors of anemia in women of reproductive age in Timor-Leste: Implications for Health Program Prioritization. PLoS ONE 9: e91252.
- Bellizzi S, Ali MM (2018) Effect of oral contraception on anemia in 12 low-and middle-income countries. Contraception 97(3): 236-242.
- Gebremedhin S, Asefa A (2019) Association between type of contraceptive use and hemoglobin status among women of reproductive age in 24 sub-Saharan Africa countries. BMJ Sexual Reprod Health 45(1): 54-60.
- Abdelrahman EG, Gasim GI, Musa IR, Elbashir LM, Adam I (2012) Red blood cell distribution width and iron deficiency anemia among pregnant Sudanese women. Diagn Pathol 7: 168.
- Federal Ministry of Health-Ethiopia (2004) National guideline for control and prevention of micronutrient deficiencies. Addis Ababa, Ethiopia.
- Coad J, Conlon C (2011) Iron deficiency in women: Assessment, causes and consequences. Curr Opin Clin Nutr Metab Care 14: 625-634.
- World Health Organization (2009) Weekly iron–folic acid supplementation (WIFS) in women of reproductive age: Its role in promoting optimal maternal and child health. Position statement. Geneva: World Health Organization.
- Haider J, Omwega AM, Muroki NM, Ayana G (2003) Daily versus weekly iron supplementation and prevention of iron deficiency anemia in lactating women. East African Med J 80(1): 11-16.
- Baykan A, Yalc SS, Yurdako KK (2006) Does maternal iron supplementation during the lactation period affects iron status of exclusively breast-fed infants? Turk J Pediatr 48(4): 301-307.
- Liabsuetrakul T, Southern Soil-transmitted Helminths and Maternal Health Working Group (2011) Is international or Asian criteria-based body mass index associated with maternal anemia, low birth weight, and preterm births among Thai population? An observational study. J Health Popul Nutr 29: 218-228.
- Blumfield M, Hure A, Wicks LMD, Smith R, Simpson S, et al. (2012) The association between the macronutrient content of maternal diet and the adequacy of micronutrients during pregnancy in the Women and Their Children’s Health (WATCH) study. Nutrients 4(12): 1958-1976.
- Mawani, M, Ali SA, Bano G, Ali SA (2016) Iron deficiency anemia among women of reproductive age, an important public health problem: Situation analysis. Reprod Sys Sexual Disord Curr Res 5(3): 1-69.
- Aspuru K, Villa C, Bermejo F, Herrero P, López SG (2011) Optimal management of iron deficiency anemia due to poor dietary intake. Int J Gen Med 4: 741-7501.
- Milman N (2011) Anemia-still a major health problem in many parts. Ann Hematol 90: 369-377.
- Siddiqui MZ, Goli S, Kumar P, Reja T, Doshi R, et al. (2017) Prevalence of anemia and its determinants among pregnant, lactating, and non-pregnant, non-lactating women in India SAGE Open July-September 7(3): 1-10.
- Weldehaweria NB, Misgina KH, Weldu MG, Gebregiorgis YS, Gebrezgi BH, et al. (2016) Dietary diversity and related factors among lactating women visiting public health facilities in Aksum town, Tigray, Northern Ethiopia. BMC Nutr 2(1): 38.
- Dreier M (2013) Quality Assessment in Meta-analysis. Methods of Clinical Epidemiology. Berlin, Heidelberg: Springer Berlin, Heidelberg, pp: 213-228.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Journal of Rheumatology Research (ISSN:2641-6999)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- Journal of Pathology and Toxicology Research
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)





