Review Article
Location of Macromolecules Coupled with Steroid Hormone Injected in Rats
5535
Views & Citations4535
Likes & Shares
In order to compare the distribution of testosterone-bovine-serum albumin-conjugate (testosterone-BSA) with that of radiolabeled testosterone in vivo, testosterone-BSA labeled with 2-nm colloidal gold (testosterone-BSA-gold) was injected into rat tail vein. The testosterone-BSA-gold with silver enhancement became visible as silver deposits under electron microscope. In rats sacrificed 2 h after the injection of testosterone-BSA-gold, silver deposits were found in the same locations as the radiolabeled testosterone, namely on the cytoplasm and nuclei of the target cells such as Leydig cells, Sertoli cells, spermatogonia, spermatocytes, spermatids in the testis, and the epithelial cells of the seminal vesicle. In an observation without silver enhancement, the gold particles representing testosterone-BSA were found in the target cell nuclei in the testis. Vesicular transportation of testosterone-BSA-gold to the nucleoplasm starts with fusion of the vesicle to a single-bilayer diaphragm in the nuclear envelope, without passing through the cytosol and nuclear pores. Few deposits were present on non-target cell nuclei in thymus. In rat injected with gold labelled-hydrocortisone-BSA, silver deposits are found on the target cell nuclei such as thymocytes. Together with the aforementioned studies on testosterone-BSA-gold, it indicates that the fate of gold labelled-steroid-BSAs may be decided at the cell membrane level. In rats injected with testosterone-BSA, hydrocortisone-BSA or corticosterone-BSA, BSA conjugation remains immunocytochemically intact in the respective target cell nuclei. These results suggest that the steroid hormones act as transporters to carry extracellular macromolecules into their target cell nuclei in vivo.
Keywords: Steroid-macromolecule conjugates, Vesicular transportation, Nuclear diaphragm, Target cell nucleoplasm, Exterior of cells, Intact antigenicity, In Vivo
INTRODUCTION
The cytoplasm and nucleus of a cell are separated from extracellular environment by the cell membrane. Extracellular signaling molecules bind to cell membrane receptors and commence the receptor-initiated intracellular signaling. Various substances targeting cell nucleus arrive from the extracellular environment as well. Many nuclear functions of such substances depend on nucleocytoplasmic transportation via nuclear pores. The nonenveloped monkey polyomavirus virions such as simian virus 40 (SV40, 45 nm in diameter) are composed of proteins and DNA, and are able to reach the nucleus from extracellular environment [1]. Besides SV40, steroid hormones and antinuclear antibodies such as immunoglobulin G (IgG) can also enter the nucleus from extracellular environment. IgG is found in some autoimmune diseases, targeting composition ingredients within the cell nucleus. However, native IgG does not pass freely the cell- or nuclear membrane.
Already back in the early 1970s, we (my team back then in Osaka City University and Osaka University, Japan) have developed a method to introduce exogenous substances into the cytoplasm of cultured cells, using inactivated Sendai virus [2]. In order to check whether IgG moves to nucleoplasm from cytoplasm, IgG was introduced into the cytoplasm of cultured cells [3, 4]; it did not. Based on this negative result, I considered three hypotheses. The first hypothesis is that there is a vesicular transportation course of extracellular macromolecules to the nucleoplasm that does not pass through the cytosol and nuclear pores. The second hypothesis is that steroid hormones may act as carriers in nuclear transportation of arbitrary macromolecules such as IgG from cell exterior as one of their membrane effects [5], possibly with endocytotic vesicles as vehicles [6]. The third hypothesis is that steroid hormones may function as transporters of extracellular macromolecules into the germ cell nuclei, such as spermatogenic cells, across blood-testis barrier in vivo.
Transportation of macromolecules between nucleoplasm and cytosol is performed through the nuclear pore complexes (NPCs). It was reported later that IgG with synthetic peptides containing nuclear localization signal (NLS) moves into the nucleoplasm from the cytoplasm by active transport through NPCs [7,8], but native IgG without NLS does not.
Transportation course into the nucleoplasm from the exterior of cell, and single-bilayer diaphragm in the nuclear envelope
SV40 particles are initially engulfed with membrane invagination and yet they are found without the membrane of endocytotic vesicles in the nuclei after 2 h [1]. Maul et al. [9] suggested that the first step of SV40 nuclear transportation is the fusion of virus-particle-containing vesicles membrane to the outer nuclear membrane. Then, the particles in the perinuclear cisterna are covered by the inner nuclear membrane to be moved into nucleoplasm [9]. Native IgG is not likely to pass neither the cell-nor nuclear membrane freely by passive transport. Accordingly, the occurrence of autoimmune diseases implies that there must be other routes which permit the nuclear entry of IgG besides the fusion of vesicular membrane to the outer nuclear membrane.
The infection processes of SV40 would transfer the cell membrane constituents to the nuclear envelope. To provide morphological evidence for the first hypothesis, i.e. the existence of vesicular transportation mechanism of extracellular macromolecules to the nucleoplasm without entering through the cytosol or nuclear pores, the nuclear migration of SV40 was pursued in cultured cells under electron microscope, using ferritin and concanavalin A as cell membrane markers [10,11]. Ferritin particles introduced directly into the cytoplasm did not enter the nucleus by themselves. In contrast, SV40-containing vesicles with ferritin particles were observed close to a single-bilayer nuclear membrane or a diaphragm [11]. The nucleoplasmic side of the diaphragm was covered with electron-dense materials, and the cell membrane markers were seen not only in perinuclear cisterna but localized along the nucleoplasmic side of the inner nuclear membrane [11,12]. These results suggest that SV40-containing vesicle membrane fuses to a single-bilayer diaphragm in the nuclear envelope in order to transport virus particles into the nucleoplasm, and that the exogenous macromolecules used as cell membrane markers here were transported into the nucleoplasm in this manner [11-13].
Nucleocapsids (45 nm x 280-300 nm) of recombinant baculovirus, Autographa californica nuclear polyhedrosis virus (enveloped DNA viruses), are formed in the nucleoplasm and migrate into the cytoplasm to bud through cell membrane [14]. To check the existence of single-bilayer diaphragm in nuclear envelope of other cells, we observed the nuclear export of baculovirus nucleocapsids in Spodoptera frugiperda clone 9 insect cells using rapid cryofixation. We proposed that the nucleocapsids move to cytoplasm from nucleoplasm, through small pores formed in the tip of protrusion of double membranes deriving from the nuclear envelope [15]. Complete fusion of two membranes such as pore formation occurs through the process of hemifusion [16]. From our study on the nuclear entry of testosterone-BSA-gold discussed below, the diaphragm seems to be formed by hemifusion of both membranes, and subsequent enlargement of the hemifused area [17].
Location of steroid hormone-BSA conjugates injected into rats
In the classical genomic model of steroid hormone action, free lipophilic hormones cross the cell membrane under passive transport, bind to their intracellular receptors in target cells to form hormone-receptor complexes which move to the nucleus to exert their genomic effects [18]. Many steroid hormones bind to plasma proteins in the blood such as albumin, and steroid-hormone-binding proteins (SHBP) such as sex hormone-binding protein and corticosteroid-binding globulin (CBG) [19,20]. Radiolabeled steroid hormones, e.g. [3H]-testosterone, can enter the target cell nuclei in vivo [21-23]. In studies that use ligated seminiferous tubules in vitro, androgen-binding protein (ABP) coupled with [3H]-testosterone (testosterone-ABP) is internalized by receptor-mediated endocytosis in spermatogenic cells, which are target cells of testosterone, and then enters the nuclei of these cells [24,25]. How testosterone-ABP moves into the nucleoplasm is unexplained in these studies. Our group showed under electron microscopy that colloidal gold embedded in epoxy resin becomes visible as silver deposits on the sections after silver enhancement [26]. The gold particles seem to be stable in lysosome. Several steroid hormone-BSA conjugates (steroid-BSAs) are commercially available. Steroid-BSAs bind to the nuclear receptors [27,28], although there is a report that cell membrane androgen receptors are unrelated to nuclear steroid receptors [29]. In order to provide evidence to the second hypothesis, i.e. that steroid hormones act as nuclear transportation carriers to extracellular macromolecules, we examined rats injected with steroid-BSA labeled with 2 nm colloidal gold (steroid-BSA-gold), before studying rats injected with bovine IgG coupled with steroid hormone.
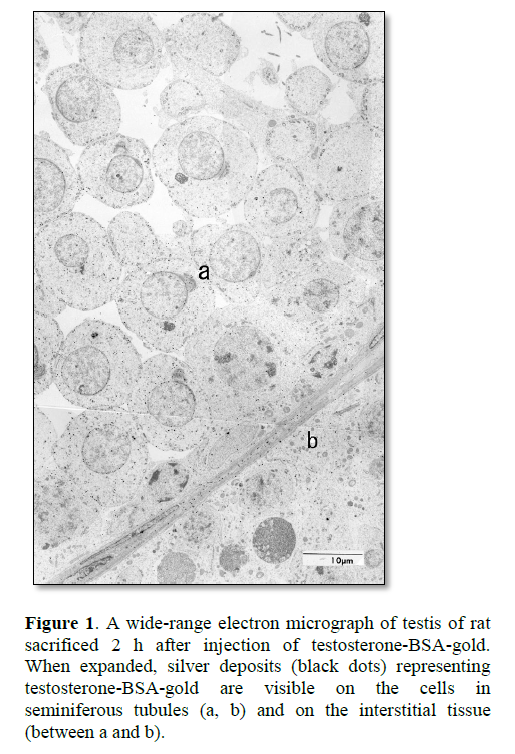
Radioactivity of [3H]-testosterone injected in rats was present in the nuclei of Leydig cells and seminiferous epithelial cells, e.g., Sertoli cells and spermatogenic cells in the testis, after 1 h and 3 h [23]. Upon injection of testosterone-BSA labeled with 2 nm colloidal gold (testosterone-BSA-gold) into the vascular system of rat, testosterone-BSA-gold was taken up by the coated pit of target cells of testosterones [30]. These results suggest that testosterone-BSA-gold is internalized in the target cells by receptor-mediated endocytosis. In the testis of rat sacrificed at 2 h after injection of testosterone-BSA-gold, many silver deposits representing testosterone-BSA-gold were visible on the interstitial cells, blood plasma and loose connective tissue of testis interstitium, and Sertoli cells and spermatogenic cells in seminiferous tubules after silver enhancement (Figure 1) [26,30]. These results of seminiferous tubules resemble the radioactivity locations of the rats injected with [3H]-testosterone [23], and of [3H]-testosterone-ABP in the studies using ligated seminiferous tubules [24,25].
In the seminal vesicle of rats injected with [3H]-testosterone, radioactivity appears in a selective location in the epithelial cell nucleus, and in the muscle layer to a lesser extent [22,31]. In the rat injected with testosterone-BSA-gold, the silver deposits representing testosterone-BSA-gold was observed in the epithelial cells, coinciding with the radioactivity [26]. In the smooth muscle layer, the deposits were mostly in the intercellular space, but a few were also on smooth muscle cells [26]. Because testosterone-BSA, being a macromolecule, remains in the blood plasma and tissue fluid, our results on the muscle layer seem not to agree with the result of rats processed by [3H]-testosterone. In blood vessels, the deposits were found in plasma, but not on erythrocytes [26,30].
In contrast, the nuclei of cells which are not targeted by testosterone such as thymocytes showed very few silver deposits representing testosterone-BSA-gold [26]. This result is also supported by the reports that no androgen receptor is detected in the nuclei of cells in thymus [32,33], and suggests that nuclear entry of testosterone-BSA-gold is specific to the target cells of testosterone. In other words, testosterone-BSA-gold does not enter the non-target cell nuclei. From the distribution of silver deposits, it has become clear that gold labeled-hydrocortisone-BSA (hydrocortisone-BSA-gold) injected into rat enters the target cell nuclei such as thymocytes and hepatocytes [34]. Together with the aforementioned studies on testosterone-BSA-gold, it indicates that the fate of gold labeled-steroid-BSAs may be decided at the cell membrane level.
Transportation of testosterone-BSA-gold to round spermatid nucleoplasm without passing through the cytosol and nuclear pores
In order to further clarify the vesicular transportation route of testosterone-BSA-gold to the nucleoplasm via nuclear envelope, the round spermatids in seminiferous tubule were observed under electron microscope. In spermiogenesis, the nuclear envelope of a round spermatid is divided into two forms as a consequence of acrosome expansion over the anterior pole of the nucleus. In the post-acrosomal region of the nuclear envelope, the nuclear pores continue to be present during the expansion of acrosome. In the subacrosomal region, the two nuclear membranes are in close apposition and devoid of nuclear pores [35,36].
After silver enhancement, silver deposits were present on the cell membrane, vesicles, nuclear envelope, perinuclear cisterna and nucleoplasm, but not nuclear pores in the post-acrosomal region [12,30]. In the region without silver enhancement, the outer nuclear membrane was irregular and invaginated in the cisterna of nuclear envelope. Some invaginating membrane seemed to contact the inner nuclear membrane. In spermatocytes, the outer nuclear membrane also seemed to be invaginated toward the inner nuclear membrane. In some nuclear envelopes, double-membrane-like vesicle consisting of inner- and outer vesicles of about 50 nm and 80 nm diameter respectively seemed to be located in the perinuclear cisterna. The gold particles were found along inner side of the inner vesicle-membrane [12,30].
In the observation of the subacrosomal region after silver enhancement, the deposits were found on cell membrane, vesicles, Golgi cisterna, acrosome, subacrosomal space, nuclear envelope and nucleoplasm [30]. From the distribution of silver deposits in the region, we suggested that testosterone-BSA-gold is also transported from the acrosome to the nucleoplasm through the subacrosomal nuclear envelope (SNE), which is devoid of pores. In an observation without silver enhancement [37], single membrane-vesicles with diameters of about 35-70 nm were observed in the subacrosomal space. Many vesicles seemed to be in contact with the inner acrosomal membrane, and some of them contained gold particles. Furthermore, some vesicles were also in contact with the SNE, of which the nucleoplasmic side was covered by electron-dense-material. In addition, partial diaphragms consisting of a single-bilayer nuclear membrane 30-50 nm in length were observed in the SNE. The nucleoplasmic side of diaphragms had low electron density, although the nucleoplasmic side of nuclear envelope excluding the diaphragm was covered by electron-dense materials which seemed to be nuclear lamina. These results indicate the possibility that the membrane of vesicles fuses to single-bilayer nuclear membrane in the SNE [12,37]. The nuclear entry of testosterone-BSA-gold, a macromolecule, resembles the entryway proposed for nuclear migration of SV40 in 1991 [11].
Immunocytochemical study on macromolecules conjugated with steroid hormones
We then immunocytochemically investigated whether BSA in the steroid-BSAs remains intact in the hormone-target cell nuclei. For this purpose, testosterone-BSA, hydrocortisone-BSA or corticosterone-BSA was injected into rats. In the rats injected with testosterone-BSA, BSA was found in the nucleus of testosterone’s target cells such as spermatogenic cells of the testis, and not in hepatocytes. This result supports the third hypothesis that steroid hormones may function as transporters of exogenous macromolecules to the target cell nuclei in vivo. In rats injected with hydrocortisone-BSA or corticosterone-BSA, BSA was found in the nucleus of target cells such as hepatocytes, but not the cells of testis. These results also suggest a possibility that steroid hormones can transport intact macromolecules into the target cell nuclei in vivo [38].
In fusion cells of cultured cells and erythrocytes, hemoglobin is still detected in the cytoplasm of fusion cells two days after the fusion [2]. When an antibody is introduced to the cytoplasm, the antibody reacts to its antigen [39,40]. Antinuclear antibodies such as IgG are found in some autoimmune diseases such as autoimmune hepatitis [41]. Foreign macromolecules introduced into cells may remain functionally stable. CBG binds specifically to cell membranes [42] and is found intact within the cells of glucocorticoid target tissues [43] and also within the nuclei of cultured cells [42]. Above results of steroid-BSAs suggest the existence of cell membrane receptors. Hydrocortisone is secreted from adrenal gland, while its targets are the hepatocytes. Because of this segregation of the hormone and its target organ, liver and hydrocortisone were chosen for the following research. Bovine IgG coupled with hydrocortisone injected into vascular system of adrenalectomized rats entered the hormone-target cell nuclei in the liver, maintaining the antigenicity [44]. This last finding also supports the second hypothesis that steroid hormones act as carriers to convey exogenous macromolecules such as IgG into the target cell nuclei in vivo.
CONCLUSION
The distribution of testosterone-BSA injected in rats is similar to that of radiolabeled testosterone in vivo. The movement of testosterone-BSA-gold into the nucleoplasm of target cells starts with the fusion of the membrane of endocytotic vesicles containing testosterone-BSA-gold to a single-bilayer diaphragm in the target cell nuclear envelope, just like the nuclear migration of SV40. The cytosol and nuclear pores do not participate in the nuclear movement of testosterone-BSA-gold. The distribution of hydrocortisone-BSA in rats is also similar to that of radiolabeled hydrocortisone in vivo. Plasma proteins such as albumin, SHBP and IgG coupled with steroid hormones would enter the hormone-target cell nuclei. In other words, steroid hormones would function as transporters for carrying extracellular macromolecules into the nucleoplasm of target cells in vivo. It is unknown whether these steroid hormones and macromolecules are functional in the target cell nuclei. Further studies are required to elucidate these actions and functions.
- Hummeler K, Tomassini N, Sokol F (1970) Morphological aspects of the uptake of simian virus 40 by permissive cells. J Virol 6: 87-93.
- Furusawa M, Nishimura T, Yamaizumi M, Okada Y (1974) Injection of exogenous substances into single cells by cell fusion. Nature 249: 449-50.
- Nishimura T, Furusawa M, Yamaizumi M, Okada Y (1976) Method for intracellular injection by cell fusion using erythrocyte ghosts. Cell Struct Funct 1: 197-200.
- Furusawa M, Yamaizumi M, Nishimura T, Uchida T, Okada Y (1976) Use of erythrocyte ghosts for injection of substances into animal cells by cell fusion. Methods Cell Biol 14: 73-80.
- Ichihara I, Santti RS, Pelliniemi LJ (1973) Effects of testosterone, hydrocortisone and insulin on the fine structure of the epithelium of rat ventral prostate in organ culture. Z Zellforsch Mikrosk Anat 143: 425-38.
- Pietras RJ, Szego CM (1984) Specific internalization of estrogen and binding to nuclear matrix in isolated uterine cells. Biochem Biophys Res Commun 123: 84-91.
- Lanford RE, Kanda P, Kennedy RC (1986) Induction of nuclear transport with a synthetic peptide homologous to the SV40 T antigen transport signal. Cell 46: 575-82.
- Yoneda Y, Hieda M, Nagoshi E, Miyamoto Y (1999) Nucleocytoplasmic protein transport and recycling of Ran. Cell Struct Funct 24: 425-433.
- Maul GG, Rovera G, Vorbrodt A, Abramczuk J (1978) Membrane fusion as a mechanism of simian virus 40 entries into different cellular compartments. J Virol 28: 936-944.
- Nishimura T, Kawai N, Kawai M, Notake K, Ichihara I (1986) Fusion of SV40-induced endocytotic vacuoles with the nuclear membrane. Cell Struct Funct 11: 135-141.
- Nishimura T, Kawai N, Ichihara I (1991) Interaction of endocytotic vacuoles with the inner nuclear membrane in simian virus 40 entries into CV-1 cell nucleus. Cell Struct Funct 16: 441-445.
- Nishimura T (2015) Steroid hormones as transporters to carry exogenous macromolecules into the target cell nuclei in vivo. Am J Life Sci 3: 53-57.
- Nishimura T (2018) Transportation Course of Macromolecules to the Nucleus from the Extracellular Environment: Steroid Hormones’ Cellular Entry Mode Revisited. Cell Biology 6: 9-12.
- Favre D, Studer E, Nishimura T, Weitz M, Michel MR (1993) Semliki Forest virus capsid protein expressed by a baculovirus recombinant. Arch Virol 132: 307-319.
- Nishimura T, Favre D, Dürrenberger M, Michel MR, Ichihara I, et al. (1994) Nuclear export of recombinant baculovirus nucleocapsids through small pore or nuclear-pore-like structure in Sf9 cells. Okajimas Folia Anat Jpn 71: 83-97.
- Chernomordik LV, Kozlov MM (2005) Membrane hemifusion: Crossing a chasm in two leaps. Cell 123: 375-382.
- Melikyan GB, White JM, Cohen FS (1995) GPI-anchored influenza hemagglutinin induces hemifusion to both red blood cell and planar bilayer membranes. J Cell Biol 131: 679-691.
- Jensen EV, DeSombre ER (1972) Mechanism of action of the female sex hormones. Ann Rev Biochem 41: 203- 230.
- Hammond GL (2016) Plasma steroid-binding proteins: Primary gatekeepers of steroid hormone action. J Endocrinol 230: R13-R25.
- Bikle DD (2020) The free hormone hypothesis: When, why, and how to measure the free hormone levels to assess vitamin D, thyroid, sex hormone, and cortisol status. JBMR Plus 5: e10418.
- Beardsley JA, Hilton FK (1975) Uptake in vivo of [3H] testosterone by the interstitial compartment in testes of normal adult mice. J Reprod Fert 42: 361-364.
- Sar M, Liao S, Stumpf WE (1970) Nuclear concentration of androgens in rat seminal vesicles and prostate demonstrated by dry-mount autoradiography. Endocrinology 86: 1008-1011.
- Sar M, Stumpf WE, McLean WS, Smith AA, Hansson V, et al. (1975) Localization of androgen target cells in the rat testis: Autoradiographic studies. Curr Top Mol Endocrinol 2: 311-319.
- Gerard A, En Nya A, Egloff M, Domingo M, Degrelle H, et al. (1991) Endocytosis of human sex steroid-binding protein in monkey germ cells. Ann NY Acad Sci 637: 258-276.
- Gerard H, Gerard A, En Nya A, Felden F, Gueant JL (1994) Spermatogenic cells do internalize Sertoli androgen-binding protein: A transmission electron microscopy autoradiographic study in the rat. Endocrinology 134: 1515- 1527.
- Nishimura T, Ichihara I (1997) Nuclear concentration of gold labeled-testosterone-bovine serum albumin conjugate injected intravenously in the hormone-target cells of rat. Cell Struct Funct 22: 433-442.
- Beppu K (1989) An electron microscopic study of the steroid hormone receptor in uterine cells by the colloidal gold-labeled steroid hormone. J. Electron Microsc 38: 430-440.
- Okuda Y, Hashimoto K, Nakayama M, Yamamoto H (1989) Histochemical study on androgen receptor in canine testis by an androgen labeled with colloidal gold. J Nara Med Assoc 40: 625-629.
- Thomas P (2019) Membrane androgen receptors unrelated to nuclear steroid receptors. Endocrinology 160: 772-781.
- Nishimura T, Nakano T (1997) Nuclear translocation of gold labeled-testosterone-bovine serum albumin conjugate through the nuclear double membranes in rat spermatids. Cell Struct Funct 22: 621-629.
- Tveter KJ (1970) An autoradiographic study on the localization of androgen in the prostate gland and the seminal vesicles of the male rat. Acta Endocr 63: 207-215.
- Iwamura M, Abrahamsson P-A, Benning CM, Cockett ATK, di Sant'Agnese PA (1994) Androgen receptor immunostaining and its tissue distribution in formalin-fixed, paraffin-embedded sections after microwave treatment. J Histchem Cytochem 42: 783-788.
- Ruizeveld de Winter JA, Trapman J, Vermey M, Mulder E, Zegers ND, et al. (1991) Androgen receptor expression in human tissues: An immunohistochemical study. J Histochem Cytochem 39: 927-936.
- Nishimura T, Nakano T (1999) Nuclear localization of gold labeled-hydrocortisone-bovine serum albumin conjugate injected intravenously into the hormone-target cells of rat. Cell Struct Funct 24: 227-235.
- Dym M (1988) The male reproductive system. In: Weiss L (ed) Cell and Tissue Biology. A Textbook of Histology pp 929-972. Urban & Schwarzenberg Inc. Baltimore Munich.
- Fawcett DW, Raviola E (1994) Male reproductive system. In: Bloom and Fawcett, a textbook of histology 12th edn pp 768-815. Chapman & Hall, New York.
- Nishimura T, Nakano T (2002) Vesicles in the subacrosomal space and partial diaphragms in the subacrosomal nuclear envelope of round spermatids of a rat injected intravenously with gold labeled-testosterone-bovine serum albumin conjugate: vesicular trafficking from acrosome to nucleus. Okajimas Folia Anat Jpn 79: 15-23.
- Nishimura T, Nakano T (2000) Immunocytochemical localization of bovine serum albumin (BSA) in the liver and testis of rats injected with testosterone-BSA, hydrocortisone-BSA or corticosterone-BSA. Cell Struct Funct 25: 161-169.
- Yamaizumi M, Uchida T, Okada Y, Furusawa M. (1978) Neutralization of diphtheria toxin in living cells by microinjection of antifragment A contained within resealed erythrocyte ghosts. Cell 13: 227-32
- Yamaizumi M, Uchida T, Takamatsu K, Okada Y (1982) Intracellular stability of diphtheria toxin fragment A in the presence and absence of anti-fragment A antibody. Proc Natl Acad Sci USA 79: 461-5.
- Nishioka M, Morshed SA (1999) Heterogeneity of anti-nuclear antibodies in autoimmune liver diseases. Biomed Pharmacother 53: 293-300.
- Rosner W, Hryb DJ, Khan MS, Singer CJ, Nakhla AM (1988) Are corticosteroid-binding globulin and sex hormone-binding globulin hormones? Ann NY Acad Sci 538: 137-l45.
- Kuhn RW, Green AL, Raymoure WJ, Siiteri PK (1986) Immunocytochemical localization of corticosteroid-binding globulin in rat tissues. J Endocrinol 108: 31-36.
- Nishimura T, Nakano T (2001) Nuclear localization of bovine immunoglobulin G (bIgG) in the liver of rats injected with hydrocortisone-bIgG conjugate intravenously. Okajimas Folia Anat Jpn 78: 107-114.



