Research Article
Overview of Physical and Chemical Greywater Treatment Technologies for Effective Recycling
5958
Views & Citations4958
Likes & Shares
It is predicted that about one third of the world population will face water shortage by 2025 which may cause a reduction in agricultural land and increased dissertation resulting in food shortage that leads to poverty, faming, and even wars. Thus, treating and reusing greywater can minimize the severity of this water shortage problem. Greywater is a wastewater originating from kitchen sinks, showers, baths, washing machines and dishwashers and accounts for 50-80% of the total wastewater produced in an average residence. The average production of greywater by a person is in the range of 30-225 L/day. Greywater is easy to treat and reuse safely for toilet flushing, landscape, crop irrigation. In our previous work, we examined the available biological treatment technologies for greywater treatment. In this study, we examined the available physical and chemical greywater treatment technologies. Physical treatment of greywater refers to separation of contaminants from the water by physical means such as sedimentation and filtration. Sedimentation is the process of allowing particles suspended in water to settle out under the effect of gravity forming sludge. Greywaters contain very low concentration of suspended solids and, therefore, sedimentation was not considered in this study. Filtration is a process of removing particulate matter from water by forcing the wastewater through a porous media that can be natural (sand, gravel and clay) or synthetic membranes made of cellulose acetate, cellulose nitrate, polyamide, polycarbonate, polypropylene, and polytetrafluoroethylene. The size of contaminants that can be removed from the water depends upon the size of the membrane pores. Based on pore size, membrane filtration processes for water and wastewater are divided into four classes: microfiltration, ultrafiltration, nanofiltration and reverse osmosis. There are several electrochemical treatment systems that have been used for treatment of greywaters for pollution load reduction and water reuse. The most used systems are chemical coagulation-flocculation, electrochemical coagulation, electrooxidation and photooxidation. In addition, adsorption is also used to treat greywater. This process creates a film of the adsorbate (Pollutants) on the surface of the adsorbent. However, when selecting a physical or a chemical treatment process, the characteristics of greywater must be taken into consideration.
Keywords: Water shortage, Greywater, Chemical Treatments, Physical treatments, Adsorption
INTRODUCTION
It is anticipated that about one third of the world population will face water shortage by 2025 [1]. This may cause a reduction in agricultural land and increased dissertation resulting in food shortage that leads to poverty, faming, illegal migration and even wars [2]. Thus, treating and reusing greywater can minimize the severity of this water shortage problems. Greywater is a wastewater discharge originating from kitchen sinks, showers, baths, washing machines and dishwashers [3-7] and accounts for 50-80% of the total wastewater produced in an average residence [8]. Greywater generation rates vary depending on the number of occupants, demographic, and personal habits [9,10]. Reported greywater values for different countries around the world are in the range of 30-225 L/p/d [1,7,11-21].
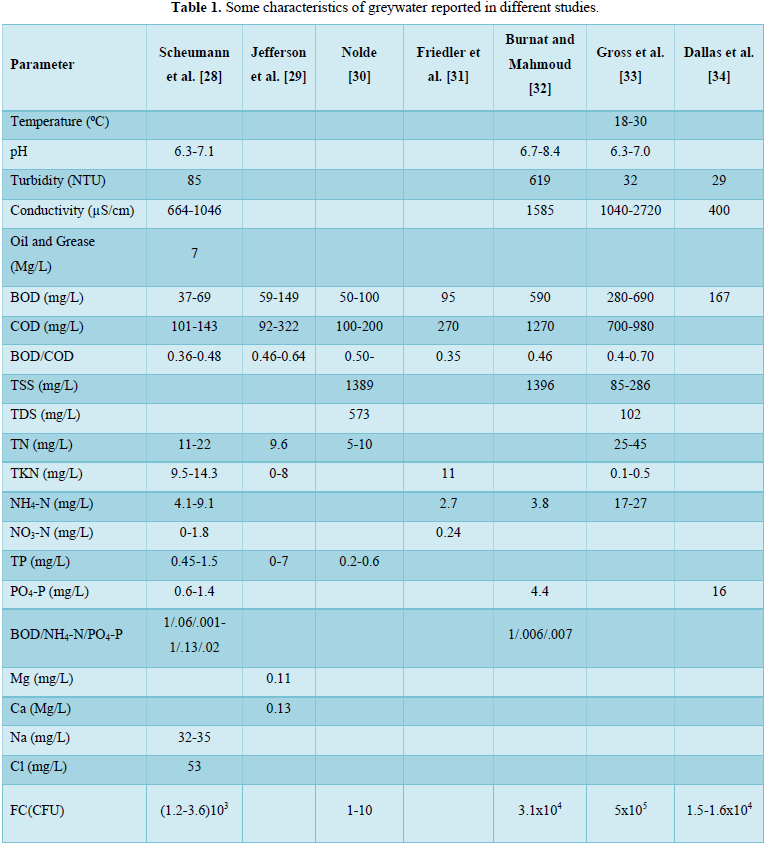
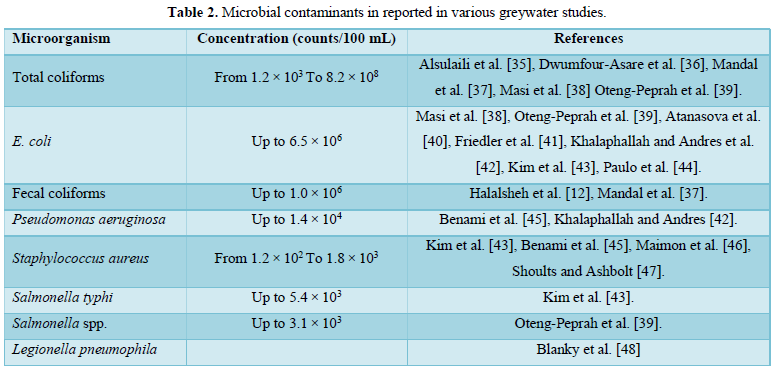
Grey water contains fewer pathogens than domestic wastewater and is generally easier to treat and reuse safely for toilet flushing, landscape, crop irrigation and other non-potable uses [9,22-26,27]. In our previous work [2], we examined the available biological treatment technologies for greywater treatment. In this study, the available physical and chemical greywater treatment technologies were reviewed. However, when selecting a physical or a chemical treatment process for greywater, the characteristics of greywater must be taken into consideration. These include pH, electric conductivity, oil and grease, surfactants, suspended solids, BOD, COD, nutrients, and microbes. Some characteristics of greywater reported in different studies are shown in Table 1 [28-34]. Microbial contaminants reported in various greywater studies are shown in Table 2 [12,35-48].


The reported pH values of greywater are in the range of 6.3-8.4 depending on the source of greywater and the level of oil and grease present [4,12,21]. The electrical conductivity of greywater is an indicative of salt (sodium, nitrates, phosphates, calcium, magnesium, chlorine and boron present in detergents and washing powders) concentration and is typically in the range of 300-1500 µS/cm [32]. The source of oil and grease in greywater are cooking grease and vegetable oil. Typical concentrations of oil and grease are in the ranges of 37-78 mg/L and 8-35 mg/L for bathroom and laundry sources, respectively [29]. Higher values (230-2000 mg/L)} were also reported [10]. The most common surfactants used in washing machines and dishwashers’ detergents and in personal cleansing products are linear alkylbenzene sulfonate, alcohol ether sulphate and alcohol ethoxylate [31,32]. The reported concentrations of surfactants in greywater are in the range of 17-60 mg/L [33]. Suspended solids concentrations of 1389-1396 mg/L were reported [30,32]. Greywater also contains BOD, COD and nutrients (phosphorous, sulfate, ammonium, sodium, and chloride). The ratio of BOD5: COD in greywater vary between 0.25 and 0.44 [3,29,36] and the average ratio of COD:NH4-N:PO4--P is 100:5:1 [14]. Typical values of nitrogen and phosphorus in greywater are within a range of 5-50 mg/L and 4-14 mg/L, respectively [4,15,37]. Greywater contains microbial contaminants that come from washing the anal area in the bath and shower [31-47].
PHYSICAL TREATMENT METHODS
Physical treatments of greywater refer to the separation of contaminants from the water by physical means such as sedimentation and filtration. Sedimentation is the process of allowing suspended particles to settle out under the effect of gravity forming sediment or sludge [8]. Since greywaters contain very low concentration of suspended solids, sedimentation will not be considered in this study. Filtration is a process of removing particulate matter from water by forcing the wastewater through a porous media that can be natural (sand, gravel and clay) or synthetic (membranes made of cellulose acetate, cellulose nitrate, polyamide, polycarbonate, polypropylene, and polytetrafluoroethylene) [49].
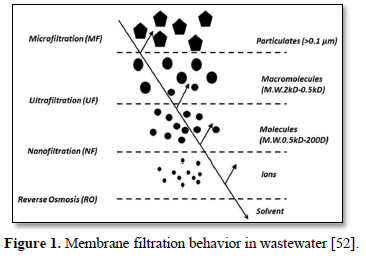
Membrane filtration is used for removal of microorganisms, particulate matter\s and organic materials which can impart color, taste and odor and react with disinfectants to form disinfection by-products [50]. The size of materials that can be removed from the water depends upon the size of the membrane pores. Based on pore size, membrane filtration processes for water and wastewater are divided into four classes: microfiltration, ultrafiltration, nanofiltration and reverse osmosis [51]. Figure 1 shows the behavior of membrane filtration in wastewater [52]. Treating and recycling greywater with granular and membrane filtration processes are technically and economically promising techniques for recycling greywater [53-54].
Granular Filtration
Granular filtration is a process where water or wastewater flows through granular material while suspended solids (sand, clay, organic particles and iron and aluminum flocs) and pathogenic microorganisms (bacteria, algae and protozoa) are removed. The granular media are made of sand, fine and course gravels, pebbles, synthetic polymers, diatomaceous earth, coal, sponge, charcoal, and cotton. Figures 2 and 3 show 2 types of granular filters made from sand and gravels [55,56].
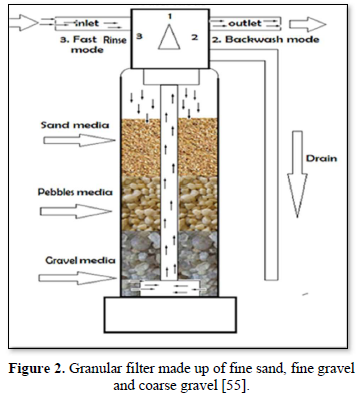
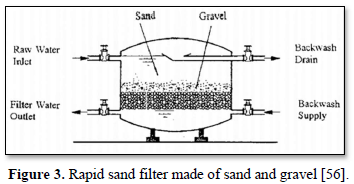



Granular filters are used in combination with sedimentation and chemical treatments. Removal efficiencies of granular filters are within the range of 90-99%. Pathogen reductions are typically >99% with pre-treatment (typically chemical coagulation) whereas with no pretreatment, 90-99% reductions of larger pathogens (helminth ova and larger protozoans) can be achieved and <90% reductions of viruses and free bacteria are achieved [57].
Sand filters are one of the oldest unit operations used in the treatment of potable water and is the most used method for the purification of effluents from wastewater treatment processes. Typical sand filter consists of predetermined column of sand and course materials. Influent is introduced to the top of the column, passes through the medium and is collected at the bottom of the column. Sand filters are typically classified in terms of their feed operation as semi continuous or continuous. Semi continuous filters can be taken offline periodically for cleaning, while continuous filter can be backwashed [58].
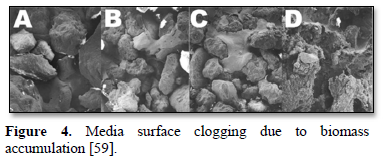
Conventional sand filters are classified as shallow bed, vertical downward flow columns of sand and/or anthracite. Since water is added to the top of these filter bed, sand filters are greatly affected by wastewater chemical and hydraulic loading rates over time. As water/wastewater containing contaminants pass through the media, crevices in the media begin to fill with particles and bioaccumulation occur (Figure 4). The accumulation of contaminants lowers the porosity of the media and over time can cause ponding on the filter bed. To ensure that granular filter beds run smoothly and maintain efficiency, filters must be regularly backwashed to loosen and remove the biofilm from the cavities in the media [59].

To backwash the sand filter, freshwater is pumped in the opposite flow of influent into the filter until the filter bed has reached full fluidization. After the filter water appears clear, the backwash cycle is discontinued, and the filter media begins to resettle. Then, the filter must go through a ripening period during which the filer media is allowed to settle and reform to its previous state [60]. However, the ripening period is influenced by many factors including porosity of filter media, depth of filter and wastewater contaminants loading rate [61].
Droste [62] reported that once the turbidity of effluent water from sand filter is below 0.2 NTU, the filter is considered online and can effectively retreat water or wastewater. However, once the turbidity of the effluent reaches 0.2 NTU, the filter should be taken offline, and backwashing commences. While most treatment operations judge sand filter run time based on effluent turbidity, some utilities use filter head loss as a need for backwash indicator. However, increased head loss can impact operation cycles and flooding of the system can occur.
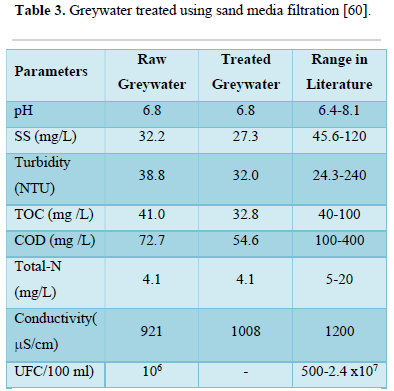
Guala et al. [60] conducted study to determine if greywater can be reused for flushing hotel toilets after treatment in sand filter. The treatment system included additional steps of osmosis rejection and pre-chlorination before entering the sand filter. Based on the results shown in Table 3, they concluded sand filters can remove greywater constituents that contribute to turbidity, COD and total suspended solids.

Droste [62] reported that typical sand filters can remove particles as small as 0.5 micrometer and harmful pathogens such as Cryptosporidium and Giardia Cysts. However, since the porosity of sand filters is limited to 0.5 micrometer many sand filters cannot remove viruses and metal ions.
Spychala et al. [63] assessed greywater volatile solid removal efficiency of a sand filter. They used 9 PVC cylinders as filter columns with medium sand as a filtering material. Samples of the sand were collected after 6, 14 and 21 d to determine deposits of volatile solids. The vertical distribution of specific deposit in the sand filters was typical for gravitationally operated sand filters and maximum specific deposit was achieved for a cumulative hydraulic load of 363.6 m. Volatile solids removal efficiency of 51-60% was achieved at relatively low cumulative hydraulic load. The average removal efficiency of COD was 26.8%.
Albalawneh et al. [64] evaluated the efficiency of a granular filtration system for greywater treatment under arid and semi-arid conditions. Six filtration systems were monitored for 13 months, each served a single rural home greywater treatment system. Volcanic tuff media were used as the filtration media in 3 systems while gravels were used as the filtration media the other three. When using gravel media, the BOD, COD and TSS removal efficiencies were 73%, 65%, and 85%, respectively. When using volcanic tuff media, the removal efficiencies for BOD, COD and TSS were 49%, 51%, and 76%, respectively. In addition, there was a significant increase in the electrical conductivity, pH, K+, Mg2+, Cl−, Na+, SO42−, HCO3−, sodium adsorption ratio, and exchangeable sodium percentage in the effluents from the volcanic tuff media systems.
Abdel-Shafy et al. [65] evaluated different designs of sand filter as a secondary treatment step in the treatment of the greywater primary sedimentation effluent. Gravel filter down flow (GFDF), gravel filter up flow (GFUF), sand filter down flow (SFDF), gravel filter followed by sand filter (GFSF), and horizontal flow sand filter (HFSF) were evaluated. GFDF, GFUF, and SFDF were operated with an influent flow rate of 173 m3/m2/d, while GFSF and HFSF were operated at influent flow rate of 86.5 m3/m2/d. The quality of final effluent from the GFSF and HFSF complied with the National Regulatory Standards for Treated Effluent Reuse in Irrigation. The residual concentrations of COD, BOD5, and TSS were 43, 16, and 7.5 mg/L for GFSF and 40, 17, and 9 mg/L for HFSF, respectively.
Jaramillo et al. [66] stated that the use of activated carbon filter has been proven to be effective in reducing heavy metals and hydrophobic organic compounds but ineffective in removal of soluble components such as dissolved organic compounds. They indicated that removal of heavy metals using activated carbon depended on: (a) properties of the activated carbon, (b) amount of surface oxygen complexes, (c) properties of metal species as solubility, ion size and ability to interact with carbon, (d) the solution pH, (e) ionic strength and (f) presence of other solutes that could compete with absorption sites of the activated carbon.
Microfiltration
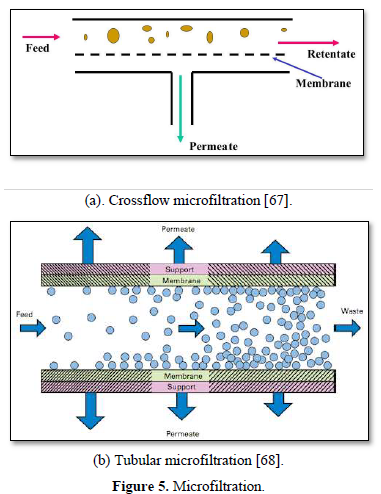
Microfiltration is a low pressure (100-400 kPa) physical separation process where a contaminated fluid is passed through a special membrane with a porosity between 0.1 to 10 μm. to separate microorganisms (Giardia lamblia and Crypotosporidium cysts, algae, and some bacterial species) and suspended particles from the liquid stream, but it does not remove virus and dissolved contaminants. Microfiltration filters are made from organic materials (polymer-based membranes) and inorganic materials (ceramic or stainless steel). Microfiltration has been used in water treatment, industrial and municipal wastewater treatment and in the dairy and food processing industry [67]. The advantages of microfiltration are limiting the concentrations and number of chemicals that are applied during water treatment and removal of natural and synthetic organic matter that cause fouling [51].
Microfiltration can be used alone as shown in Figure 5 [67,68] or in combination with biological process (membrane bioreactor) as shown in Figure 6 [69,70]. In the case of membrane bioreactor, the membranes are either submerged directly in the bioreactor or kept outside the bioreactor. The membrane bioreactors are economically attractive, compact, trouble-free operations and have options for water reuse with fast delivery time [71].
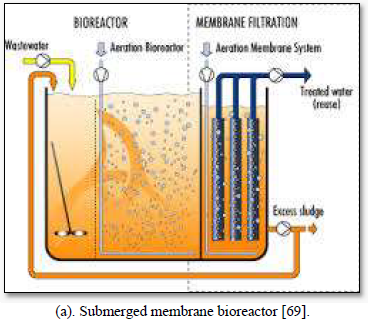
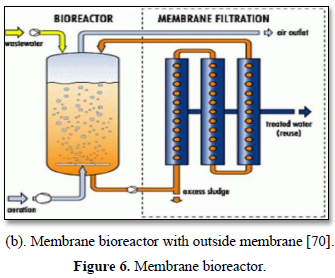



Bhattacharya et al. [72] investigated the performance of a low-cost ceramic membrane for treatment of graywater with high concentration of organics. They evaluated the efficiencies of microfiltration (MF) and ultrafiltration (UF) processes individually and as a two-stage treatment (MF followed by UF). About 73-90% COD reduction was achieved by UF in single-stage after 30 min of filtration with operating pressure of 2 bar, whereas a COD reduction of 84-94% was achieved by the two-stage treatment.
Kim et al. [43] treated graywater through a microfiltration membrane and an oxidation process, and evaluated the removal efficiencies of color, turbidity, COD, suspended solids, E. coli, total coliform, Salmonella and Staphylococcus. The pH of the treated graywater was in the range of 7-7.7. The removal efficiency of the microfiltration was 98% for color, 99% for turbidity, 99% for COD, 99% for suspended solids and 30% for E. coli, total coliform, Salmonella and Staphylococcus. Following the membrane filtration process, the removal efficiency of the oxidation process was 100% for color, 99% for turbidity, 99% for COD, 99% for suspended solids and 100% for E. coli, total coliform, Salmonella and Staphylococcus. The treated graywater was reused for firefighting, watering plants, flushing toilets and car washing.
de Oliveiraa et al. [73] investigated the use of a dual membrane process for greywater treatment (microfiltration membrane followed by a reverse osmosis process). The microfiltration pretreatment tolerated unfavorable variations in greywater and presented high removal efficiencies of apparent color, turbidity, and suspended particles thereby allowing the reverse osmosis membrane system to operate at a higher permeate flux and lower frequency of chemical cleaning. The system achieved 90% removals of turbidity, apparent color, total suspended solids, linear alkylbenzene sulfonate, and organic matter.
Manoucheri and Kargari [74] treated domestic laundry wastewater through a cross membrane filtration process using a mixed cellulose ester (MCE) microfiltration membrane with 0.22 μm pore size. The effect of trans-membrane pressure and feed flow rate on permeate flux, rejection characteristics, membrane fouling and the formed resistances against permeation that caused flux decline (including membrane, cake layer, reversible and irreversible resistances) were studied. The results indicated that changes in trans-membrane pressure and feed flow rate affected the permeate flux and membrane rejection performance. The overall resistance increased with an increase in trans-membrane pressure and decreased with an increase in feed flow rate. The highest removal efficiency (93.9, 90.8 and 98.7% for BOD, COD, TSS and turbidity, respectively) was obtained at a trans-membrane pressure of 1 bar and feed flow rate of 44 L/h.
Ahn and Song [75] evaluated the applicability of microfiltration in treating domestic wastewater for reuse. They used a hollow fiber microfiltration membrane with a pore size of 0.1 μm and effective surface area was 20 m2. The maximum capacity of the system was 10 m3/d and the system could be operated for 120 days without cleaning. The bi-directional agitation in the membrane tank significantly reduced fouling. Chemical cleaning was effective in the recovery of permeate flux and resistance. However, fouling slowly progressed despite chemical cleaning. The particles in the membrane tank were transformed into smaller size by the agitator induced shear force. About 60% of dissolved TOC removal was attributed to biodegradation, indicating its important role. Suspended solids and colloidal matter were removed by the sieve mechanism of the membrane. The effluent had lower than 30 mg/L of COD, 10 mg/L of BOD, 10 mg/L of TOC, 1 NTU of turbidity and 2 mg/L of SS, respectively. The effluent quality satisfied the standard for wastewater reuse.
Ultrafiltration
Ultrafiltration is a pressure-driven physical separation process in which a hydrostatic pressure forces a liquid against a semi permeable membrane to produce water with very high purity. An ultrafiltration membrane has a pore size of about 0.01-0.02 μm which can remove large particles, most microorganisms (bacteria, protozoa, algae and virus) and some natural minerals (divalent ions), but cannot remove dissolved substances. Most ultrafiltration membranes use polymeric materials (polysulfone, polypropylene, polyvinylidene fluoride, polyacrylonitrile, cellulose acetate, polylactic acid), but ceramic membranes are used for high temperature applications [49,76].
Ultrafiltration is frequently used to pre-treat surface water, seawater, carwash wastewater and biologically treated municipal water upstream. Figure 7 shows flat and tubular ultrafiltration units [77,78]. The primary advantages of ultrafiltration process include no need for chemicals (coagulants, flocculants, disinfectants, pH adjustment), constant quality of the treated water (Removal of particles and microbes), compactness of process and simplicity of automation [79,80]. However, fouling can cause difficulties in using ultrafiltration membrane technology for water and wastewater treatment [51-54].
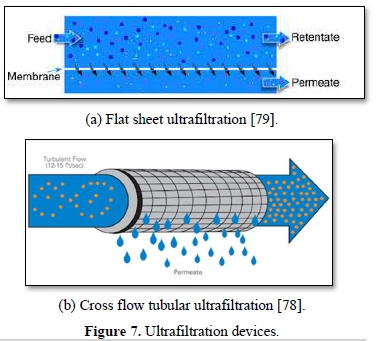

Bhattacharya et al. [72] evaluated the efficiency of ultrafiltration (UF) process individually and with microfiltration (MF). Reduction in COD in the permeate stream and permeate flux were observed. About 73-90% COD reduction was achieved in the single-stage UF at 30 min of filtration with operating pressure of 2 bar. The combined two stage MF-UF treatment achieved 84-94% COD reduction and the permeate quality complied with the discharge standards for agricultural reuse of treated water.
Li et al. [80] evaluated a decentralized greywater treatment system that used a submerged spiral-wound ultrafiltration (UF) membrane module. The UF membrane filtration system was able to maintain a permeate flux between 6 and 10 L/m2/h. The TOC was reduced from 161 to 28.6 mg/L (83.4% removal efficiency). The soluble nutrients (ammonia and phosphorus) passed through the UF membrane and the total nitrogen and total phosphorus in the permeate were 16.7 and 6.7 mg/L respectively. The permeate was free of suspended solids, had a turbidity below 1 NTU, and can be used after disinfection for irrigation of gardens and agricultural lands or for toilet flushing.
Kaminska and Marszalek [81] treated greywater in a 3L sequential biological reactor (SBR) operated in a 24 h cycle followed by a crossflow ultrafiltration system. The addition of ultrafiltration provided high-quality water with very low COD (5.8-18.1 mg/L), TOC (0.47-2.19 mg/L), absorbance UV254 (0.015-0.048 1/cm), color (10-29 mgPt/L), nitrate (0.18-0.56 mg/L), phosphate (0.9-2.1 mg/L), ammonium (0.03-0.11 mg/L), and total nitrogen (3.3-4.7 mg/L) as well as lack of E. coli and enterococci. The values of these quality parameters did not exceed permissible values for treated wastewater discharged to agricultural soil and water bodies.
Schafer et al. [82] investigated the performance of submerged and direct ultrafiltration (UF) of synthetic greywater with regards to bisphenol A (BPA)) and fouling. The synthetic greywater consisted of inorganic particulates (kaolin), organic fibers (cellulose), protein (casein), the surfactant sodium dodecyl sulphate (SDS), humic acid (HA), calcium, sodium chloride electrolyte and sodium bicarbonate buffer. The results indicated that UF removed 30-45% of BPA. Humic acid and calcium were the main contributors to fouling and affected BPA retention. Fouling increased with the increase in HA concentration.
Sumish et al. [83] investigated the treatment of laundry wastewater using hydrophilic polyvinylpyrollidone (PVP) modified polyethersulfone (PES) ultrafiltration membranes. The performances of PES/PVP membranes were assessed using commercial PES ultrafiltration membrane with 10 kDa. The influence of transmembrane pressure (TMP) and stirring speed on the wastewater flux were assessed. A higher permeate flux of 55.2 L/m2h was obtained for PES membrane with high concentration of PVP at TMP of 500 kPa and stirring speed of 750 rpm. The PES membrane with 10% of PVP had higher permeate flux, faster flux recovery and less fouling when compared with other membranes. Higher COD (88%) and TDS (82%) were observed for modified membranes due to the improved surface property.
Lodge et al. [84] described the fouling of a bench-scale, hollow-fiber, pressurized ultrafiltration (UF) membrane in terms of simple cake resistance theory. The fouling properties of biologically pretreated greywater matrix were compared with reference to measured values of specific cake resistance and cake compressibility. The results obtained from constant pressure runs (between 125 mbar and 1 bar), constant flux runs (between 30 and 90 L/m2/h, and constant flux runs with periodic backwashes were compared. Specific cake resistance values were between 1015.5 and 1016.5 m/kg throughout. It was not possible to distinguish between the values of specific cake resistance for the two matrices which, suggests that the bulk properties of the two cakes were similar. The cakes were highly compressible, and the specific cake resistance increased by an order of magnitude as the pressure was increased from 125 mbar to 1 bar. The calculated compressibility factor was 0.65 for both matrices. Specific cake resistance calculated over short periods between backwashes was found to increase much more rapidly with pressure than for non-backwashed runs.
Vani et al. [85] reported on the indigenously synthesized titanium dioxide (TiO2) NPs and their amalgamation of 1-5% (by weight) into polyphenyl sulfone (PPSU) mixed matrix membranes (MMMs) for greywater purification by ultrafiltration (UF) process. The 3% of TiO2-loaded MMMs were optimized. Based on the results obtained from scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), 40.20% of porosity and 31.14 nm pore size by 49.4 contact angle with 16.97 MPa tensile strength, 10.71% of elongation-at-break, 92.59 L/m2 h permeation and 99.43% of bovine serum albumin (BSA) rejection with an 89.50% of flux recovery. The optimized membrane exhibited a low fouling tendency with a minimal flux declination. From the experimental results, the PPSU-T3 achieved a maximum flux of 45.63 L/m2 h, with 80% water recovery and the removal of 95.54% turbidity, 94.20% color, 95.54% TSS and complete E. coli bacterial eradication at an applied pressure of 2 bar.
Bahaei et al. [86] investigated a new hybrid system using multi-layer slow sand filter (MSSF), micro filter (MF) and ultrafilter (UF) for removal of the COD, linear alkylbenzene sulfate (LAS), TSS and turbidity from the greywater at different organic loading (3.15-19.28 gCOD/ (L.d) in a laboratory scale over a period of 157 days. The scanning electron microscopy (SEM) images confirmed that biofilm grew appropriately in the media. The best removal efficiencies of MSSF-MF-UF hybrid system for COD, LAS, TSS, and turbidity were 98.22, 99.97, 99.99, and 99.98 %t, respectively. Increasing the organic loading rate decreased the removal efficiency levels of COD, LAS, TSS, and turbidity.
Nghiema et al. [87] stated that most research on the utilization of membrane filtration for treatment of various wastewaters have shown that UF is the preferred method. They also indicated that the biggest contributors to membrane fouling of typical greywater were the organic matter and calcium present as these can cause ‘caking’ and precipitation on the membrane surface. Calcium concentrations greater than 3 mM causes significant fouling of the membrane. Also, acids and bases can cause significant damage to most membranes.
Nanofiltration
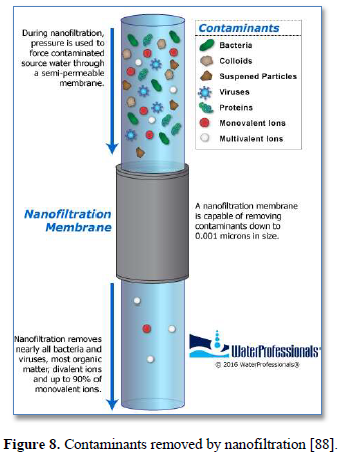
Nanofiltration membrane has a pore size of 0.001 μm and can remove most organic molecules, viruses, cysts, bacteria, a wide range of salts and humic materials as shown in Figure 8 [88]. Pushing water through smaller membrane pores requires higher operation pressure of 600-1000 kPa [89]. Nanofiltration is used to remove dissolved contaminants and viruses from surface and ground water [90,91] and various wastewaters [88]. It provides high rejection of multivalent ions such as calcium and low rejection of monovalent ions such as chloride. The nanofiltration membrane rejects various salts in proportion to their molecular size, the order of rejection is Na2SO4>CaCl2>NaCl [89-91].
The advantages of nanofiltration include lower discharge volumes, lower retentate concentrations for low salt concentrations, reduction of salt and dissolved matter contents in brackish water, reduction in heavy metals, reduction of nitrates and sulphates and reduction in color, tannins and turbidity. The disadvantages are high energy consumption (0.3 to 1 kWh/m³), prefiltration is needed forsome heavily polluted waters, limited retention for salts and univalent ions and high cost of membranes [88].
Hourlier et al. [92] conducted a study aimed at selecting a tubular nanofiltration membrane to treat greywater in buildings for reuse. Three membranes (AFC30, AFC40 and AFC80) having distinct molecular weight cut-offs were used to treat synthetic greywater at 25°C and two transmembrane pressures (20 and 35 bar). The best results were obtained with AFC80 membrane at 35 bars and a flux was 50 L/ m² h. COD and anionic surfactants retentions of 95% were observed and no Enterococcus was detected in the permeate. The performance of AFC80 was then evaluated on a real greywater and the flux and retention were similar to those observed with synthetic greywater.

Ramona et al. [93] studied the potential of nanofiltration membranes (200 Da MWCO) for treating low load graywater (collected from public showers of a sports center) for onsite reuse. The graywater had 29.8 mg/L TSS and 170.3 mg/L COD. Particle distribution analysis showed that colloidal size particles were the dominant fraction, while the fewer larger particles make up most of the particle volume (mean particle diameter was 0.1 μm). Permeate produced by nanofiltration was of high quality with high rejection of soluble organic matter (>90%) and ionic species (50%).
Guilbaud et al. [94] investigated the feasibility of implementing nanofiltration process on board a ship to treat laundry greywater and recycle 80% of treated water for washing of machines. The laundry greywater had a pH of 7, a COD of 1300 mg/L and a TSS of 80 mg/L. A direct nanofiltration process (without pre-treatment) using a tubular PCI-AFC80 membrane at a pressure of 35 bars, a temperature of 25°C, and a volume-reduction-factor of 5 produced a permeate free of microorganisms and SS and with only 48 mg/L COD and 7 mg/L TOC.
Guilbaud et al. [95] investigated the influence of nanofiltration operating conditions on COD rejection rates and permeate fluxes. The pH and temperature of greywater and the transmembrane pressure were fixed to 7 or 9, 25 or 40°C and 35 or 40 bars, respectively. The AFC80 membranes showed different COD rejection rates whereas permeate fluxes were quasi similar when the same greywater (pH 7) was nonfiltered at 35 bars and 25°C. Amongst all the tested operating conditions, the nanofiltration of greywater by AFC80 membrane at pH 7, 35bars and 25°C achieved the highest COD rejection rate (93%) but the best permeate flux (85.5 L/h/m2) was obtained at 40 bar and 40°C. Increases in temperature (above 25°C) or pressure (above 35 bar) lead to a drop in the COD rejection rate.
Van der Bruggen et al. [96] measured the water flux for two nanofiltration membranes (UTC-20 and NF70) using aqueous solutions of 11 organic compounds of different concentrations. The flux of aqueous solutions declined by more than 50% for solutions containing less than 1 g/L of some organic compounds as compared to the pure water flux. The flux declined as a function of the concentration of the organic compound and was related to adsorption on the membrane material.
Reverse Osmosis
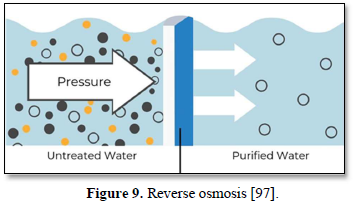
Reverse osmosis is the tightest membrane separation process in which water is separated from dissolved salts by filtering through a semipermeable membrane at a pressure greater than osmotic pressure as shown in Figure 9 [97]. Reverse osmosis membrane has a pore size around 0.0001 μm which removes all organic molecules, pesticides, cysts, bacteria, viruses and all minerals including monovalent ions. Reverse osmosis allows removal of dissolved individual ions (sodium, chlorine, calcium, and magnesium), metal ions, minerals and organics. It produces water that meets most demanding specifications [98].
The advantages of reverse osmosis are: (a) removal of nearly all contaminant ions and most dissolved non-ions, (b) insensitive to flow and dissolved solids concentration, (c) suitable for small systems with a high degree of seasonal fluctuation in water demand, operates immediately without break-in period, low effluent concentration of dissolved solids, (d) removes bacteria and viruses, and (e) simplicity of operational and automation, (f) require minimum operator attention and (g) suitable for small system applications.

Some of the limitations of reverse osmosis are: (a) high capital and operating costs, (b) managing the effluent (brine solution) is a potential problem, (c) high level of pretreatment is required in some cases, (d) membrane fouling and (e) clean water produced for use is only 25-50 % of the feed [50,53,99].
Singh et al. [100] investigated the treatment of greywater containing detergent and salinity (∼2,000 ppm) by RO. Reusable water with 8-C24 primary/secondary ethoxylates based detergent. The membrane selectivity for salt rejection was slightly higher for the feed with detergent than that of the detergent-free feed because the surface-active agent of the detergent altered the surface potential of the membrane.
de Oliveira et al. [73] used a dual membrane process for greywater treatment made of microfiltration (MF) followed by a reverse osmosis (RO). The MF pretreatment tolerated variations in feed greywater and achieved high removals of apparent color, turbidity and suspended solids while the RO membrane treatment operated at a higher permeate flux and lower frequency of chemical cleaning. The dual process achieved 90% removals of turbidity, apparent color, total suspended solids, linear alkylbenzene sulfonate, and organic matter.
Senthilmurugan and Venkatesh [101] reported on membrane-based greywater treatment for surfactant recovery and water reuse. The greywater from washing machine discharge had turbidity and contained 45 ppm surfactant and 720 ppm total dissolved solids. It was processed first through a polymeric ultrafiltration (UF) membrane to remove the turbidity and then through reverse osmosis (RO) membrane for surfactant recovery. The surfactant trapped inside the RO spiral wound membrane module was recovered through various membrane physical regeneration techniques (backwashing, simultaneous backwash–back-flush and ozone back-flush). The backwash-back-flush was found to be the most effective process for surfactant recovery. The surfactant recovery was affected by feed detergent concentration, backwash pressure, backwash temperature and back-flush flow rate. By implementing optimal process conditions, the integrated UF-RO system produced 300 L of reusable pure water and 80 L of concentrated detergent solution and 20 L of turbid water while treating 400 L of greywater. The maximum surfactant recovery was 82 %.
Boddu et al. [102] treated graywater using RO filtration after microfiltration treatment. The results showed that microfiltration in combination with RO treatment can achieve adequate reduction of COD but at the cost of progressively decreasing water flux through the RO membrane. Flux decline during microfiltration was attributed to the presence of colloids (>0.2 μm). Microfiltration maintained the water permeation capacity, but the permeate COD level was higher.
Reang and Nath [103] used a combination of spiral wound ultrafiltration and spiral wound reverse-osmosis membranes to treat greywater from washing machine to recover the surfactant solution and water. The dirt and dust particles were separated from the greywater using the ultrafiltration process and later the surfactant solution and water were separated from the mixture using the reverse osmosis membrane. Several backwash and backwash-backflush methods were used to decrease the fouling problem and increase the membrane performance. The ultrafiltration membrane decreased the turbidity of the mixture but allowed the surfactant solution to pass. The reverse osmosis membrane decreases both the turbidity and TDS of the solution.
Engin et al. [104] used a compact household RO unit to determine the feasibility of treating greywater reuse. The results showed COD and BOD removal rates around 80%. The conductivity of permeate was reduced to 15 mS/cm within 15 min. The permeate obtained was free of suspended solids and had an excellent physical appearance.
DiPaolo [105] stated that RO systems use a pump to increase the pressure on the feed side of the equipment and forces the water across and through a semipermeable membrane, a process that results in approximately 96-99 % total dissolved solids removal. A correctly functioning RO system can effectively reduce levels of salt, hardness and minerals, and provides a pure mineral-free water.
ELECTROCHEMICAL TREATMENT METHODS
There are several electrochemical treatment systems that have been used for treatment of greywaters for pollution load reduction and water reuse. These are chemical coagulation-flocculation, electrochemical coagulation, electrooxidation and photooxidation.
Chemical Coagulation-Flocculation
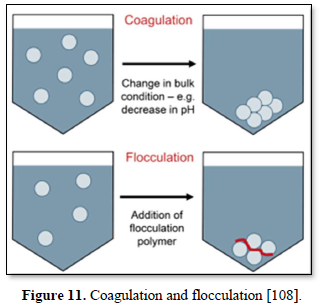
Coagulation and flocculation processes are used for treatment of a variety of wastewaters containing colloids and metal ions. In coagulation, particles aggregate with themselves by a change in pH while in flocculation, particles aggregate using polymers to bind them together [10,107].
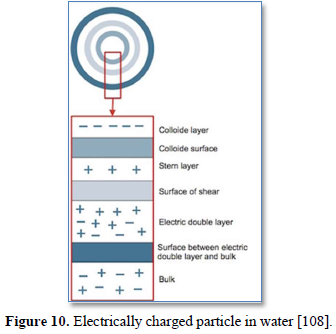
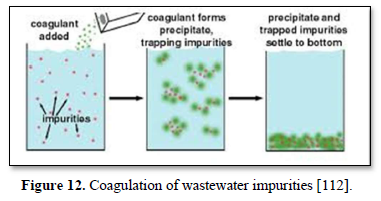
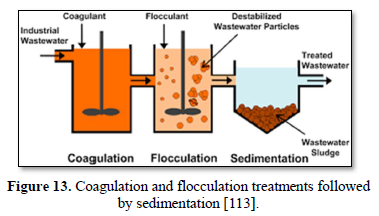
Particles in water are electrically charged as shown in Figure 10 [108]. The area nearest to the particle is divided into two layers: (a) the first layer is the closest to the electrically charged particle and in which counter ions gather to create the stern layer and (b) the next layer is composed of both counter-ions and co-ions, but with a surplus of counter-ions. The bulk (the surrounding water) has an equal distribution of counter-ions and co-ions [109-111]. In coagulation, the two layers around the particle cause it to be stable in the water. When the conditions within the water are changed by a change in pH or conductivity, the number of ions in the water changes and affects the amounts of ions in the two layers, thereby affecting the stability of the particles and force them to settle as shown in Figure 11-top [108]. In flocculation, electrically charged particles precipitate by flocculation polymers with charged sites. By using a polymer with the opposite charge to that of the particles to be flocculated, the particles will bound to the polymer making larger particles that cannot stay suspended as shown in Figure 11-bottom [108]. When particles are precipitated from the solution (Figure 12), further filtration treatment is necessary to obtain the desired water quality [112]. Figure 13 shows a system having the processes of coagulation, flocculation, and sedimentation [113].
Polymers are a large range of natural and synthetic water-soluble macromolecular compounds that can enhance flocculation of the water constituents. Natural polymers have long been used as flocculants because they are free of toxins, biodegradable and often locally available. However, the use of synthetic polymers is more widespread because they are more effective and easier to control. Synthetic polymers are available in various forms including solutions, powders, beads, oils and water-based emulsions. One problem with synthetic polymers relates to potential toxicity issues arising from unreacted monomer residual [107,109].
The commonly used metal coagulants fall into two categories: those based on aluminum (Al) and those based on iron (Fe). The Al coagulants include aluminum sulfate, aluminum chloride, and sodium aluminate. Pre-hydrolyzed aluminum forms include aluminum chlorohydrate, poly-aluminum chloride, polyaluminum sulfate chloride, polyaluminum silicate chloride and forms of polyaluminum chloride with organic polymers. The Fe coagulants include ferric sulfate, ferrous sulfate, ferric chloride, and ferric chloride sulfate. Pre-hydrolyzed iron forms include polyferric sulfate and ferric salts with polymers and also polymerized aluminum-iron blends. The Al and Fe coagulants are effective because of their ability to form multi-charged polynuclear complexes with enhanced adsorption characteristics.




The nature of the complexes formed can be controlled by the pH of the system. When Al and Fe coagulants are added to water, the metal-ions hydrolyze rapidly forming a series of metal hydrolysis species. Rapid mixing, pH, temperature and the coagulant dosage determine which hydrolysis species is effective. Usually, lower dosages of pre-hydrolyzed coagulants are required to achieve treatment goals and fewer chemical residuals are produced resulting in lower final water total dissolved solids [108-110].
Bolto et al. [112] reported that organic polymeric flocculants were used in water purification as coagulant aids or floc builders to replace inorganic coagulants like alum, iron salts and lime. The increased use of cationic polyelectrolytes as primary coagulants instead of inorganic is due to their faster processing, lower content of insoluble solids to handle (by sedimentation, filtration, flocculation or biological conversion) and smaller volume.
Oldegard [113] showed that coagulation with metal salts was very efficient but can lead to excessive sludge production and demonstrated how the use of cationic polymers can reduce sludge production considerably.
Jekel and Heinzmann [114] reported that coagulation and flocculation can be used for removal of dissolved solids and suspended particles including pathogens (Giardia and Cryptosporidium, a parasite that cases diarrhea), virus, arsenic, phosphorus, and fluoride. They showed that the efficiency of the coagulation-flocculation process was dependent on the type of coagulant, coagulant dosage, coagulant feed concentration, type and dosage of chemical additives, sequence of chemical addition, pH and time lag between dosing points, intensity and duration of mixing, velocity gradients applied during flocculation stage, flocculator retention time, type of stirring device and flocculator geometry.
Pidou et al. [115] investigated the potential of coagulation and magnetic ion exchange resin processes for the treatment of low and high strength greywaters. The results revealed that magnetic ion exchange resin and coagulation were suitable treatment solutions for low strength greywater but were unable to achieve the required level of treatment for the reuse of the high strength greywaters The effectiveness of coagulants was greatly dependent upon contact time, pH, temperature, the dose of the coagulant, and mixing speed.
De Feo et al. [116] conducted several tests to determine the optimal pH and dose of various coagulants (aluminum sulfate, Ecofloc CP, PAC, ferric chloride, sodium aluminate) used in both wastewater and drinking water treatments. The coagulants doses were 10, 20, 30, 40 and 50 mg/L. The coagulants were rapidly mixed for 5 min at 120 rpm, followed by slow mixing for 5 min at 30 rpm and then allowed to flocculate for 15 min. Most inorganic salts used as coagulants have a high removal rate (60-80%) of natural organic molecules.
Pidou et al. [115] stated that natural organic molecules are typically comprised of anionic hydrophobic humic and fulvic acids that are easily removed by typical coagulants. On the other hand, typical greywater organics are mainly hydrophilic which are difficult to form larger particle groups due to their lower molecular weight and composition.
Thompson et al. [117] compared wood-based biochar (a low-cost sorbent) to activated carbon in removing dissolved organic carbon (DOC) from graywater and evaluated the impact of pretreatments by coagulation and biodegradation on the final greywater quality. The results indicated that the biochar was effective for graywater treatment, but the activated carbon removed more dissolved organic carbon. However, graywater regulations could not be met by sorption alone but could be met with pre-treatment before sorption.
Vinitha et al. [118] treated greywater by chemical coagulation using polyaluminium chloride. They conducted 140 jar tests on greywater of varying characteristics to determine the optimum coagulant dosage. The average removal efficiencies of turbidity, COD and TSS were 91, 73 and 83% using alum and 93, 74 and 89%, respectively.
Ghaitidak and Yadav [119] investigated the effect of coagulation treatment using alum on greywater characteristics under variable pH conditions (8.5, 7.5, 6.5 and 5.5). Turbidity removal was above 88%, BOD reduction was 53-77%, and Escherichia coli removal was 95-99% under the pH conditions examined. The alum-treated greywater satisfied most of the reuse standards for the discharge of effluents into land for irrigation and industrial cooling in India.
Chitra and Muruganandam [120] evaluated the coagulating efficiencies of various natural coagulants for greywater treatment. Powdered coagulants obtained from tamarind seeds, moringa oleifera, banana peels and fly ash were compared with the conventional commercial coagulant alum. The results showed that turbidity removal efficiency for alum, tamarind seeds, moringa oleifera, banana peels and fly ash were 96.49, 61.33%, 85.75%, 90.42% and 94.27%, respectively.
Alharbi et al. [121] used alum coagulation followed by batch and continuous activated carbon adsorption to treat ablution greywater (greywater produced at mosques from cleaning certain parts of the body before performing prayers) for recycling. In coagulation experiments, optimal removals of turbidity (95.8%), COD (31.6%) and BOD (50.0%) were achieved at an alum dose of 20 mg/L. Using the activated carbon adsorption further enhanced the overall removal efficiencies for COD by 70.8% and BOD by 57.2% at 20 min adsorption equilibrium time with 0.2 g/L of optimal activated carbon dose. Maximum adsorption capacities were 175 mg/g COD and 88 mg/g BOD. Continuous treatment of greywater resulted in residual turbidity less than 1 NTU and COD and BOD values less than 10 mg/L. The treated greywater was suitable for irrigation, toilet flushing, and firefighting.
Bielski and Giermek [122] treated greywater from a small household using coagulation and oxidation with hydrogen peroxide and ultra-violet radiation (H2O2/UV). Doses of coagulant (poly-aluminum chloride) ranging from 12.5 to 200 g Al3+/m3 were used. The results indicated that coagulant doses within the range of 25-100 g Al3+/m3 produced the best removal of turbidity, COD and TOC. The lowest concentration of residual aluminum (about 0.1 g Al3+/m3) was found during coagulation with the doses of 25-50 g Al3+/m3. The lowest turbidity (1 NTU) was observed with the doses of 50-100 g Al3+/m3.
Electrocoagulation
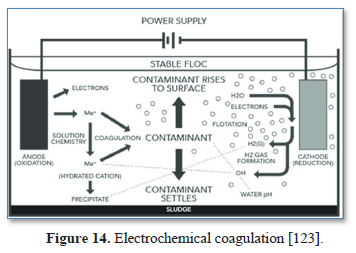
Electrocoagulation (EC) is an electrochemical process that simultaneously removes heavy metals, suspended solids, emulsified organics and many other contaminants from water and wastewater using electricity instead of chemicals. The EC device operates continuously and performs automated coagulation, flocculation, flotation, separation, and removal of contaminants in a single enclosed reactor as shown in Figures 14 [123]. No polymer addition, settling or flotation tanks or filters are required [124].
The advantages of EC are: (a) it removes any size of suspended solids including the destructive >30 µm particles that pose environmental hazard (b) it requires no filters, no daily maintenance and no additives, (c) it removes suspended solids, oil, grease and heavy metals, (d) it requires simple equipment and is easy to operate (e) it results in clear, colorless and odorless water with low total dissolved solids content, (f) the formed sludge tends to be settable and easy to de-water, (g) formed flocs tend to be much larger, contain less bound water, acid- resistant, more stable, and can be separated faster by filtration, (h) it has little if any impact on sodium and potassium ions in solution and (i) the gas bubbles produced during electrolysis can conveniently carry the pollutants to the top of the solution where it can be more easily concentrated, collected, and removed by skimmer [124,125].

EC technology has been increasingly used worldwide for treatment of wastewater from metal processing industries, mining industry, pulp and paper industry, wastewater containing foodstuff and oil, wastewater containing dyes and synthetic detergent, wastewater from public transit, marinas, chemical and mechanical polishing industries, land fill leachates and carwash wastewater [123,124,126-133].
An et al. [123] used a simple and efficient electrocoagulation treatment method for the removal of oil from wastewater. The process involved the electro-dissolution of sacrificial anodes and formation of hydroxo-metal products as coagulants, while simultaneously producing hydrogen at the cathode to facilitate the removal of pollutants by flotation. The electrocoagulation treatment was effective in destabilizing oil-in-water emulsions by neutralizing charges and bonding oil to generated flocs and hydrogen bubbles.
Ansari and Shrikhande [134] reviewed the recent electrocoagulation studies on greywater treatment, examined electrode arrangement, cell design, treatment facilities and economic concern. They also suggested recommendations to boost the technology to maximize resource conservation.
Barzegar et al. [135] applied an electrocoagulation/ozone process for the removal of COD and TOC from greywater. The results showed that 85% of COD and 70% of TOC were removed during 60 min electrolysis time at a pH of 7.0 and a current density of 15 mA/cm2, using 47.4 mg/L ozone. EC with Fe electrode exhibited high catalytic activity for ozone activation in contrast with Al electrode. Ozone was superior compared to other chemical oxidant such as peroxydisulfate, peroxymonosulfate and hydrogen peroxide.
Barisci and Turkay [136] investigated the treatment of greywater by an electrocoagulation process and evaluated eight different electrode combinations and the effect of current density, initial pH and supporting electrolyte concentration on the treatment efficiency. The highest COD removal was obtained with the Al–Fe–Fe–Al hybrid combination and a current density of 1 mA/cm2. The original pH value (7.62) was found to be the most suitable condition and the supporting electrolyte concentration did not affect the removal efficiencies.
Sahu et al. [137] reviewed the mechanism, affecting factors, process, and application of the electrocoagulation process. They found the electrocoagulation process to be widely accepted over other physicochemical processes due to its ability to treat large volume and its low cost. The electrocoagulation process was able to remove pollutants such as pesticides, radionuclides and harmful microorganisms.
Karichappan et al. [138] used electrocoagulation process to treat grey wastewater under different pH values (4-8), current densities (10-30 mA/cm2), electrode distances (4-6 cm) and electrolysis times (5-25 min) using stainless steel anode in batch mode. The process variables had significant effects on the efficiency of the electrocoagulation treatment process. The optimal operating conditions were initial pH of 7, current density of 20 mA/cm2, electrode distance of 5 cm and electrolysis time of 20 min.
Electrooxidation Treatment
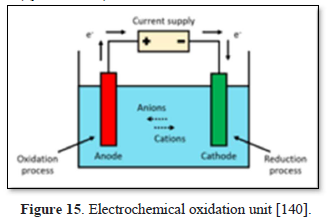
Oxidation is the loss of electrons whereas reduction is the acquisition of electrons. The species being oxidized is known as the reducing agent or reductant, and the species being reduced is called the oxidizing agent or oxidant. Electrooxidation (EO) is an advanced oxidation process used for wastewater treatment [139]. The most general layout comprises two electrodes (anode and cathode) connected to a power source as shown in Figure 15 [140]. When an energy input and sufficient supporting electrolyte are provided to the system, strong oxidizing species are formed and interact with the contaminants to degrade them into water and CO2 by complete mineralization [141-145].
EC has grown in popularity because it’s easy to set-up, is effective in treating harmful and recalcitrant organic pollutants which are difficult to degrade with conventional wastewater remediation processes, and does not require external addition of chemicals because the required reactive species are generated at the anode surface [146-148].
EC has been used to treat harmful and non-biodegradable contaminants including aromatics, pesticides, drugs, and dyes [144-149]. Electrochemical oxidation has also been used in several studies to treat different types of wastewaters and carwash wastewater [146-149]. However, due to its relatively high operating costs, it is often combined with other technologies such as biological remediation [143].
Butkovskvi et al. [151] identified six compounds frequently found in personal care and household products (methylparaben, propylparaben, bisphenol A, triclosan, galaxolide, and 4- methylbenzilidene camphor (4-MBC)) in the effluent of the aerobic graywater treatment system. The effluent was then treated with an electrochemical cell operated in batch mode with 3 different anodes and 5 different cathodes. Among the anodes, Ru/Ir mixed metal oxide showed the best performance. Ag and Pt cathodes worked slightly better than Ti and mixed metal oxide cathodes. Compounds containing a phenolic ring (parabens, bisphenol A, and triclosan) were completely transformed on this anode at a low electric charge (Q = 0.03 Ah/L) whereas compounds containing a benzene ring and multiple side methyl groups (galaxolide, 4-MBC) required high energy input (Q ≤ 0.6 Ah/L) for transformation.

Ghanbari and Martinez-Huitle [152] treated a washing machine effluent by four electrochemical advanced oxidation processes (electro-Fenton (EF) and photoelectro-Fenton (PEF) processes, EF combined with peroxymonosulfate (PMS) and DEF combined with PMS). Heterogeneous iron-based catalyst (Fe3O4, MNPs) was synthesized as catalyst for all processes. PEF/PMS process removed 99.5% of COD and 97.1% of total organic carbon under a pH of 5.0, current density of 30 mA/cm2, MNPs of 100 mg/L, PMS of 2 mM and reaction time of 180 min.
Drennan et al. [153] evaluated the impacts of EC cell configuration, current density, and cathode material on COD removal and disinfection by-product (DBP) formation in greywater. The formation and/or cathodic removal of active chlorine, perchlorate, halo acetic acids, and trihalomethanes were assessed during EC treatment. The results showed that DBP formation was proportional to current density in undivided EC cells. Sequential anodic-cathodic treatment in divided EC cells resulted in COD removal in the catholyte and anolyte. The anodic COD removal rate (using a mixed metal-oxide anode) was greater than the cathodic removal rate (using boron-doped diamond (BDD) or graphite cathodes). However, anodic and cathodic COD removal was similar when a stainless-steel cathode was used. The overall energy demand required for 50% COD removal was 24% less in the divided cells using the graphite or BDD cathodes (13 W-h./L) compared to undivided cells (20 W-h/L). Perchlorate formation was observed in undivided cells (>50 μg/L), but not detected in divided cells. While halo acetic acids (HAAs) and trihalomethanes (THMs) were generated anodically, they were removed on the cathode surface in the divided cell.
Patidar and Srivastava [154] performed critical analysis of reported studies from 1996 to 2020 on the treatment of wastewater using the sono-electrooxidation process for the degradation of the persistent organic pollutants. They found that coupling these two techniques (sonolysis and electrooxidation) increases the mineralization degree by increasing the mass transport rate and the chemical reaction rate and reduces the electrode passivation and fouling problem.
Zhang et al. [155] studied photocatalytic (PC) and electrochemical (EC) oxidation of ammonia/ammonium pollutants in water/wastewater. Depending on the contamination level, water matrix characters, and regulatory consideration, the PC and EC oxidation of wastewater pollutants exhibit their own advantages and disadvantages at specific conditions. The PC oxidation of ammonia primarily relies on in situ generated strong oxidants such as hydroxyl radicals and holes, but their reactivities with ammonia are relatively slower at environmentally relevant pH conditions. In contrast, EC oxidation of ammonia based on active chlorine species is more efficient and exhibits some advantages compared to the chemical chlorination approach.
Photooxidation
Photooxidation is an advanced oxidation processes for effective treatment of recalcitrant organic products in wastewaters. In this process, the hydroxyl radical (OH•), which is highly reactive due to its high oxidation potential, is formed. In the presence of organic matter, these radicals trigger a series of chemical reactions that end up in the complete mineralization of organic compounds to CO2 and water. The advanced oxidation processes have several advantages including a high reactivity with most organic compounds, complete oxidation of both organic and inorganic compounds and emission of harmless compounds since all oxidants are destroyed in the process [156-158].
Photolysis is based on irradiating the effluent with ultraviolet light (170-230 nm) so the chemical compounds absorb it and form free radicals. The lower the radiation wavelength, the more energy is absorbed and the greater the efficiency of destroying contaminants. Radiation causes oxidation reactions by forming free radicals in the presence of oxidizing species (ozone and hydrogen peroxide). The combination of ultraviolet radiation with ozone or hydrogen peroxide is very effective in providing a free radical source for non-selective oxidation of most organic molecules. They are also environmentally sustainable compounds as they break down into oxygen and water [159].
Photocatalytic oxidation destroys contaminants by using ultraviolet radiation with catalysts (salts of iron such as chlorides, fluorides and bromides, or semiconducting oxides such as TiO2, Al2O3 or ZnO.) to increase the formation of hydroxyl radicals which oxidise the chemical contaminants. Titanium dioxide is particularly efficient as it has another free radical production mechanism for the OH. radical. In the presence of ultraviolet radiation in aqueous medium, the electrons in one valence band of TiO2 migrate to a conduction band, leaving a corresponding hole in the valence band thereby producing so-called electron-hole pairs (h+- e-). The energy required to excite TiO2 is 3.2V, corresponding to the absorption of ultraviolet light (l < 385nm). Electron-hole pairs can recombine (and thus cancel each other out) or move to the catalyst surface. To prevent the h+- e- pairs from recombining, an oxidant (usually oxygen) acting as an electron acceptor is required, which forms the superoxide ion (O2-•). An organic molecule (MO) adsorbed in the holes can also be oxidised by electron transfer as shown in Figure 16 [160,161].
The destruction of contaminants by photooxidation has a number of advantages including: (a) toxic pollutants are destroyed by converting them into harmless substances (water, CO2 and mineral salts), (b) the process is non-selective and can decompose virtually any organic molecules, (c), additional pre-treatment or post-treatment processes are not required, (d) energy consumption is very low as the process takes place at moderate temperatures (30-80°C), (e) the radiation source could be solar energy, and (f) the chemicals used are relatively low cost and available. Therefore, photooxidation treatment technique is of great importance for different sectors (including municipal, chemical, food, pharmaceutical, textile and electroplating industries) for removing species such as cyanide, Zn, Ni, antibiotics, hormones, organochlorides, organic polyphosphates, heterocycloaliphatics, nitrogenous and aromatic organics and heteroaromatic compounds [156-158].
Chong et al. [161] stated semiconductor photocatalytic process are a low-cost, environmentally friendly and sustainable treatment technology for the water and wastewater industries and can remove persistent organic compounds and microorganisms in water. They reviewed the R&D progresses of engineered-photocatalysts, photoreactor systems, process optimizations and modellings of the photooxidation processes and discussed several commercial photocatalytic reactor configurations. The effects of key photoreactor operation parameters and water or wastewater quality on the photo-process performances in terms of the mineralization and disinfection were also assessed.
Reviro et al. [162] investigated the process of photooxidation as an attractive technology for treating recalcitrant organic compounds in greywater. The photocatalytic process oxidized organic reactants at the catalyst surface in the presence of ultraviolet light into carbon dioxide and water.
Lopez et al. [163] reported that treating greywater using photooxidation over TiO2 films resulted in catalytic reactions, which can be either enhanced or inhibited by xenobiotic contaminants in the greywater. Analysis of TiO2 film and reaction conditions indicated that photoactivation was caused in part by contact of the films with water and degradation products were produced during the initial cycle of photooxidation. Aging of these films over a prolonged period did not alter film photocatalytic activity and prior exposure of the films to UV irradiation in water decreased subsequent photocatalytic activity of the films compared with the un-irradiated films.



Alrousan et al. [164] investigated the mineralization of total organic carbon (TOC) in greywater using TiO2-based advanced photooxidation processes in a stirred tank reactor. The combinations of H2O2, O3, and immobilized TiO2 under either dark or UVA irradiation conditions were evaluated. This included TiO2/dark, O3/dark (ozonation), H2O2/dark (peroxidation), TiO2/UVA (photocatalysis), O3/UVA (Ozone photolysis), H2O2/UVA (photo-peroxidation), O3/TiO2/dark (catalytic ozonation), O3/TiO2/UVA (photocatalytic ozonation), H2O2/TiO2/dark, H2O2/TiO2/UVA, H2O2/O3/dark (peroxonation), H2O2/O3/UVA (photo-peroxonation), H2O2/O3/TiO2/dark (catalytic peroxonation), and H2O2/O3/TiO2/UVA (photocatalytic peroxonation). The results indicated that combining different treatment methods with UVA irradiation dramatically enhanced the organic mineralization efficiency. The optimum TiO2 loading was 0.96 mg/cm2 with the highest TOC removal (54%) achieved using photocatalytic peroxonation under optimal conditions (0.96 mg TiO2/cm2, 25 mg O3/min, and 0.7 H2O2/O3 molar ratio).
Boyjoo et al. [165] performed a pilot scale study of photocatalytic degradation of impurities in shower water using a 31 L reactor with titanium dioxide as the photocatalyst. The reactor was operated in a continuous slurry recirculation mode and several operational parameters (slurry initial pH, catalyst concentration, air flow rate, and slurry recirculation rate) were evaluated. Up to 57% of total organic carbon elimination was obtained after 6 h of treatment at optimum condition (initial pH of 3, catalyst concentration of 0.07 mg/cm2, air flow rate of 1.8 L/min, and slurry recirculation rate of 4.4 L/min).
Dubowski et al. [166] investigated the removal of triclosan and oxybenzone micropollutants in deionized water and greywater using combined UVC/VUV or UVC only radiation in a continuous-flow reactor. Degradation kinetics of these micropollutants and their transformation products were evaluated as well as bacterial growth inhibition of the resulting effluents. In deionized water, micropollutants degradation was much faster under the combined UVC/VUV irradiation. In treated greywater, the combined radiation successfully removed both micropollutants but at lower efficiency. as particles and dissolved organic matter acted as radical scavengers. Filtration prior to irradiation improved the process efficiency and reduced energy requirements under the combined radiation (from 1.6 and 167 to 1.1 and 6.0 kWh/m3 for triclosan and oxybenzone, respectively).
Grcic et al. [167] investigated the possibility of treating greywater by solar photocatalysis followed by flocculation with special emphasis on hair colorants and accompanying chemicals. The greywater was collected from the bathroom after hair dyeing, washing and rinsing. Then, surface tension, light absorption and scattering, TOC, COD, BOD5 and toxicity toward yeast and Vibrio fischeri were determined. Greywater was then treated in a reactor with a constant recirculation over photocatalytic layer exposed to direct sunlight. The photocatalytic layer consisted of TiO2-coated textile fibers prepared by applying TiO2–chitosan pasteous dispersion on polyester/wool blend textile (75% polyester, 25% wool). The results showed significant decrease in organic content, COD, toxicity, emulsifying compounds and surfactants and complete degradation of dye molecules and certain aromatic compounds over a period of 4 h.
Amanious et al. [168] treated aqueous solutions of tetraethyleneglycol dimethylether, spiked with different inorganic salts in concentrations found in biologically treated greywater, with TiO2 flocculation and photocatalytic oxidation. Flocculation of the photocatalyst primarily depended on pH (which was affected by the salts). Photocatalyst agglomeration was maximum at a pH of 5.5. With salt concentrations >7 mmol/ L, flocculation was strong due to electric double layer compression. Increasing pH caused deterioration of photocatalytic oxidation efficiency as a result of impaired absorbability of negatively charged oxidation intermediates and the enhanced CO2 absorption with increasing pH and subsequent formation of HCO3− anions which are OH radical scavengers.
Agullo-Barcelo et al. [169] assessed the disinfection of a secondary effluent from a municipal wastewater treatment plant using H2O2 (20-50 mg/L), TiO2 (100 mg/L) and photo-Fenton under natural solar radiation in compound parabolic collector photo-reactors. The naturally occurring Escherichia coli, spores of sulfite-reducing clostridia, Somatic coliphages and F-specific RNA bacteriophages were tested before and along the different solar treatments. The best treatments efficiency for E. coli was photo-Fenton at a pH of 3 followed by H2O2 (20 mg/L)/solar, TiO2/solar and solar photo-inactivation. On the other hand, for viral indicators the ranking was: photo-Fenton pH 3>TiO2/solar>H2O2(20 mg/L)/solar>solar photo-inactivation. Sulfite-reducing clostridia was the most resistant microorganism in all the evaluated processes.
ADSORPTION TREATMENT
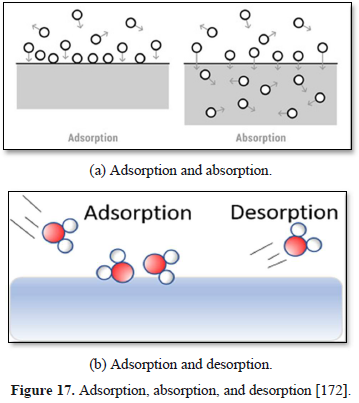
Adsorption is the adhesion of atoms, ions or molecules from a gas or liquid to a surface, creating a film of the adsorbate on the surface of the adsorbent. Adsorption differs from absorption in which a fluid (the absorbate) is dissolved by a liquid or solid (the absorbent). Thus, adsorption is a surface phenomenon, while absorption involves the whole volume of the material (Figure 17). Sorption encompasses both adsorption and absorption processes, while the desorption is the reverse of it [170-172].
Adsorption is a consequence of surface energy. In a bulk material (ionic, covalent or metallic), all the bonding requirements of the constituent atoms of the material are filled by other atoms in the material. However, atoms on the surface of the adsorbent are not wholly surrounded by other adsorbent atoms and therefore can attract adsorbates. The exact nature of the bonding depends on the details of the species involved, but the adsorption process is generally classified as physisorption or chemisorption. It may also occur due to electrostatic attraction [173,174].
Siyal et al. [175] stated that treating wastewater containing surfactants by adsorption is effective and activated carbon is the most suitable adsorbent for removing surfactants. However, activated carbon is expensive and, therefore, a variety of new adsorbents such as zeolites, nanomaterials, resins, biomaterials and clays have been used as alternatives.
Thompson [117] compared biochar to activated carbon for removing dissolved organic carbon from graywater and evaluated the impact of pre-treatments (coagulation, biodegradation) on the removal efficiency. A wood-based biochar was effective for graywater treatment, but activated carbon removed more dissolved organic carbon. Graywater regulations could not be met by sorption alone but could be met with pretreatment before sorption and the treated water was suitable for After pretreatment, irrigation and toilet flushing targets could be achieved with activated carbon doses less than 0.7 g/L, while a biochar dose of about 1 g/L was needed to achieve the irrigation treatment targets.
Sales et al. [176] carried out a qualitative and quantitative investigation on the effluent of the water treatment plant by determining its physical and chemical parameters, comparing the results to what is allowed by the current local legislation. They prepared activated charcoal of coco-da-baia mesocarp and tested it as adsorbent material in a filter system in a column with a continuous flow. The results showed reductions of 50% in hardness, 87.5% in chloride and 66.6% in acidity. The effluent was qualified for use in agricultural.

Patel et al. [177] reported on batch and continuous adsorption studies for the treatment of greywater using activated carbons prepared from sawdust, sugarcane bagasse and pine needles. The optimum conditions in batch mode for the removal of contaminants were a pH of 7, a contact time of 240 min. and an adsorbent dose of 8 g/L with initial greywater COD of 554 mg/L and BOD of 120 mg/L. The sawdust activated carbon was found to be the most efficient.
Topkava et al. [178] treated greywater from laundry washing by adsorption process using different adsorbents. To synthesize the adsorbents, walnut shell, seed hull (SH), hazelnut shell (HS) and rice husk (RH) were carbonized and then supported with polyaniline (PA). KIO3 and K2S2O8 were used as oxidizing agents in the polymerization of aniline monomers. Adsorption experiments were conducted with 100 mL of wastewater and 1 g of adsorbent, for a 2 h reaction time at room temperature and a mixing speed of 150 rpm. The highest removal efficiencies for the PA/RH+KIO3 adsorbent were 98%, 70% and 96% for color, turbidity and detergent, respectively. The highest removal efficiencies for the PA/SH+K2S2O8 adsorbent were 58%, 3% and 95% for the same parameters, respectively.
Guo et al. [179] studied the adsorption mechanisms of mercury ion (Hg2+) in contaminated water by different fractions (inorganic carbon (IC), organic carbon (OC), hydroxyl-blocked carbon (BHC), and carboxyl-blocked carbon (BCC)) of biochar prepared from corn straw. The reaction mechanisms of biochar for Hg2+ removal included electrostatic adsorption, ion exchange, reduction, precipitation, and complexation. The equilibrium adsorption capacity of biochar for Hg2+ was 75.56 mg/g, and the adsorption contribution rates of IC and OC were 22.4% and 77.6%, respectively. Despite the lower rate, IC shows the largest adsorption capacity of 92.63 mg/g which is attributed to all the mechanisms involved in Hg2+ adsorption by IC, with ion exchange being the main reaction mechanism accounting for 39.8%. The main adsorption mechanism of OC is the complexation of carboxyl and hydroxyl groups with Hg2+ which accounted for 71.6% of the total OC contribution. BHC and BCC adsorbed mercury mainly via the reduction–adsorption mechanism, accounting for 54.6% and 54.5%, respectively.
SUMMARY AND CONCLUSION
One third of the world population will face water shortage by 2025 that may result in reductions in agricultural lands, increased dissertation and inadequate food supply leading to poverty, faming, migration and even wars. Treating and reusing greywater, originating from kitchen sinks, showers, baths, washing machines and dishwashers, can minimize the severity of the water shortage problems. There are several physical and chemical greywater treatment technologies for safe reuse.
Physical treatment of greywater refers to the separation of contaminants from the water by physical means such as sedimentation and filtration. Sedimentation is the process of allowing particles in water to settle out of suspension by gravity and cannot be considered for greywater treatment due to its very low concentration of suspended solids. Filtration is a process of removing contaminants from water and wastewater by forcing the water through a porous media that can be natural (sand, gravel and clay) or synthetic (membranes made of cellulose acetate, cellulose nitrate, polyamide, polycarbonate, polypropylene, and polytetrafluoroethylene). Based on pore size, membrane filtration is divided into four classes: microfiltration, ultrafiltration, nanofiltration and reverse osmosis. Electrochemical treatment systems that have been used for treatment of greywaters for pollution load reduction and water reuse include: chemical coagulation-flocculation, electrochemical coagulation, electrooxidation and photooxidation. In addition, the adsorption process has been used very successfully for treating greywater, sand filters have been proven to be an effective method of water and wastewater treatment, have low capital and maintenance costs, and require little skill for operation. However, the efficiency of sand filters decreases with increased hydraulic and chemical loading rates and they require large quantities of water for backwashing and large land areas to house the filter beds.
Microfiltration is a low-pressure physical separation process used for treating water, industrial wastewater and dairy and food processing wastewaters municipal wastewater and greywater. The use of microfiltration limits the concentrations and number of chemicals that are applied during treatment and removes natural and synthetic suspended particles and microorganisms.
Ultrafiltration is a pressure-driven physical separation process used to pre-treat surface water, seawater, carwash wastewater and greywater. It removes large particles, bacteria, protozoa, algae, virus and divalent ions. It does not require chemicals (coagulants, flocculants, disinfectants, pH adjustment), maintains constant quality of the treated water and is simple to operate and automate but fouling can cause difficulties.
Nanofiltration is a high-pressure physical separation process that can remove dissolved solids, most of the organic molecules, all viruses, cysts, bacteria and wide range of salts and humic materials from surface and ground water as well as various types of wastewaters. Nanofiltration provides high rejection of multivalent ions such as calcium and low rejection of monovalent ions such as chloride. It has lower discharge volumes, lower retentate concentrations for low value salts, high reduction of salt content and dissolved matter content in brackish water, high reduction of heavy metals, nitrates and sulphates and high reduction in color, tannins, and turbidity. However, it has higher energy consumption, requires pre-treatment for heavily polluted waters and membranes are expensive.
Reverse osmosis is the tightest membrane separation process in which water is separated from dissolved salts at a pressure greater than osmotic pressure. It removes all organic molecules, pesticides, cysts, bacteria, viruses and all minerals including monovalent ions. The advantages of reverse osmosis include removal of nearly all contaminant ions and most dissolved non-ions, insensitive to flow and total dissolved solids levels, suitable for small systems with a high degree of seasonal fluctuation in water demand, operates immediately without break-in periods, and simplicity of operational and automation allow for less operator attention. However, it has high capital and operating costs, managing the effluent is a potential problem, requires high level of pre-treatment, and is prone to fouling.
Coagulation and flocculation processes are used for treatment of a variety of wastewaters and can be used for removal of dissolved and suspended solids including pathogens (Giardia and Cryptosporidium), virus, arsenic, phosphorus, and fluoride. In coagulation particles aggregate with themselves by a change in pH while in flocculation particles aggregate using polymers to bind them together. Polymers are natural and synthetic water-soluble macromolecular compounds that enhance flocculation of constituents. Natural polymers are free of toxins, biodegradable and locally available. Synthetic polymers are more effective and easier to control. The metal coagulants fall into two categories: aluminum based (aluminum sulfate, aluminum chloride, and sodium aluminate.) and iron based (ferric sulfate, ferrous sulfate, ferric chloride, and ferric chloride sulfate). The efficiency of the coagulation-flocculation process is dependent on the type of coagulant, coagulant dosage, coagulant feed concentration, type and dosage of chemical additives, sequence of chemical addition, pH and time lag between dosing points, intensity and duration of mixing, flocculator retention time, type of stirring device used and flocculator geometry.
Electrocoagulation is an electrochemical process that removes heavy metals, suspended solids, oil, grease, emulsified organics and other contaminants from water and wastewaters using electricity instead of chemicals. The electrocoagulation device performs coagulation, flocculation, flotation, separation, and removal in a single enclosed reactor. Electrocoagulation requires no filters and no daily maintenance, and is simple and easy to operate. Wastewater treated by electrocoagulation results in clear, colorless and odorless water and the sludge formed tends to be settable and easy to de-water.
Electrooxidation is an advanced oxidation process comprising of two electrodes (anode and cathode) connected to a power source and sufficient electrolyte and in which strong oxidizing species are formed, interacting with the contaminants and converting them into water and CO2 by complete mineralization. Electrochemical oxidation is easy to set-up, effective in treating harmful and recalcitrant organic pollutants and does not require addition of chemicals. It has been used to treat a wide variety of harmful and non-biodegradable contaminants including aromatics, pesticides, drugs, and dyes. However, due to its relatively high operating costs, it is often combined with other technologies such as biological remediation.
Photooxidation is an advanced oxidation processes that is used in treating recalcitrant organic products in wastewaters by forming the hydroxyl radical (OH•) which triggers a series of chemical reactions that end up in complete mineralization of organic compounds to CO2 and water. Photolysis is based on irradiating the effluent with ultraviolet light (170-230 nm) which is absorbed by the chemical compounds to form free radicals. The lower the radiation wavelength, the more energy is absorbed and the greater the efficiency in destroying the contaminants. For the oxidation reactions to occur, oxidizing species (ozone and hydrogen peroxide) must be present. Photocatalytic oxidation can destroy contaminants using ultraviolet radiation with catalysts (salts of iron, usually chlorides, fluorides and bromides, or semiconducting oxides such as TiO2, Al2O3 or ZnO) to increase the formation of hydroxyl radicals. Photooxidation has several advantages including: the process is non-selective and can decompose virtually any organic molecules, pre- or post-treatment processes are not required, energy consumption is very low, the radiation source could be solar energy, the chemicals used are relatively low cost and freely available, complete oxidation of organic and inorganic compounds and emission of harmless compounds.
Adsorption is the adhesion of atoms, ions or molecules in the medium to a surface, creating a film of the adsorbate on the surface of the adsorbent. Adsorption is widely used in industrial applications such as catalysts, activated charcoal capturing and use of waste heat to provide cold water for air conditioning, synthetic resins, ion exchange, chromatography, water purification, pharmaceutical industry applications and water and wastewater treatments.
Treating and recycling greywater with physical or chemical treatment technologies is economically and environmentally sustainable. However, when selecting a physical or a chemical treatment process for greywater treatment, the characteristics of greywater must be taken into consideration.
ACKNOWLEDGEMENTS
The authors appreciate the assistance provided by the Ms. D. M. El Nakib, the Manager of the Bioengineering Laboratory and staff of the Department of Agricultural Engineering, Faculty of Agriculture, Cairo University.
AUTHORS’ CONTRIBUTIONS
This work was carried out in collaboration among all authors. All authors read and approved the final manuscript.
- Morel A, Diener S (2006) Greywater management in low and middle-income countries, review of different treatment systems for households or neighborhoods. Sandec Report No. 14/06, Department of Water and Sanitation in Developing Countries, Swiss Federal Institute of Aquatic Science and Technology, Zurich, Germany. Accessed on: September 15, 2020. Available online at: https://www.susana.org/_resources/documents/default/2-947-en-greywater-management-2006.pdf
- Ghaly AE, Abdelrahman EN, Emam RH, Mahmoud NS, Ibrahim MM, et al. (2021) Overview of biological treatment technologies for greywater reuse. Adv Environ Waste Manag Recycl 4(3):165-191.
- Dixon AM, Butler D, Fewkes A (1999) Guidelines for greywater re-use: Health issues. J Chart Inst Water Environ Manag 13(5): 322-326.
- Eriksson E, Auffarth K, Henze M, Ledin A (2002) Characteristics of grey wastewater. Urban Water 4(1): 85-104.
- Ledin A, Eriksson E, Henze M (2001) Aspects of groundwater recharge using grey wastewater. In: P. Lens, G. Zeemann and G. Lettinga (Editors), Decentralized Sanitation and Reuse, London, UK.
- Otterpohl R, Grottker M, Lange J (1997) Sustainable water and waste management in urban areas. Water Sci Technol 35(9): 121-133.
- Ottoson J, Stenstrom TA (2003) Fecal contamination of greywater and associated microbial risks. Water Res 37(3): 645-655.
- Metcalf and Eddy Inc. (2003) Wastewater Engineering-Treatment and Reuse (Tchobanoglous, G., F. L. Burton and H. D. Stensel, Eds). McGraw-Hill Companies, Boston, USA.
- Behzadian K, Kapelan Z (2015) Advantages of integrated and sustainability-based assessment for metabolism based strategic planning of urban water systems. Sci Total Environ 527-528: 220-231.
- Al-Jayyousi OR (2003) Greywater reuse: Towards sustainable water management. Desalination 156(2): 181-192.
- Al-Hamaiedeh H, Bino M (2010) Effect of treated grey water reuse in irrigation on soil and plants. Desalination 256: 115-119.
- Halalsheh M, Dalahmeh S, Sayed M, Suleiman W, Shareef M, et al. (2008) Grey water characteristics and treatment options for rural areas in Jordan. Bioresour Technol 99: 6635-6641.
- Faraqui N, Al-Jayyousi O (2002) Greywater reuse in urban agriculture for poverty alleviation. A case study in Jordan. Water Int 27: 387-394.
- Casanova LM, Gerba CP, Karpiscak M (2001) Chemical and microbial characterization of household greywater-Part A: Toxic/Hazardous Substances and Environmental Engineering. J Environ Sci Health 34: 395-401.
- Friedler E (2004) Quality of individual domestic greywater streams and its implication for onsite treatment and reuse possibilities. Environ Technol 25(9): 997-1008.
- Martin C (2005) Ecological sanitation greywater demonstration project at Hui Sing Garden. Urban Environmental Management System Report, Project Natural Resources and Environment Board, Kuching, Sarawak, Malaysia.
- Alderlieste MC, Langeveld JG (2005) Wastewater planning in Djenne, Mali. A pilot project for the local infiltration of domestic wastewater. Water Sci Technol 51: 57-64.
- Shresta RR (2000) Application of constructed wetlands for wastewater treatment in Nepal. Water Sci Technol 44(11-12): 381-386.
- Jamrah A, Al Omari A, Al Qasem L, Ghani NA (2011) Assessment of availability and characteristics of greywater in Amman. Water Sci Technol 50: 157-164.
- Adendorff J, Stimie C (2005) Food from used water-making the previously impossible happen. South African Research Commission. Accessed on: November 10, 2020. Available online at: https://journals.co.za/content/waterb/4/1/EJC115497;jsessionid=eN3T0-vyFBBLzkyrSxLhrihD.sabinetlive
- Busser S, Pham TN, Morel A, Nguyen VA (2006) Characteristics and quantities of domestic wastewater in urban and peri-urban households in Hanoi. Accessed on: October 30, 2020. Available online at: http://ir.library.osaka-u.ac.jp/dspace/bitstream/11094/13204/1/arfyjsps2006_395.pdf
- Barker AV, English JE (2011) Recycling greywater for home gardens. Agriculture and Land Scape Program Publication, University of Massachusetts, Amherst, Massachusetts, USA. Accessed on: September 30, 2020. Available online at: https://web.archive.org/web/20120901010624/http:/extension.umass.edu/landscape/fact-sheets/recycling-gray-water-home-gardens
- Sharvelle S, Roesner LA, Qian Y, Stromberger M (2010) Long term study on landscape irrigation using household greywater-Experimental study. Interim Report, Horticulture and Landscape Architecture, Colorado State University, Fort Collins, Colorado, USA. Accessed on: September 15, 2020. Available online at: https://web.archive.org/web/20130409040529/http:/www.decentralizedwater.org/documents/06-CTS-1CO/06CTS1COInterimweb.pdf
- Finley S, Barrington S, Lyew D (2009) Reuse of domestic greywater for the irrigation of food crops. Water Air Soil Pollut 199: 235-245.
- Gross A, Azulai N, Oron G, Ronen Z, Arnold M, et al. (2005) Environmental impact and health risks associated with greywater irrigation: A Case Study. Water Sci Technol 52: 161-169.
- Qian YL, Mecham B (2005) Long-term effects of recycled wastewater irrigation on soil chemical properties on golf course fairways. Agronomy J 97: 717-721.
- Shafran AW, Ronen Z, Weisbrod N, Adar E, Gross A (2006) Potential changes in soil properties following irrigation with surfactant-rich greywater. Ecol Eng 26: 348-354.
- Scheumann R, Masi F, El Hamouri B, Kraume M (2007) Greywater treatment options for effective wastewater management in small communities. Desalin Water Treat 4: 33-39.
- Jefferson B, Judd S, Diaper C (2000) Treatment methods of grey water. In: Decentralized Sanitation and Reuse - Concepts, Systems and Implementation (P. Lens, Editor), IWA publishing, London, UK.
- Nolde E (1999) Greywater reuse systems for toilet flushing in multi-story buildings - over ten- year experiences in Berlin. Urban Water 1(4): 275-284.
- Friedler E, Schatrtzman Z, Ostfeld A (2008) Assessment of the reliability of on-site MBR system for greywater treatment and associated aesthetic and health risks. Water Sci Technol 57(7): 1104-1110.
- Burnat JMY, Mahmoud N (2005) Evaluation of on-site gray wastewater treatment plants performance in Bilien and Biet-Diko villages. Environment Protection Committee Report, Palestine.
- Gross A, Shmueli O, Ronen Z, Raveh E (2007) Recycled vertical flow constructed wetland- a novel method of recycling greywater for landscape irrigation in small communities and households. Chemosphere 66(5): 916-923.
- Dallas S, Scheffe B, Ho G (2004) Reedbeds for greywater treatment - Case study in Santa Elena-Monteverde, Costa Rica, Central America. Ecol Eng 23(1): 55-61.
- Alsulaili AD, Hamoda MF, Al-Jarallah R, Alrukaibi D (2017) Treatment and potential reuse of greywater from schools: A pilot study. Water Sci Technol 75: 2119-2129.
- Dwumfour-Asare B, Adantey P, Nyarko KB, Appiah-Effah E (2017) Greywater characterization and handling practices among urban households in Ghana: The case of three communities in Kumasi Metropolis. Water Sci Technol 76: 813-822.
- Mandal D, Labhasetwar P, Dhone S, Dubey AS, Shinde G, et al. (2011) Water conservation due to greywater treatment and reuse in urban setting with specific context to developing countries. Resour Conserv Recycl 55: 356-361.
- Masi F, El Hamouri B, Shafi HA, Baban A, Ghrabi A, et al. (2010) Treatment of segregated black/grey domestic wastewater using constructed wetlands in the Mediterranean basin: The zero-m experience. Water Sci Technol 61: 97-105.
- Oteng-Peprah M, de Vries NK, Acheampong MA (2018) Greywater characterization and generation rates in a peri urban municipality of a developing country. J Environ Manag 206: 498-506.
- Atanasova N, Dalmau M, Comas J, Poch M, Rodriguez-Roda I, et al. (2017) Optimized MBR for greywater reuse systems in hotel facilities. J Environ Manag 193: 503-511.
- Friedler E, Kovalio R, Ben-Zvi A (2006) Comparative study of the microbial quality of greywater treated by three on-site treatment systems. Environ Technol 27: 653-663.
- Khalaphallah R, Andres Y (2012) The effect of various abiotic factors on the survival growth of Escherichia coli and Pseudomonas aeruginosa in bathroom greywater. J Water Reuse Desalin 2: 92-101.
- Kim J, Song I, Oh H, Jong J, Park J, et al. (2009) Laboratory-scale graywater treatment system based on a membrane filtration and oxidation process-characteristics of graywater from a residential complex. Desalination 238: 347-357.
- Paulo PL, Begosso L, Pansonato N, Shrestha RR, Boncz MA (2009) Design and configuration criteria for wetland systems treating greywater. Water Sci Technol 60: 2001-2007.
- Benami M, Gillor O, Gross A (2015) The question of pathogen quantification in disinfected graywater. Sci Total Environ 506: 496-504.
- Maimonm A, Friedler E, Gross A (2014) Parameters affecting greywater quality and its safety for reuse. Sci Total Environ 487: 20-25.
- Shoults DC, Ashbolt NJ (2017) UV disinfection of hand-rinse greywater and performance testing using indigenous Staphylococcus Water 9(12): 1-9.
- Blanky M, Sharaby Y, Rodriguez-Martinez S, Halpern M, Fridler E (2017) Grey water reuse - assessment of the health risk induced by Legionella pneumophila. Sustain Earth Technol 125: 410-417.
- Hakim MW, Alkhudhiri A, Al-Batty S, Zacharof MP, Maddy J, et al. (2020) Ceramic microfiltration membranes in wastewater treatment: Filtration behavior, fouling and prevention. Membranes 10(9): 1-34.
- AMTA (2020) Membrane filtration for water reuse. American Membrane Technology Association, Stuart, Florida, USA. Accessed on: January 10, 2020. Available online at: https://www.amtaorg.com/Membrane_Filtration_for_Water_Reuse.html
- WWD (2000) Membrane filtration for water and wastewater. Water and Wastewater Digest, Arlington Heights, Illinois, USA. Accessed on: January 10, 2020. Available online at: https://www.wwdmag.com/desalination/membrane-filtration-water-and-wastewater
- Boussu K, Eelen D, Vanassche S, Vandecasteele C, Van der Bruggen B, et al. (2008) Technical and economic evaluation of water recycling in the carwash industry with membrane processes. Water Sci Technol 57(7): 1131-1135.
- BSS (2020) Sand filter. Benbell Softener Systems, Nolda, Uttar Pradesh, India. Accessed on: January 28, 2020. Available online at: http://www.benbellsoftenersystems.in/sand-filter/
- SSWMT (2020) Rapid sand filtration. Sustainable Sanitation and Water Management Toolbox, Willisau, Switzerland. Accessed on: January 28, 2020. Available online at: https://sswm.info/sswm-university-course/module-6-disaster-situations-planning-and-preparedness/further-resources-0/rapid-sand-filtration
- WHO (2000) Guidelines for the safe use of wastewater, excreta and greywater, Volume 2: wastewater use in agriculture. World Health Organization, Geneva, Accessed on: October 12, 2020. Available online at: https://www.who.int/water_sanitation_health/publications/gsuweg2/en/
- Abdelmoez W, Barakat NAM, Moaz A (2013) Treatment of wastewater contaminated with detergents and mineral oils using effective and scalable technology. Water Sci Technol 68(50): 974-981.
- Zaneti R, Etchepare R, Rubio J (2011) Car wash wastewater reclamation: Full-scale application and upcoming features. Resour Conserv Recycl 55(11): 953-959.
- Metcalf & Eddy, AECOM (2007) Water Reuse: Issues, Technologies and Applications. McGraw-Hill Professional, New York, New York, USA.
- Rodgers M, Mulqueen J, Healy MG (2004) Surface clogging in an intermittent stratified sand filter. Soil Sci Soc Am J 68: 1827-1832.
- Guala G, Moiàb A, Marcha JG (2008) Monitoring of an indoor pilot plant for osmosis rejection and greywater reuse to flush toilets in a hotel. Desalination 219: 81-88.
- Steviki TK, Ausland G, Deinboll P, Robert J, Siegrist L (1999) Removal of Coli during intermittent filtration of wastewater effluent as affected by dosing rate and media type. Water Res 33(9): 2088-2098.
- Droste R (2004) Theory and Practice of Water and Wastewater Treatment, John Wiley and Sons, New York, New York, USA.
- Spychala M, Niec J, Zawadeki P, Matz R, Nguyen TH (2019) Removal of volatile solids from greywater using sand filters. Appl Sci 9: 1-13.
- Albalawneh A, Chang TK, Alshawabkeh H (2017) Greywater treatment by granular filtration system using volcanic tuff and gravels media. Water Sci Technol 75(10): 2331-2341.
- Abdel-Shafy HI, El Khateeb MA, Shehata M (2013) Greywater treatment using different designs of sand filters. Desalin Water Treat 52(28-30): 5237-5242.
- Jaramillo J, Gomez-Serrano V, Alvarez PM (2009) Enhanced adsorption of metal ions onto functionalized granular activated carbons prepared from cherry stones. Hazard Mater161(2-3): 270-276.
- Lenntech (2020) Submerged membrane bioreactor. Lenntech Water Treatment and Purification, South Miami, Florida, USA. Accessed on: December 20, 2020. Available online at: https://www.lenntech.com/processes/submerged-mbr.htm
- Hydroblue (2012) How membrane bioreactor is important for wastewater treatment plan, Hydro Blue Membrane Technology Company, JiangNan Industry Park, NanChun, China. Accessed on: December 20, 2020. Available online at: https://www.hydrobluemem.com/how-membrane-bioreactor-is-important-for-wastewater-treatment-plant/
- Moazzem S, Ravishankar H, Fan L, Roddick F, Jegatheesan VJ (2020) Application of enhanced membrane bioreactor for the reuse of carwash wastewater. Environ Manag 254: 109780.
- Boluarte IAR, Andersen M, Pramanik BK, Chang CY, Bagshaw S, et al. (2016) Reuse of car wash wastewater by chemical coagulation and membrane bioreactor treatment processes. Int Biodeterior Biodegradation 113: 44-48.
- Pinto ACS, Drossi LB, Melo RAC, Assis TM, Ribeiro VM, et al. (2017) Carwash wastewater treatment by micro and ultrafiltration membranes: Effects of geometry, pore size, pressure difference and feed flow rate in transport properties. J Water Process Eng 17: 143-148.
- Bhattacharya P, Sarkar S, Ghosh S, Majumdar S (2013) Potential of ceramic microfiltration and ultrafiltration membranes for treating greywater for effective reuse. Desalin Water Treat 51(22-24): 4323-4332.
- de Oliveira TM, Benattib CT, Tavaresc CRG (2020) Pilot system of microfiltration and reverse osmosis membranes for greywater reuse. Desalin Water Treat 201: 13-19.
- Manoucheri M, Kargari A (2017) Water recovery from laundry wastewater by the crossflow microfiltration process: A strategy for water recycling in residential buildings. J Clean Product 168: 227-238.
- Ahn KH, Song KG (1999) Treatment of domestic wastewater using microfiltration for reuse of wastewater. Desalination 126: 7-14.
- Jamil A, Umer M, Karim SS, Amaduddin M (2017) Design of a car wash wastewater treatment process for local car wash stations. J Pakistan Inst Chem Eng 45(2): 83-95.
- Baker RW (2004) Membrane technology and applications. Membrane Technology and Research, Inc. Menlo Park, California, USA.
- Cakmakci M, Basoinar AB, Balaban U, Uyak V, Koyumcu I, et al. (2009) Comparison of nanofiltration and adsorption techniques to remove arsenic from drinking water. Desalin Water Treat 9(1-3): 149-154.
- Skrzypek M, Burger M (2010) Isoflux ® ceramic membranes - Practical experiences in dairy industry. Desalination 250: 1095-1100.
- Li F, Guyas H, Wichmann K, Otterpohi R (2009) Treatment of household grey water with a UF membrane filtration system. Desalin Water Treat 5(1-3): 276-282.
- Kaminska G, Marszalek A (2020) Advanced treatment of real greywater by SBR followed by ultrafiltration: Performance and fouling behavior. Water 12(1): 154-161.
- Schafer A, Nghiem LD, Oschmann N (2006) Bisphenol A retention in the direct ultrafiltration of greywater. J Membr Sci 283(1-2): 233-243.
- Sumish A, Arthanareerswaran G, Thuyavan YL, Ismail AE, Chakraborty S (2015) Treatment of laundry wastewater using polyethersulfone/polyvinylpyrollidone ultrafiltration membranes. Ecotoxicol Environ Safe 121: 174-179.
- Lodge B, Judd SJ, Smith AJ (2004) Characterization of dead-end ultrafiltration of biotreated domestic wastewater. J Membr Sci 231(1-2): 91-98.
- Vani B, Shivakumar M, Kalyani S, Sridhar S (2021) TiO2 nanoparticles incorporated high-performance polyphenyl sulfone mixed matrix membranes for ultrafiltration of domestic greywater. Iran Polym J 30: 917-934.
- Bahaei F, Ehrampoush MH, Eslami H, Ghaneian MT (2019) Removal of linear alkylbenzene sulfonate and turbidity from greywater by a hybrid multi-layer slow sand filter microfiltration ultrafiltration system. J Clean Produc 211: 922-931.
- Nghiema LD, Oschmanna N, Schäfera AI (2006) Fouling in greywater recycling by direct ultrafiltration. Desalination 187: 283-290.
- Hilal N, Al-Zoubi H, Darwish NA, Mohamed A (2007) Performance of nanofiltration membranes in the treatment of synthetic and real seawater. Sep Sci Technol 42(3): 493-515.
- Smbekke HDM, Voorhoeve DK, Hiemstra P (1997) Environmental impact assessment of ground water treatment with nanofiltration. Desalination 113(2-3): 293-296.
- Water Professional (2020) Nanofiltration. Water Professionals, North Carolina, Raleigh, USA. Accessed on: December 2020. Available online at: https://www.waterprofessionals.com/learning-center/nanofiltration/
- Koyuncu I, Arikan OA, Wiesner MR, Rice C (2008) Removal of hormones and antibiotics by nanofiltration membranes. J Membr Sci 309(1-2): 94-101.
- Hourlier F, Masse A, Jaouen P, Lakel A, Gerente C, et al. (2010) Membrane process treatment for greywater recycling: Investigations on direct tubular nanofiltration. Water Sci Technol 62(7): 1544-1550.
- Ramona G, Green M, Semiat R, Dosoretz C (2004) Low strength graywater characterization and treatment by direct membrane filtration. Desalination 170: 241-250.
- Guilbaud J, Masse A, Andres Y, Combe F, Jaouen P (2010) Laundry water recycling in ship by direct nanofiltration with tubular membranes. Resour Conserv Recycl 55(2): 148-152.
- Guilbaud J, Masse A, Andres Y, Combe F, Jaouen P (2012) Influence of operating conditions on direct nanofiltration of greywaters: Application to laundry water recycling aboard ships. Resour Conserv Recycl 62(2): 64-70.
- Van der Bruggen B, Vandeccasteele C (2001) Flux decline during nanofiltration of organic components in aqueous solution. Environ Sci Technol 17: 3535-3540.
- Panpanit S, Visvanathan C, Muttamara S (2000) Separation of oil-water emulsion from car washes. Water Sci Technol 41(10): 109-116.
- Sayers D (2002) Coming clean at the carwash customers value a spot-free wash. Modern Car Care 9(1): 1.
- KAY (2020) Reverse osmosis water and clean water management. Kay Plumbing Services, Lexington and Columbia Plumbers, Lexington, North Carolina, USA. Accessed on: December 25, 2020. Available online at: https://kayplumbing.com/plumbing-blog/reverse-osmosis-water/
- Singh PS, Ray P, Trivedi JJ, Rao AP, Parashuram K, et al. (2014) RO membrane treatment of domestic greywater containing different detergent types. Desalin Water Treat 52(22-24): 4071-4078.
- Senthilmurugan S, Venkatesh T (2017) Greywater treatment and simultaneous surfactant recovery using UF and RO processes. Sep Sci Technol 52(14): 2262-2273.
- Boddu VN, Paul T, Page MA, Byl C, Wade L, et al. (2016) Effect of pretreatment technologies on low pressure reverse osmosis treatment. J Environ Chem Eng 4(4): 4435-4443.
- Reang S, Nath H (2021) Greywater treatment with spiral wound UF and RO membranes. Mater Today 46(14): 6253-6257.
- Engin GO, Ucar BS, Senturk C (2011) Reuse feasibility of pretreated greywater and domestic wastewater with a compact household reverse osmosis system. Desalin Water Treat 299(1-3): 103-109.
- DiPaolo R (2016) A carwash ’s guide to reverse osmosis. Professional Car washing and Detailing magazine. Akron, Ohio, USA.
- Aquarden Technologies (2020) Coagulation and flocculation in wastewater treatment. Aquarden Technologies, Skaeving, Denmark. Accessed on: January 12, 2021. Available online at: https://aquarden.com/technologies/coagulation-flocculation/
- Ives KJ (1978) The scientific basis of flocculation. Springer, New York, New York, USA.
- Hudson HE (1981) Water clarification processes. Practical design and evaluation. Van Nostrand Reinhold, New York, New York, USA.
- van Loosdrecht MCM, Keller J, Nielsen PH, Lopez-Vazquez CM, Brdjanovic D (2014) Experimental methods in wastewater treatment. IWA Publishing, London, UK.
- AES (2020) Coagulation/flocculation packages. AES Arabia Limited Water and Wastewater Treatment Systems. Riyadh, Kingdom of Saudi Arabia. Accessed on: January 12, 2020. Available online at: http://www.aesarabia.com/coagulation-flocculation-packages/
- Teh CY, Budiman PM, Shak KPY, Wu TY (2016) Recent advancement of coagulation-flocculation and its application in wastewater treatment. Indust Eng Chem Res 16: 4363-4389.
- Bolto BA, Dixon DR, Gray SR, Ha C, Harbour PJ, et al. (1996) The use of soluble organic polymer in waste treatment. Water Sci Technol 43(9): 117-124.
- Ødegaard H (1998) Optimized particle separation in the primary step of wastewater treatment. Water Sci Technol 37(10): 43-53.
- Jekel MR, Heinzmann B (1989) Residual aluminum in drinking-water treatment. J Water Supply Res Technol-Aqua 38: 281-288.
- Pidou M, Avery L, Stephenson T, Jeffrey P, Parsons SA, et al. (2008) Chemical solutions for greywater recycling. Chemosphere 71: 147-155.
- De Feo G, De Gisi S, Galasso M (2008) Definition of a practical multi-criteria procedure for selecting the best coagulant in a chemically assisted primary. Desalination 230: 229-238.
- Thompson KA, Valencia EW, Summers RS, Cook SM (2020) Sorption, coagulation, and biodegradation for graywater treatment. Water Sci Technol 81(10): 2152-2162.
- Vinitha EV, Abammed MM, Gadekar NR (2018) Chemical coagulation of greywater: Modelling using artificial neural networks. Water Sci Technol 2017(3): 869-877.
- Ghaitidak D, Yadav K (2014) Effect of coagulant in greywater treatment for reuse: Selection of optimal coagulation condition using analytic hierarchy process. Desalin Water Treat 55(4): 913-925.
- Chitra D, Muruganandam L (2020) Performance of natural coagulants on greywater treatment. Recent Innov Chem Eng 13(1): 81-92.
- Alharbi SK, Shafiquazzaman M, Heider H, Al Saleem SS, Ghumman A (2019) Treatment of ablution greywater for recycling by alum coagulation and activated carbon adsorption. Arab J Sci Eng 44: 8389-8399.
- Bielski A, Giermek A (2019) Coagulation of greywater from a small household. Sewer Environ Monit 24: 31-155.
- An C, Huang G, Yao Y, Zhao S (2017) Emerging usage of electrocoagulation technology for oil removal from wastewater: A review. Sci Total Environ 579: 537-556.
- Lai CL, Lin SH (2003) Treatment of chemical mechanical polishing wastewater by electrocoagulation: System performance and sludge settling characteristics. Chemosphere 54(3): 235-242.
- Al-Shannag M, Al-Qodah Z, Bani-Melhem K, Qtaishat MR, Alkasrawi M (2015) Heavy metal ions removal from metal plating wastewater using electrocoagulation: Kinetic study and process performance. Chem Eng J 260: 749-756.
- Woytowich DL, Dalrymple CW, Britton MG (1993) Electrocoagulation treatment of ship bilgewater for the US Coast Guard in Alaska. Marine Technol Soc J 27(1): 92.
- Mohamud AA, Çalışkan Y, Bektaş N, Yatmaz HC (2018) Investigation of shipyard wastewater treatment using electrocoagulation process with Al electrodes. Sep Sci Technol 53(15): 2468-2475.
- de Santana MM, Zanoelo EF, Benincá C Freire FB (2018) Electrochemical treatment of wastewater from a bakery industry: Experimental and modeling study. Process Saf Environ Prot 116: 685-692.
- Zhang H, Luo Z, Zhang Z, Liu Y, Chen Y (2020) Electro-flocculation pre-treatment experiments of shale gas drilling wastewater. Nat Gas Indust B 7(4): 309-316.
- Chu JY, Li YR, Li N, Haung WH (2012) Treatment of car-washing wastewater by electrocoagulation-ultrasound technique for reuse. Adv Mater Res 433-440: 227-232.
- Gonder ZB, Balcioglu G, Vergili I, Kaya Y (2007) Electrochemical treatment of carwash wastewater using Fe and and Al electrode: Techno-economic analysis and sludge characterization. J Environ Manag 200: 380-390.
- Moulood I, Abdul-Majeed BA (2019) Treatment of simulated carwash wastewater by electrocoagulation with sonic energy. J Eng 25(9): 30-40.
- Atiyah IM, Abdul-Majeed BA (2019) Carwash wastewater treatment by electrocoagulation using aluminum foil electrodes. J Eng 25(10): 50-60.
- Ansari K, Shrikhande AN (2019) Feasibility on Grey Water Treatment by Electrocoagulation Process: A Review. Int J Emerg Technol 10(1): 85-92.
- Barzegar G, Wu J, Ghanbari F (2019) Enhanced treatment of greywater using electrocoagulation/ozonation: Investigation of process parameters. Process Saf Environ Prot 121: 125-132.
- Barisci S, Turkay O (2016) Domestic greywater treatment by electrocoagulation using hybrid electrode combinations. J Water Process Eng 10: 56-66.
- Sahu O, Mazumdar B, Chandhari PK (2014) Treatment of wastewater by electrocoagulation: a review. Environ Sci Pollut Res 21: 2397-2413.
- Karichappan T, Venkatachalam S, Jeganathan PM (2014) Optimization of electrocoagulation process to treat grey wastewater in batch mode using response surface methodology. J Environ Health Sci Eng 12: 20-37.
- Sarkka H, Bhatnagar A, Sillanpaa M (2015) Recent developments of electro-oxidation in water treatment: A review. J Electroanal Chem 754: 46-56.
- Robles-Molina J, de Vidales MJM, García-Reyes JF, Cañizares P, Sáez C, et al. (2012) Conductive-diamond electrochemical oxidation of chlorpyrifos in wastewater and identification of its main degradation products by LC–TOFMS. Chemosphere 89(10): 1169-1176.
- Gurung K, Ncibi MC, Shestakova M, Sillanpaa M (2018) Removal of carbamazepine by electrochemical oxidation using a Ti/Ta2O5-SnO5 Appl Catal B: Environ 221: 329-338.
- Basile A, Cassano A, Rastog NK (2015) Advances in membrane Technology for wastewater treatment: Materials, processes and application. Woodhead Publishing, Cambridge, UK.
- Chu Y, Wang W, Wang M (2010) Anodic oxidation process for the degradation of 2, 4-dichlorophenol in aqueous solution and the enhancement of biodegradability. J Hazard Mater 180(1-3): 247-252.
- Bogdanowicz R, Fabiańska A, Golunski L, Sobaszek M, Gnyba M, et al. (2013) Influence of the boron doping level on the electrochemical oxidation of the azo dyes at Si/BDD thin film electrodes. Diam Relat Mater 39: 82-88.
- Ramírez C, Saldaña A, Hernández B, Acero R, Guerra R, et al. (2013) Electrochemical oxidation of methyl orange azo dye at pilot flow plant using BDD technology. J Indust Eng Chem 19(2): 571-579.
- Luu TL (2020) Tannery wastewater treatment after activated sludge pre-treatment using electro-oxidation on inactive anodes. Clean Technol Environ 22: 1701-1713.
- Nayir TY, Kara S (2017) Container washing wastewater treatment by combined electrocoagulation-electrooxidation. J Sep Sci Technol 53(8): 1-12.
- Liu YJ, Hu YJ, Lo SL (2018) Direct and indirect electrochemical oxidation of amine-containing pharmaceuticals using graphite electrodes. J Hazard Mater 366: 592-605.
- Ganiyu S, Dos santos EV, de Araújo Costa ECT, Martínez-Huitle CA (2018) Electrochemical advanced oxidation processes (EAOPs) as alternative treatment techniques for carwash wastewater reclamation. Chemosphere 211: 998-1006.
- Ulucan-Altuntas K (2021) Fluidized electrooxidation process using three-dimensional electrode for decolorization of reactive blue. Akcademik Platform Muhendislik ve Fen Bilimelrri Dergisi 9(1): 53-59.
- Butkovskvi A, Jeremiasse AW, Leal LH, Zande TV, Rijnaartso H, et al. (2014) Electrochemical conversion of micropollutants in graywater. Environ Sci Technol 48(3): 1893-1901.
- Ghanbari F, Martinez-Huitle CA (2019) Electrochemical advanced oxidation processes coupled with peroxymonosulfate for the treatment of real washing machine effluent: A comparative study. J Electroanal Chem 847: 113182.
- Drennan DM, Koshy RE, Gent DB, Schefer CE (2019) Electrochemical treatment for greywater reuse: effects of cell configuration on COD reduction and disinfection byproduct formation and removal. Water Supply 19(3): 891-898.
- Patidar R, Srivastava VC (2020) Understanding of ultrasound enhanced electrochemical oxidation of persistent organic pollutants. J Water Process Eng 37: 101378.
- Zhang G, Ruan J, Du T (2021) Recent advances on photocatalytic and electrochemical oxidation for ammonia treatment from water/wastewater. ACS EST Eng 1(3): 310-325.
- Borrell P, Bulltjes P, Grennfelt P (1997) Photooxiants, acidification and tools: Policy application of eurotrac results, Springer Publisher, Berlin Heidelberg, Germany.
- McNaught AD, Wilkinson A (1996) Glossary of terms used in photochemistry. Blackwell Science Publication, Oxford, UK.
- Ranby BG, Rabeck JF (1975) Photodegradation, photooxidation and photo stabilization of polymers. Principles and applications, John Wiley and Sons, New York, USA.
- Schrauzer GN, Guth TD (1977) Photolysis of water and photoreduction of nitrogen on titanium dioxide. J Am Chem Soc 99(23): 7189-7193.
- Thiruvenkata R, Vigeneswaran S, Moon S (2008) A review on UV/TiO2 photocatalytic oxidation process. Korean J Chem Eng 25: 64-72.
- Chong MN, Lin B, Chow CWK, Saint C (2010) Recent developments in photocatalytic water treatment technology: A review. Water Res 44(10): 2997-3027.
- Rivero MJ, Parsons SA, Jeffrey P, Pidou M, Jefferso B (2006) Membrane chemical reactor (MCR) combining photocatalysis and microfiltration for grey water treatment. Water Sci Technol 53(3): 173-180.
- Lopez L, Panther BC, Tumey TW (2015) Contaminant effects on the photo-oxidation of greywater over titania film catalysts. J Water Process Eng 7: 46-53.
- Alrousan D, Afkhami A, Bani-Melhem K, Dunlop P (2020) Organic degradation potential of real greywater using TiO2-based advanced oxidation processes. Water 12(10): 2-18.
- Boyjoo Y, Ang M, Pareek V (2012) Photocatalytic treatment of shower water using a pilot scale reactor. Int J Photoenergy 12: 578916.
- Dubowski Y, Alfiya Y, Gilboa Y, Sabach S, Friendler E (2020) Removal of organic micropollutants from biologically treated greywater using continuous-flow vacuum-UV/UVC photo-reactor. Environ Sci Pollut Res 27: 7578-7587.
- Grcic I, Vrsaliko D, Katancic Z, Papic S (2015) Purification of household greywater loaded with hair colorants by solar photocatalysis using TiO2-coated textile fibers coupled flocculation with chitosan. J Water Process Eng 5: 15-27.
- Amanious A, Ozkan A, Sobmen U, Gulyas H (2011) Inorganic greywater matrix impact on photocatalytic oxidation: Does flocculation of TiO2 nanoparticles impair process efficiency? Water Sci and Technol 63(12): 2808-2813.
- Agullo-Barcelo M, Polo-Lopez MI, Lucena F, Jofre J, Frnandez-Ibanez P (2013) Solar Advanced Oxidation Processes as disinfection tertiary treatments for real wastewater: Implications for water reclamation. Appl Cataly B: Environ 136-137: 341-350.
- Tien C (2019) Introduction to adsorption: Basics, analysis, and applications. Wiley and Son Inc., New York, USA.
- Toth J (2002) Adsorption: Theory, modelling and analysis. Marcel Dekker Inc, New York, USA.
- Atkins PW, Paula JD, Keeler J (2018) Atkin’s physical chemistry, Oxford, U K.
- Calvert JG (1990) Glossary of atmospheric chemistry terms. Pure Appl Chem 62(11): 2167-2219.
- Ferrari L, Kaufmann J, Winnefeld F, Plank J (2010) Interaction of cement model systems with superplasticizers investigated by atomic force microscopy, zeta potential, and adsorption measurements. J Colloid Interface Sci 347(1): 15-24.
- Siyal AA, Shamsuuddin MR, Low A, Rabat NE (2020) A review on recent developments in the adsorption of surfactants from wastewater. J Environ Manag 254: 109797.
- Sales FRP, Serra RBG, de Figueiredo GJA, da Hora PHA, de Sousa AC (2019) Wastewater treatment using adsorption process in column for agricultural purposes. Revista Ambiente Água 14(1): 2178.
- Patel P, Muteen A, Mondal P (2019) Treatment of greywater using waste biomass derived activated carbons and integrated sand column. Sci Total Environ 711: 134586.
- Topkava E, Veli S, Arslan A, Kurtkulak H, Zeybek S, et al. (2018) Investigation of greywater treatment by adsorption process using polymeric composites supported with activated carbon. Eurasian J Environ Res 2(2): 14-19.
- Guo X, Li M, Lin A, Jiang M, Niu X, et al. (2020) Adsorption mechanisms and characteristics of Hg2+ removal by different fractions of biochar. Water 12(8): 2105.



















