Research Article
Antibacterial Activity of Sanicula Elata Crude Extracts Invitro Against on Streptococcus Mutans Isolated from Dental Decays
3570
Views & Citations2570
Likes & Shares
Dental decay is the dissolution of tooth mineral by acids derived from bacterial fermentation of sucrose and other dietary carbohydrates. The aim of this study was to isolate and identify Streptococcus mutans from decayed teeth; and to evaluate antibacterial activity of Sanicula elata crude extracts invitro against Streptococcus mutans.
10 dental samples were taken from Turinesh Beijing General Hospital to isolate dental decay bacteria. The primary culture was done by culturing bacteria on nutrient broth and agar. Sub culturing was done on selective media blood agar until pure colony was obtained. Various biochemical tests were performed to identify Streptococcus mutans. Antibacterial activity of Sanicula elata was evaluated by extracting crudes from plants leaf using organic solvents (chloroform, ethanol and distilled water). Antibacterial assay was performed on Muller Hinton Agar using well-diffusion method.
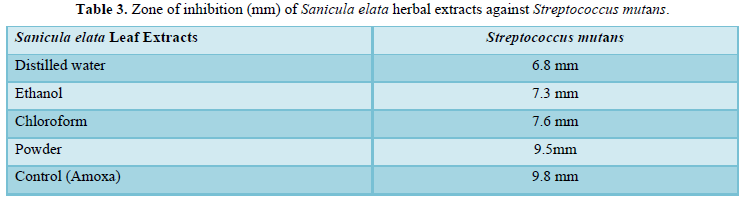
Result from all tests indicates that among 10 dental samples streptococcus mutans was founded in eight teeth patients. After Streptococcus mutans identified, Antibacterial activity of crude extracts showed that zone of inhibition: distilled water (6.3mm), ethanol (7.3mm), chloroform (7.6mm) and powder (9.5mm). The standard control Amoxicillin shows (9.8mm) against Streptococcus mutans.
Further investigations should conduct to evaluate the efficacy of Sanicula elata on several antimicrobial groups within range of concentration. Finally, Extra study is needed to characterize phyto-constitute of Sanicula elata for use as a potential source of drug.
Keywords: Crude extract, Dental decays, Inhibition zone, Sanicula elata, Streptococcus mutans
INTRODUCTION
Dental decay is occurred due to the irreversible solubilization of tooth mineral by acid produced by certain bacteria that adhere to the tooth surface in bacterial communities known as dental plaque. The tooth surface normally loses some tooth mineral from the action of the acid formed by plaque bacteria after ingestion of foods containing fermentable carbohydrates. This mineral is normally replenished by the saliva between meals. However, when fermentable foods are eaten frequently, the low pH in the plaque is sustained and a net loss of mineral from the tooth occurs. Streptococcus mutans is the main cause of dental decay. This low pH is selected by acid- loving bacteria specially S. mutans store polysaccharide and continue to secrete acid that demineralize teeth long after the food has been swallowed [1].
Herbs have been a valuable source of medication in virtually all cultures and societies worldwide due to their important antimicrobial constituents and wider therapeutic potentials. The natural products obtained from plants contain very rich biologically active constituents which have great potential against bacterial species and they act as main ingredient in various pharmaceutical products. Extracts or phytoconstituents derived from various parts of medicinal plants are applicable for prevention and cure of several diseases. It provides for therapeutic modalities with broad spectrum antimicrobial activities against various pathogenic microorganisms [2]. Due to adverse effects of chemical-based remedies the use of plant and plant-based products are organic and emerged out as a best alternative.
The herbal medicines have broad spectrum antibiotic effect, less cost and easily available, less side effects and it difficult to develop resistance by pathogen [3]. The aim of this study was to isolate and identify Streptococcus mutans from decayed teeth; and to evaluate antibacterial activity of Sanicula elata crude extracts invitro against Streptococcus mutans.
MATERIAL AND METHODS
Sample Collection
Ten dental samples (n=10) were collected from patients who were clinically diagnosed as suffering from dental disease at Tirunesh Beijing General Hospital, Addis Ababa, Ethiopia. All the samples were immediately taken using transport media in sterile neck tube to avoid contamination. It was sent to the Addis Ababa Science and Technology University, Microbiology laboratory. Then, it was kept at +4°C prior for isolation and identification of dental carries bacteria species.
Primarily dental samples were spread on sterile nutrient broth and nutrient agar (Hi Media) anaerobically at 37°C for 24 h. Sub-culture was done repeatedly at 37°C for 24-36 h anaerobically on the Blood agar and Brian heart infusion until pure colony was obtained.
The identification of Streptococcus mutans is based on Bergeys Manual of Determinative Bacteriology 9th ed., 1994 distinctive colonial morphology on selective media, Gram staining, distinctive cell shape on light microscopy, specific growth characteristics, and sugar fermentation. Furthermore, biochemical tests were conducted to confirm the bacterial species including growth on high sucrose concentration, salt and acid media, dextran production, catalase tests.
Gram Test
Morphological characterization was done using differential staining method. After incubation, bacterial colonies were smeared onto microscopic slides then stained with gram stain and observed under light microscope [4].
Dextran Production Test
A 2ml of Nutrient broth medium was inoculated with loop full of bacterial culture then incubated anaerobically at 37°C for two days. The culture was centrifuged at 3000xg for 10 min, 0.1ml of supernatant of each culture was added to each three tubes and stirred with 0.3 ml of 10% sodium acetate. A 0.8-fold volume of acetone was added to tube 1; a 1.2-fold volume of ethanol to tube 2 and 1.5-fold volume of methanol was added to tube 3. Each tube was shacked for 3 min and observed. The flocculation in each tube indicates dextran production [5].
The Fermentation of Different Carbohydrates Sources
The bacterial isolates can ferment different carbohydrates sources were determined follow the method described by Fingold and Barone [6]. Brain heart infusion broth supplemented with 10% of sucrose. Brain heart infusion broth medium used as negative control, while Sucrose as positive control and added aseptically to the autoclaved brain heart infusion broth and incubated anaerobically at 37°C for 72 h. The PH of media has lowered to 4.5 as compared with the controls indicated the ability of these bacteria to ferment and convert it to lactic acid these carbohydrates sources.
Tolerance to High Concentration of Sodium Chloride
The ability of bacterial isolates to growth in 4% NaCl was tested using nutrient broth medium and incubated anaerobically at 37°C for 48 hrs. Turbidity was indicated for the ability of the bacterial isolates to tolerate this concentration of NaCl as compared with control which did not contains concentration of NaCl [7].
Catalase Test
Nutrient agar was placed in slant and bacterial species were inoculated and spread on slant tube and was incubate at optimum temperature for 24‐48 h. 3% of H2O2 was dropped on the slant culture. There was no any bubble formed in two test tubes [4].
Plant Material
Plant specimens were gathered from the Akaki Kality sub-city around the Tulu Dimtu site. In the study area, the vernacular name was termed as ‘Gororsa’ in the Afan Oromo language. Its Scientific Name is Sanicula Elita.
Preparation of Sanicula Elata Crude Extract
Sanicula elata leaves sample was collected from Baseka River, Addis Ababa. Antibacterial crudes were extracted from its leaf by chloroform, ethanol and its leaf powder. According to Biarwa [8] those solvents have potential to extract crudes from plant parts. The leaves of Sanicula elata was cleaned and sterilized with 70% alcohol and dried. After drying the plant parts was macerated using mortal and pistil. 3g of leaf powder was extracted exhaustively at room temperature. 1 gram of leaf powder was immersed in each of three conical flasks containing 20ml, 95% ethanol, chloroform, and distilled water solvent. It was stayed for 24 h with in shaker. The of each suspension were filtered in separate flasks by using standard filter paper Whatman number 1 and extracts was concentrated in mg/ml. Extracts were delivered in sterile screw cap test tube with suitable labeling and were kept at 4°c until used.
Antibacterial Assay
Antibacterial susceptibility of streptococcus mutans strains was determined invitro after culturing Streptococcus mutans on Muller Hinton Agar (HI Media). Antibacterial assay was performed using agar well diffusion method. Muller Hinton agar was prepared according to manufacturer instruction, and 0.1ml (0.5 Mc Farland; 1.5 × 108 CFU/ml) of bacteria suspensions was pipetted and spread uniformly with sterile spreader.
Then, left to dry for five up to ten min. A five pore was made in the plate with the help of sterile cork borer (0.65cm). 100µl of plant extracts (distilled water, ethanol, chloroform and 500mg of solid plants powder) and control antibiotic (Amoxicillin) compound was introduced into the respective wells aseptically. It was kept in refrigerator for 20 up to 30 min until diffuse and incubated overnight at 37oC. Zone of inhibition was measured (mm) by subtracting the well diameter.
DATA ANALYSIS
Antibacterial activity of Sanicula elata leaf crude extracts against Streptococcus mutans was calculated on average. The outcome of antibacterial effect was analyzed using Microsoft spreadsheet 2010, and finally interpreted in a simple table.
RESULTS AND DISCUSSION
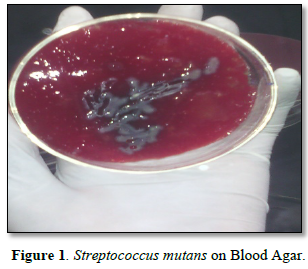
Dental decay is due to the dissolution of tooth mineral by acids derived from bacterial fermentation of sucrose and other dietary carbohydrates. These bacteria live in bacterial communities known as dental plaque which accumulates on the tooth surface. Dental caries is one of the most common chronic infectious diseases in the world [1]. Streptococcus mutans is the main cause of dental decay (Figure 1). Lactobacillus casei and Streptococcus faecalis are also associated with progression of the lesion. Streptococcus mutans is acidogenic bacteria that ferment sucrose produce acids, which in vitro lower the pH value to below 5.0 (Table 1). The major pathway is concerned with energy metabolism [9].
The natural products (Figure 2) derived from medicinal plants have proven to be an abundant source of biologically active compounds, many of which have been the basis for the development of new lead chemicals for pharmaceuticals [10].
In dextran production tests, the occurrences of flocculation in all three tubes and turbidity in the alcohols have indicated that formation of dextran. The bacteria also grow on high concentration of 10% sucrose and ferment it to lactic acid, and become lower pH up to 4.5. It also grows on high salt concentration at 4% Sodium chloride. High turbidity which indicates high bacterial growth was observed in nutrient broth. During catalase test bacteria didn’t indicate foam. Hence, it is catalase negative. Gram tests also showed that purple color which indicates gram positive. The study outcome is similar with Essam [7] and Nada [4]. Then, we decide the bacterium (Table 2) is streptococcus mutans.
Antibacterial effect of Sanicula elata extracts (Table 3) showed that variable results. The highest zone of inhibition was recorded from dry powder (9.5mm) without mixing any extraction agent. Extracts of Ethanol (7.3mm) and Chloroform (7.6mm) have moderate inhibition zone. The lowest inhibition zone was observed from Distilled water. According to Alazar and Endeshaw [11], Ethanol leaf extract from Hagenia abyssinica (Bruce) JF Gmel was effect against Shigella flexneri (2mm), Staphylococcus aureus (3mm), and Escherichia coli (4mm) through well diffusion method. As Seleshe [12] stated that the antimicrobial activities of ethanol, methanol, chloroform, and water extracts of Moringa stenopetala Leaves against Escherichia coli pathogens were 8mm, 12mm, 11.5mm, and 8mm respectively through disc diffusion method.







CONCLUSION
The dominant bacterium species that responsible for dental decay was Streptococcus mutans. It was majorly isolated from eight dental decay patients. Sanicula elata leaf shows high antibacterial activity invitro against Streptococcus mutans. When compare powder (9.5mm) of Sanicula elata with the control amoxicillin (9.8mm). It has almost relatively equal inhibition zone. Over all, Sanicula elata may potentially source for synthesizing antibiotic for future dental drugs.
Further investigations should conduct to evaluate the efficacy of Sanicula elata on several antimicrobial groups within range of concentration. In the future phytochemical composition analysis and in-vivo bioassays should implement to know its efficiency.
ACKNOWLEDGEMENT
We would like to thank Tirunesh Beijing Hospital Administration and Dental Case Team for their collaboration during sample collection. We would like to express our deep gratitude to Addis Ababa Science and Technology University for financial support and microbiology laboratory staff for their valuable assistance, guidance and support for the success of this research.
CONFLICTS OF INTEREST
The authors declare that no conflict of interest.
- Chandrabhan D, Hemlata R, Renu B, Pradeep V (2012) Isolation of dental caries bacteria from dental plaque and effect of tooth pastes on acidogenic bacteria. Open J Med Microbiol 2: 65-69.
- Dhama K, Tiwari R, Chakraborty S, Saminathan M, Kumar A, et al. (2014) Evidence based antibacterial potentials of medicinal plants and herbs countering bacterial pathogens especially in the era of emerging drug resistance. Int J Pharmacol 10: 1-43.
- Chaiya A, Saraya S, Chuakul W, Temsiririrkku R (2013) Screening for Dental Caries: Preventive Activities of Medicinal Plants against Streptococcus mutans. Mahidol Univer J Pharm Sci 40(1): 9-17.
- Al-Mudallal N, Al-Jumaily EFA, Muhimen NAA, Al-Shaibany AAl-W (2008) Isolation and identification of mutan's streptococci bacteria from human dental plaque samples. Al-Nahrain J Sci 11(3): 98-105.
- Guthof O (1970) The presence and demonstration of ektopolysaccharides in streptococci. Central sheet for bacteriology, parasite science, infectious diseases and hygiene. Originale 215(4): 435-440.
- Fingold S, Barone E (1986) Method of identification of etiological agent of infection disease. In: "Bailey and Scotts diagnostic microbiology". 7th C. V. Mosby; C. St. Louis. pp: 382.
- Al-Jumaily EFA, AL-Seubehawy HMZ, Al-Toraihy FA (2014) Isolation and Identification of Streptococcus mutans (H5) produced glucosyltransferase and cell-associated glucosyltransferase isolated from dental caries. Int J Curr Microbiol App Sci 3(6): 850-864.
- Bairwa R, Gupta P, Srivastava B (2012) Traditional Medicinal Plants: Use in Oral hygiene. Int J Pharm Chem Sci 1(4): 2277-5005.
- Pathak A, Sardar A, Kadam V, Pekadwad B, Karuppayil M (2012) Efficiency of some medicinal plants against human dental pathogens. Indian J Nat Prod Resour 3(1): 123-127.
- Yadav R, Yadav SK (2012) Dental disease and its cure. Asian J Pharm Clin Res 6: 974-2441.
- Alazar Y, Endeshaw A (2020) Antibacterial Effect of Hagenia Abyssinica (Bruce) JF Gmel Against on Selected Pathogens. Adv Biotechnol Microbiol 15(4): 84-90.
- Seleshe S, Kang SN (2019) In Vitro Antimicrobial Activity of Different Solvent Extracts from Moringa stenopetala Prev Nutr Food Sci 24(1): 70-74.




