Research Article
Carwash Wastewater Treatment and Reclamation Technologies for Water Conservation and Pollution Load Reduction: A Review
28087
Views & Citations27087
Likes & Shares
Carwash stations use large volumes of water and release wastewater containing harmful chemicals into the environment. The type and quantity of cleaning solutions and finish products used and the soil present on the vehicle add harmful pollutants to the water and affect the characteristics of the carwash wastewater. Therefore, understanding how much water is used by the carwash industry and the pollution load of wastewater produced is necessary to ensure adoption of water conservation measures and design wastewater recycling systems. The growing public concern for water conservation, and the environmental health of waterways has led to several environmental regulatory structures designed to protect watersheds and enhance wastewater reclamation. Professional carwash wastewater reclamation has been in use for many years and is growing in sophistication. Most reclaim systems that have been installed are used to reduce freshwater consumption or reduce sewer discharge (volume and pollution load) and meet regulatory demands or a combination of both. The desire by the professional carwash operator to conserve water or reduce discharges will dictate the choice treatment and water reclaim equipment to be installed. This study describes the treatment and recycling options for carwash wastewaters used to achieve pollution reduction, water conservation and economic benefits for carwash operators. The general categories of treatment include electro chemical treatments (chemical coagulation-flocculation, electrocoagulation, electrooxidation), physical treatments (granular filtration, microfiltration, ultrafiltration, nanofiltration, reverse osmosis and adsorption), biological treatments (biofilters, bioreactors and wetlands). The environmentally friendly, modern carwash requires a good washing technology with compatible washing chemicals followed by advanced water treatment method with proper recycling system. Professional carwash reclaiming systems use water treated in one or more of these manners although technology may differ from installation to installation. It is important to note that choosing the wrong combination of cleaning solutions or treatment processes can create more problems than it solves. It is imperative for the professional carwash operator to understand each element of the reclaim system and its intended use.
Keywords: Carwash, Water Use, Water Conservation, Wastewater Characteristics, Wastewater Treatments
INTRODUCTION
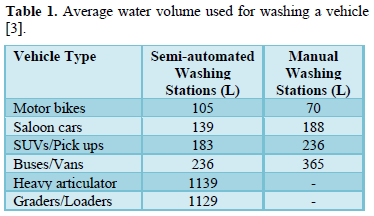
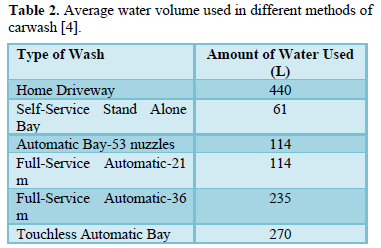
Carwash stations use large volumes of water and release wastewater containing harmful chemicals (cleaning solutions and finish products used to clean mobile vehicles) and soil and salt particles into the environment [1-6]. The amount of water used to wash a vehicle depend on the size of the vehicle (Table 1) and the type of washing system (Table 2) and the pollution load depends on the type of chemicals used in washing and amount of dirt on vehicles [3,4].
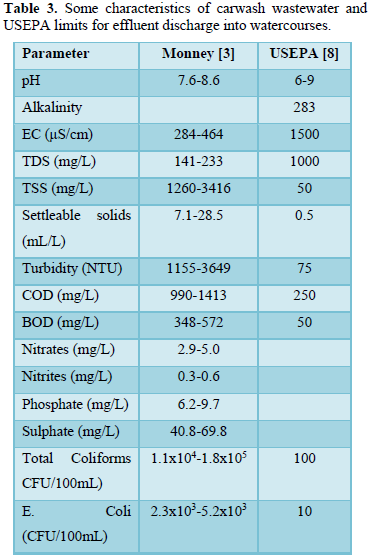
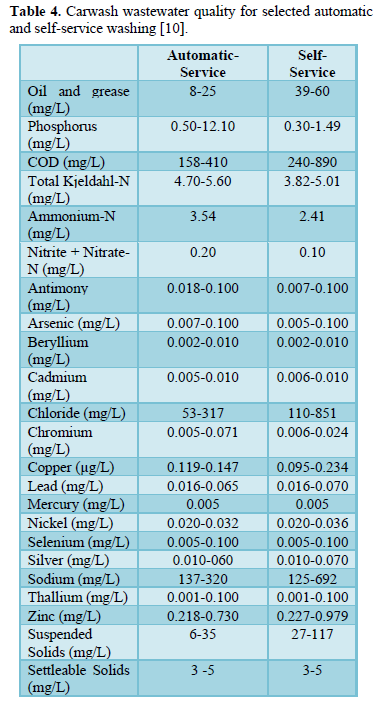
Carwash wastewater effluents contain dissolved, suspended and settleable solids, oil and grease, surfactants, nitrogen (ammonium, nitrite and nitrate), phosphorus (phosphate), sulfur (sulfate) metals (antimony, arsenic, beryllium, cadmium, chloride, chromium, copper, lead, mercury, nickel, selenium, silver, sodium, thallium and zinc) and microorganisms (total coliform, E. coli, Aeromonas, Pseudomonas. Proteobacteria, Bacteroidetes, Firmicutes, Acidobacteria, and Verrucomicrobia [3,5-10]. Table 3 shows some characteristics of carwash wastewater reported by Monney [3] and the effluent limits for discharge into watercourses prepared by USEPA [8]. Table 4 shows carwash wastewater quality of automatic and self-service washing operations [10]. The presence of pollutants in carwash wastewater affects the wastewater characteristics including pH [3,6,11-13], temperature [14-16], electric conductivity [9,12,16,17], turbidity [3,5,6,8,], COD and BOD [3,8,10,16-19], all of which have significant impact on human health and aquatic life.


More than 99 percent of professional car washing operations in USA [8], Canada [20], England [21], Australia [22], Brazil [23], Germany [24] and elsewhere [5,7,14] discharge their effluents to sanitary sewers (SS) and publicly owned treatment works (POTW). Only the POTW provides pretreatment guidance for discharge limits which is usually accomplished through local Municipal Regulations. However, there are many carwash stations that are located outside municipal sewer system zones and as such discharge their wastewaters into nearby waterbodies [11,14,16-18].
Morel and Diener [24] stated that global water resource supplies are worsening, and water shortages will affect 2.7 billion people by 2025 which means 1 out of every 3 people in the world will be affected by the water shortage problem. Therefore, reclaiming carwash wastewater is becoming of paramount importance in solving the problem of water shortage. Understanding how much water is used for car washing and the pollution loads of wastewater resulting from carwash operations are necessary to ensure adoption of water conservation measures and to design/select sustainable wastewater treatment and recycling systems [5,6].
Janik and Kupiec [4] and Brown [25] stated that effective car washing technology results in high quality wash, but also results in high-water consumption and wastewater with high pollution load. On the other hand, Barnes [1] and Manney [3] indicated that the growing public concern for water conservation, the health and safety of the public water supply and the environmental health of streams, rivers and other waterways led to several environmental regulatory structures designed to protect drinking water supplies and watersheds. Therefore, treatment of carwash wastewater is critical not only for the prevention of environmental contamination but also for recycling of such high-volume water resource and reducing the cost of car washing.
Fall [6] reported that a pre-treatment step of carwash wastewater may be accomplished first through a series of tanks buried underground known as an oil-water separator. Wastewater from carwash drains by gravity into the first in which oil is separated and only water from within the tank flow into subsequent tanks.


The grit collected in the oil/water separator is pumped out on a periodic basis, dewatered, and sent to a properly licensed landfill. Further treatment of carwash wastewater can be carried out using one or a combination of several treatment and recycling options. These options include chemical coagulation/flocculation, electrocoagulation, electrooxidation, granular filtration, microfiltration, ultrafiltration, nanofiltration, reverse osmosis, biofilters, bioreactors, wetland and adsorption. The main aim of this study was to review the available technologies for the treatment and recycling of carwash wastewater and shed some light on their uses and performances.
ELECTRO-CHEMICAL TREATMENTS
There are several carwash treatment systems that have been used for treatment of carwash wastewaters for pollution load reduction and reuse in same operation. The most commonly used systems are chemical coagulation-flocculation (CC), electrochemical coagulation (EC) and electrooxidation (EO).
Chemical Coagulation-Flocculation
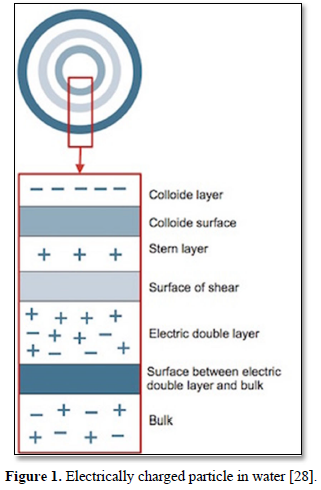
Coagulation and flocculation are techniquesused for treatment of wastewaters containing colloids (suspended particles) and metal ions. In coagulation, particles aggregate with themselves by the influence of a change in pH while in flocculation, particles aggregate using polymers that binds them together [26,27]. Particles in water are electrically charged as shown in Figure 1 [28]. The area nearest to the particle is divided into two layers. Closest to the electrically charged particle counter, ions will gather and create the first layer which is called the stern layer. The next layer is composed of both counter-ions and co-ions but with a surplus of counter-ions. The bulk which is the surrounding water has an equal distribution of counter-ions and co-ions [29-31].
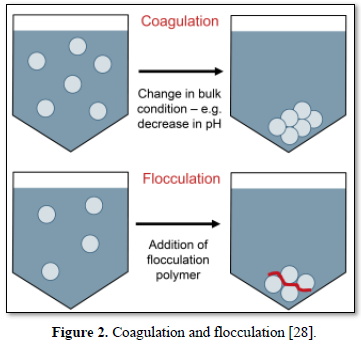
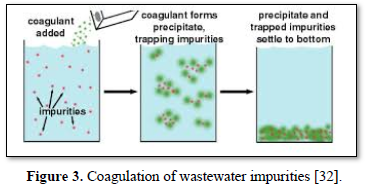
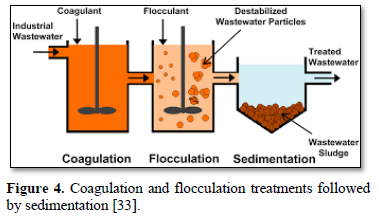
In coagulation, the two layers around the particle cause it to be stable in the water. When the conditions within the water are changed by a change in pH or conductivity, the number of ions in the water changes and affects the amounts of ions in the two layers, thereby affects the stability of the particles and force them to settle as shown in Figure 2-top [28]. In flocculation, electrically charged particles precipitate by using flocculation polymers with charged sites. By using a polymer with the opposite charge as that of the particles to be flocculated, the particles will bound to the polymer, combining together into larger particles that cannot stay suspended as shown in Figure 2-bottom [28]. When particles are precipitated from the solution (Figure 3), further filtration treatment is necessary to obtain the desired water quality [32]. Figure 4 shows a system having the processes of coagulation, flocculation, and sedimentation [33].
Coagulation and flocculation are essential processes in water and wastewater treatments. Clarification of water using coagulating agents has been practiced from ancient times. As early as 2000 BC, the Egyptians used almonds smeared around vessels to clarify River Nile water. The use of alum as a coagulant by the Romans was mentioned in around 77 AD. By 1757, alum was being used for coagulation in municipal water treatment in England. In modern water treatment, coagulation and flocculation are essential components of the overall treatment processes. Many water utilities are committed to consistently producing treated water with turbidity of less than 0.1 NTU to guard against pathogen contamination. Coagulation and flocculation are also important in municipal wastewater treatment operations including chemical phosphorus removal and chemically enhancing primary treatment to reduce suspended solids as well as carwash wastewater treatment [31].


Polymers are a large range of natural and synthetic water-soluble macromolecular compounds that have the ability to enhance flocculation of the water constituents. Natural polymers have long been used as flocculants because they are virtually free of toxins, are biodegradable in the environment and the raw products are often locally available.


However, the use of synthetic polymers is more widespread because they are more effective and easier to control. Polymers are available in various forms including solutions, powders, beads, oils and water-based emulsions. One problem with synthetic polymers is relates to potential toxicity issues arising from residual of unreacted monomers [2,6,27,29].
The commonly used metal coagulants fall into two categories: those based on aluminum (Al) and those based on iron (Fe). The aluminum coagulants include aluminum sulfate, aluminum chloride, and sodium aluminate. The iron coagulants include ferric sulfate, ferrous sulfate, ferric chloride, and ferric chloride sulfate. Other chemicals used as coagulants include hydrated lime and magnesium carbonate. The aluminum and iron coagulants are effective because of their ability to form multi-charged polynuclear complexes with enhanced adsorption characteristics. The nature of the complexes formed can be controlled by the pH of the system. When Al and Fe coagulants are added to water, the metal-ions hydrolyze rapidly forming a series of metal hydrolysis species. Rapid mixing, pH, and the coagulant dosage determine which hydrolysis species is effective for treatment [29,30].
There has been considerable development of pre-hydrolyzed aluminum and iron coagulants to produce the correct hydrolysis species regardless of the treatment process conditions. These include aluminum forms such as aluminum chlorohydrate, poly-aluminum chloride, polyaluminum sulfate chloride, polyaluminum silicate chloride and forms of polyaluminum chloride with organic polymers. Iron forms include polyferric sulfate and ferric salts with polymers. There are also polymerized aluminum-iron blends. The principal advantages of these pre-polymerized inorganic coagulants are: they are able to function efficiently over wide ranges of pH and water temperatures, lower dosages are required to achieve treatment goals, they produce lower metal residuals, and fewer chemical residuals are produced resulting in lower final water total dissolved solids (TDS) [28-30].
Bolto [34] stated that organic polymeric flocculants have been used in water purification for several decades as coagulant aids or floc builders to replace inorganic coagulants like alum, iron salts and lime. The increased use of cationic polyelectrolytes as primary coagulants instead of inorganic is due to their significant inherent advantages which include: faster processing, lower content of insoluble solids to handle (by sedimentation, filtration, flocculation or biological conversion) and a much smaller volume. Organic polymers have often been used in chemically assisted sedimentation of sewage solids to enhance the removal of suspended matter. The concept is applicable to the primary coagulation of industrial wastewaters where the separation may be based on flotation as in the leather, steel, wood scouring, cosmetic, detergent, plastic, dyeing, paper, food processing and brewing industries.
Odegaard [35] stated that enhancing particle separation in the primary step of wastewater treatment should be the focus because the pollutants in wastewaters are associated with particulate compounds. The study showed that coagulation with metal salts was very efficient but can lead to excessive sludge production and demonstrated how the use of cationic polymers can reduce the sludge production considerably. The study also showed how improved flocculation, either chemically by the addition of flocculants or physically by improvements in the settling tank, can lead to better separation as well as smaller footprint of the treatment plant.
Jahel and Heinzmann [36] reported that coagulation and flocculation can be used for removal of dissolved solids and suspended particles including pathogens (Giardia and Cryptosporidium, a parasite that cases diarrhea), virus, arsenic, phosphorus, and fluoride. They also indicated that the efficiency of the coagulation-flocculation process is dependent on the type of coagulant used, coagulant dosage, coagulant feed concentration, type and dosage of chemical additives, sequence of chemical addition, pH and time lag between dosing points, intensity and duration of mixing, velocity gradients applied during flocculation stage, flocculator retention time, type of stirring device used and flocculator geometry.
Monney [37] assessed contaminants removal potential of a low-cost alum synthesized from bauxite slime waste as compared to industrial grade alum [Al2(SO4)3.18H2O] in treating carwash wastewater using standard jar tests. The results showed that removals of up to 99%, 34%, and 75% were achieved with 90 mg/L of the synthesized alum compared to 100%, 37%, and 74% for industrial grade alum for turbidity, anionic surfactants, and COD, respectively. The results of this study demonstrated the potential of alum synthesized from bauxite slime waste as a cheaper alternative for industrial grade alum in wastewater recycling in the carwash industry.
Li [38] separated the suspended particles from carwash wastewater by a hollow fiber membrane aided by an enhanced coagulation and activated carbon processes. Their study demonstrated that addition of KMnO4 to the coagulant polyaluminum chloride could enhance the efficiency of coagulation and in turn help reduce clogging of the ultrafiltration membrane and activated carbon. The existence of linear alkylbenzene sulfonate (LAS) can loosen the gel layer on the membrane and improve the flux. Adsorption of organic matter and oil was the main reason causing membrane flux decrease. When carwash wastewater was pretreated, the permeation flux of membrane showed a higher value. LAS, odor and color were removed by granules activated carbon adsorption treatment and the COD, BOD, LAS and oil values of recycled water were 33.4 mg/L, 4.8 mg/L, 0.06 mg/L and 0.95 mg/L, respectively.
Moazzem [39] reported that carwash centers all over Australia use 17.5 billion liters of water and discharge it as wastewater, spending $75 million a year for purchasing fresh water and discharging wastewater. They stressed the importance of developing simple and reliable systems to treat and reuse carwash wastewaters. Their study evaluated the performance of granular and membrane filtration systems combined with coagulation/flocculation and sedimentation in treating carwash wastewater for reuse. Overall, 99.9% of turbidity, 100% of suspended solids and 96% of COD were removed from the carwash wastewater using this treatment combination.
Zaneti [40] investigated the treatment of carwash wastewater from a typical carwash station in Brazil by flocculation-column flotation (FCF) plus sand filtration and chlorination. They performed a quantitative microbial risk assessment with a dose-response model. An Escherichia coli limit of 200 UFC/100 mL in the reclaimed water was chosen as acceptable microbiological risk. The results revealed that the chloride and TDS concentrations in reclaimed water were below 350 and 900 mg/L, respectively. The cost-benefit analysis showed that water reclamation was highly competitive, and the payback period might be as short as one year, depending on water price and daily wash demand.
Al-Gheethi [41] developed an integrated treatment system for carwash wastewater in Johor, Malaysia. The system was based on coagulation and flocculation using organic coagulant (dried seed powder of Moringa oleifera) and ferrous sulphate (FeSO4.7H2O) as inorganic coagulant followed by filtration. The coagulation and flocculation were carried out using different dosages (35, 70, 105 and 140 mg/L) of M. oleifera and FeSO4.7H2O. The integrated treatment system was effective in treating raw carwash wastewater and the treated water met the Environmental Quality Act (EQA 1974) Regulation of Malaysia.
Rodriguez-Boluarte [42] demonstrated the efficiency of the coagulation, ozonation and membrane bioreactor (MBR) in removing both solids and chemical contaminants from carwash wastewaters. They showed that the coagulants alum and polyaluminum chloride reduced all types of contaminants from carwash wastewater and the quality of the permeate produced by the MBR was extremely high. The work led to a new direction relating to water conservation and cost saving initiatives for the carwash industry.
Rubio and Zaneti [43] evaluated a new technique for flocculation and flotation called flocculation-column flotation (FCF) for the treatment of carwash wastewaters for reuse. The system was composed of a compact flocculation–flotation unit utilizing an inline flocculator device, a centrifugal multiphase pump which generates microbubbles and a column flotation for solid/liquid separation. A tannin derivative (water-soluble phenol derivative) was employed as a flocculant and aerated flocs (0.8-1.6 mm diameter and 45-150 m/h rise rate) were rapidly formed at 10 s residence time. Due to the rapid formation of these very light flocs, the FCF system was able to handle a high hydraulic-load capacity (>18 m/h) with a reduced footprint and reduced energy consumption.
Aboulhassan [44] employed a coagulation-flocculation process to treat carwash wastewaters for removal of surfactants. Jar-test experiments were employed to determine the optimum conditions (pH and effective dose) for the removal of surfactants, COD and turbidity. Treatment with FeCl3 proved to be effective in a pH range of 7-9 and showed reduction of 99% in surfactants and 88% in COD and increased the BOD5/COD index from 0.17 to 0.41.
Etchepare [45] assessed a flocculation–flotation treatment followed by sand filtration and ozonation (FFO) for the treatment of carwash wastewaters in Brazil to enhance the quality of reclaimed water. The FFO process provided disinfected (Escherichia coli < 1.8 CFU/100 mL) and clarified water (turbidity of 10 NTU) with minor foaming (residual surfactants of 1.30 mg) and no odor. The results revealed that the conductivity and dissolved solids concentrations of the ozone-treated water were lower than those of the chlorinated water. A cost-benefit analysis showed that the payback period for the FFO equipment might be as short as one year, depending on water price and daily wash demand.
Mohamed [46] evaluated the efficiency of commercial and natural coagulants in treating carwash wastewater in Malaysia over a period of 10 weeks. Two types of chemical coagulants [alum (KAl(SO₄)₂·12H₂O and ferrous sulphate (FeSO4)] and natural coagulants [seeds of Moringa oleifera and Strychnos potatorum] were evaluated using different dosages (30-200 mg/L). Moringa oleifera is a large tree native to North India and all parts of the tree are eaten or used in traditional herbal medicines. Strychnos potatorum is a moderate sized tree found in southern and central parts of India, Sri Lanka, and Burma and the seeds are used in traditional medicine. These coagulants were evaluated for their effect on pH, chemical oxygen demand, phosphorus, total suspended solid and turbidity. The results showed that the seeds of Moringa oleifera and Strychnos potatorum contained coagulating substances capable of removing up to 99 % of turbidity. The removal efficiencies of both natural coagulants were higher than those of chemical coagulants at low dosages of 30-80mg/L. Moringa oleifera showed removal of 90% in turbidity, 60% in COD and75% in phosphorus, whereas Strychnos Potatorum showed removal of 96% in turbidity, 55% in COD, 65% in phosphorus. Meanwhile, when using 150 mg/L alum and FeSO4, removals of 87% and 77% in turbidity, 74% and 71% in COD, and 81% and 65% in phosphorus, respectively.
Electrocoagulation
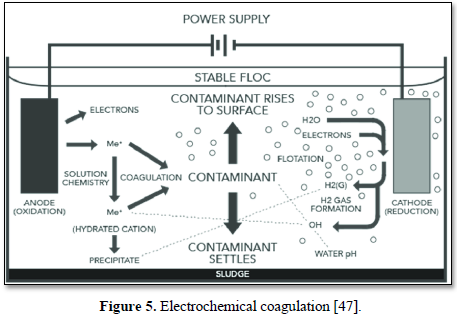
Electrocoagulation (EC) is an electrochemical process that simultaneously removes heavy metals, suspended solids, emulsified organics and many other contaminants from water and wastewater using electricity instead of expensive chemical reagents. The electrocoagulation device operates continuously and performs automated contaminant coagulation, flocculation, flotation, separation, and removal in a single enclosed reactor as shown in Figure 5 [47]. No polymer addition, settling or flotation tanks or filters are required [48].
The advantages of electrocoagulation are: (a) it addresses any size of suspended solids including the destructive >30 µm particles that can cause wear and tear to pressure washers and pose an environmental and employee hazard (b) it requires no filters, no daily maintenance and no additives and removes any size of suspended solids, oil, grease and heavy metals, (c) it requires simple equipment and is easy to operate with sufficient operational latitude to handle most problems encountered during operation, (d) wastewater treated by electrocoagulation results in clear, colorless and odorless water, (e ) sludge formed by electrocoagulation tends to be settable and easy to de-water, (f) flocs formed by electrocoagulation tend to be much larger, contain less bound water, acid- resistant, more stable, and can be separated faster by filtration, (g) it produces effluent with less total dissolved solids (TDS) content as compared with chemical treatments, (h) has little if any impact on sodium and potassium ions in solution and (i) the gas bubbles produced during electrolysis can conveniently carry the pollutants to the top of the solution where it can be more easily concentrated, collected, and removed by skimmer [48-50].
Treatment of wastewater by EC has been practiced for most of the 20th century with increasing popularity. In the last decade, EC technology has been increasingly used worldwide for treatment of wastewater from metal processing industries, mining industry, pulp and paper industry. EC treatment has also been applied to treat wastewater containing foodstuff, oil wastes, ink, dyes, synthetic detergent, wastewater from public transit, marinas, chemical and mechanical polishing and land fill leachates [47,48,50-55] as well as carwash wastewater [15,56-62].

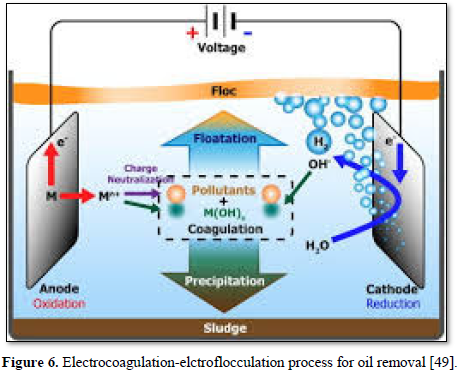
An [49] used a simple and efficient electrocoagulation treatment method for the removal of oil (Figure 6). The process involved the electro-dissolution of sacrificial anodes and formation of hydroxo-metal products as coagulants, while simultaneously producing hydrogen at the cathode to facilitate the removal of pollutants by flotation. The electrocoagulation treatment was effective in destabilizing oil-in-water emulsions by neutralizing charges and bonding oil to generated flocs and hydrogen bubbles.

Lai [50] investigated the treatment of copper chemical-mechanical polishing (CMP) wastewater from a semiconductor plant by electrocoagulation. The CMP wastewater was characterized by high turbidity, high suspended solids and chemical oxygen demand concentration of 500 mg/L as well as a copper concentration of 100 mg/L. The results indicated that electrocoagulation with Al/Fe electrode pair was very efficient and achieved 99 % copper ion removal, 96.5% turbidity removal and 85 % COD removal in less than 30 min. The effluent wastewater was very clear, and its quality exceeded the direct discharge standards.
Al-Shannag [51] studied the removal of heavy metal ions (Cu2+, Cr3+, Ni2+ and Zn2+) from metal plating wastewater by electrocoagulation using an electro-reactor with six carbon steel electrodes of monopolar configurations. The results showed that the removal efficiency of heavy metal ions increases with increasing both electrocoagulation residence time and direct current density. Over 97% of heavy metal ions were removed by the treatment at a current density of 4 mA/cm2 and a pH of 9.56 in 45 min.
Woytowich [52] investigated the treatability of bilge water by electrocoagulation-electroflocculation processes. The bilge water contained petroleum oil and hydrocarbons in high concentrations. Due to interference of seawater, it also had high chloride concentration and high conductivity that led to increment of current intensity and decrement in voltage and energy costs. The results showed that optimum removals of COD (90.3%) and oil & grease (81,7%,) were obtained under 9,87 mA/cm2 current density at a temperature of 29ºC and a pH of 6.95 in 13 min.
Mohamud [53] treated a shipyard oily wastewater by electrocoagulation using aluminum electrodes in a batch reactor. The results showed a maximum chemical oxygen demand removal efficiency of 88.83% at a current density of 3 mA/cm2. The removal efficiency was gradually improved with increasing current density and decreased with increasing COD concentration but was not affected by the initial pH value.
de Santana [54] evaluated the efficiency of electrocoagulation in treating wastewater from the bakery industry. They used iron and aluminum electrodes in the pH range of 4.6-7.0 at 6 and 12 V for 1200 and 2400 s. The best electrode was the aluminum electrode and the optimum values of pH and voltage were 7.0 and 12 V, respectively. The results revealed that the removal of chemical oxygen demand was 6-8% and the removal of turbidity was 32-98% using aluminum electrodes which were about twice as high as those obtained with iron electrodes.
Zhang [55] applied electrocoagulation for the pretreatment of shale gas drilling wastewater. They investigated the effects of current densities and reaction time on hardness, turbidity and organic matter removal. The drilling wastewater was rich in dissolved salts, among which the hardness ion Ca2+. The Ca2+ concentration varied little at the reaction time of 10 min but decreases significantly with increases in reaction time. Lower current densities and longer reaction times were suitable for higher hardness removal rates, while higher current densities decreased the turbidity quickly before 10 min. The pH value of the wastewater was negatively correlated with the concentration of Ca2+ and iron ions. The TOC decreased with increases in reaction time and the current density. At the current density of 8 mA/cm2 and the reaction time of 20 min, the removal rates of Ca2+, turbidity and TOC were 53.4%, 98.3%, and 62.7%, respectively.
El Ashtoukky [15] investigated the use of electrocoagulation of wastewater produced from a carwash station using a new cell design featuring a horizontal spiral anode placed above a horizontal disc cathode in batch mode. The results indicated that aluminum was superior to iron as a sacrificial electrode material in treating carwash wastewater. The COD and turbidity reductions increased with increasing the current density and NaCl concentration. The optimum pH for treating the wastewater was in the range of 7-8 and the temperature had an insignificant effect on the process. Energy consumption based on COD reduction ranged from 2.32 to 15.1 kWh/kg COD removed, depending on the operating conditions.
Chu [56] used a combined technique of electrocoagulation coupled with ultrasound to treat the carwash wastewater for reuse. The results indicate that the highest removal efficiencies of COD (68.77%) and turbidity (96.27%) were obtained at a current intensity of 1.2 A, a pH of 6.0, an electrode distance of 1.5 cm and a treatment time of 20 min. The quality of treated wastewater met the COD and turbidity requirements in Water Quality Standards for Urban Water Consumption. This combined technique achieved a higher removal rate of pollutants from car washing wastewater than the single electrocoagulation method.
Gonder [57] investigate the treatment of carwash wastewater using electrocoagulation process with Fe and Al electrodes. Higher removal efficiencies were found at a pH of 8, a current density of 3 mA/ cm2 and an operating time of 30 min for Fe electrode and at a pH of 6, a current density of 1 mA/cm2 and an operating time of 30 min for Al electrode. The removal efficiencies under the optimum conditions for COD, oil and grease and chloride were 88%, 90% and 50% for Fe electrode and 88%, 68% and 33% for Al electrode, respectively. The total operating costs at the optimum conditions were 0.6 $/m3 and 0.3 $/m3 for Fe and Al electrodes, respectively. This study revealed that EC process using Fe electrode is a feasible technology for higher COD and oil-grease removals from carwash wastewaters.
Moulood and Abdul-Majeed [58] investigated the effectiveness of a combined electrocoagulation treatment with ultrasonic energy (Sono-Electrocoagulation) in decreasing the contaminants in an oily carwash wastewater containing high organics and chemicals. The effect of the voltage and time of treatment on the removal of COD, turbidity, conductivity, and TDS were studied at a constant initial pH of 7 and an electrode distance of 2 cm. The best removal results of COD, turbidity, TDS and electrical conductivity were obtained at a voltage of 30 V and a treatment time of 90 min. The ultrasound waves increased the mass transfer of species, thereby creating rapid mixing.
Atiyah and Abdul-Majeed [59] used a novel electrocoagulation treatment with a thin foil electrode to remove COD, turbidity, TDS and electric conductivity EC} from contaminated carwash wastewater. The contaminated carwash wastewater contained large quantities of chemicals from detergents, oil and grease, heavy metals, suspended solids, hydrocarbons, and biological contaminants. The best result was found at a voltage of 30 V and treatment time of 90 min where the removal efficiencies of COD, turbidity, TDS, and EC were 97.94%, 99.90%, 25.31%, 15.57%, respectively.
Takdastan [60] evaluated the efficiency of electrocoagulation process in removal of COD, turbidity, detergent and phosphate from carwash effluent. Iron and aluminum electrodes (AL-AL, AL-Fe, Fe-Fe) were connected to a power supply using bipolar method to convert alternative electricity to direct current. The initial pH of samples was between 7 to 9 and the removal percentage was calculated at several pH values (11, 7, 3), electrical potentials (30, 20, 10 volts) and reaction times (90, 60, 30 min) with middle intervals of 2 cm. The best COD removal (99%) was observed at a pH of 3, a voltage of 30 and a retention time of 90 minutes for the aluminum electrode. However, the removal efficiency of detergent by the iron electrodes was higher than that achieved by the aluminum electrode. It was found that this method can be used as a safe and convenient method for treating carwash effluent for safe discharge into the environment.
Panizza and Cerisola [61] used a combined two-step process consisting of electrochemical coagulation with iron anodes and electrochemical oxidation with boron-doped diamond anode (BDD) for the treatment of carwash wastewater. The effects of current density, electrolysis time and pH on the surfactant oxidation, COD removal and energy consumption were explored. The optimal experimental conditions were observed at a pH of 6.4, an electrolysis time of 6 min and an applied current of 2 mA/cm2. At these conditions, the electrocoagulation method removed 75% of COD with a low energy consumption of 0.14 kWh/m3. The complete COD removal was achieved by the overall combined process where the residual organics coming from the electrocoagulation were degraded by electrochemical oxidation when applying a current of 10 mA/cm2. The energy consumption and the electrolysis time for the complete mineralization of the carwash wastewater were 12 kWh/m3 and 100 min, respectively.
Priya and Jevanthi [62] investigated the removal of COD, oil and grease and turbidity from the automobile wash wastewater using electrocoagulation technique (ECT) with varying the position of the sacrificial electrode materials (Al, Fe, St, and Cu). The influences of distance among the electrodes (2,5 - 10 cm), current density (5 - 30 A/m2), reaction time (10 - 60 min), pH (4 - 10) and aeration were evaluated. The maximum COD reduction was attained with a Cu (anode) - Al (cathode) electrodes at the pH of 6.5. The higher removals of 95.1%, 92.5% and 99% of COD, oil and grease and turbidity were attained with an optimized distance among the electrodes of 5 cm, a current density of 25 A/m2, a reaction time of 40 min and a pH of 6.
Electrooxidation Treatment
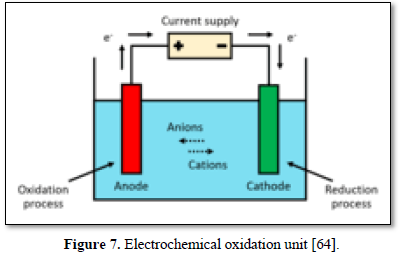
Oxidation is the loss of electrons whereas reduction is the acquisition of electrons. The species being oxidized is known as the reducing agent or reductant, and the species being reduced is called the oxidizing agent or oxidant. Electrooxidation (EO) is a technique used for wastewater treatment and is a type of advanced oxidation process (AOP) [63]. The most general layout comprises two electrodes (anode and cathode) connected to a power source as shown in Figure 7 [64]. When an energy input and sufficient supporting electrolyte are provided to the system, strong oxidizing species are formed, which interact with the contaminants and degrade them. The refractory compounds are thus converted into reaction intermediates and ultimately into water and CO2 by complete mineralization [65-69].
Electrochemical oxidation has grown in popularity because it’s easy to set-up, effective in treating harmful and recalcitrant organic pollutants which are difficult to degrade with conventional wastewater remediation processes and does not require external addition of chemicals because the required reactive species are generated at the anode surface [70-72].
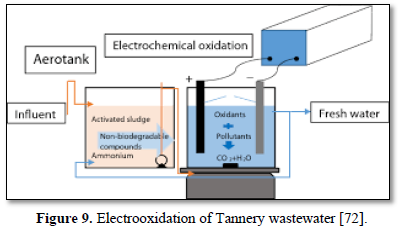
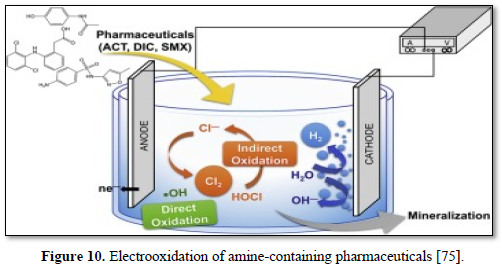
Electrochemical oxidation has been used to treat a wide variety of harmful and non-biodegradable contaminants including aromatics, pesticides, drugs, and dyes [68-75]. Figure 8 shows an electrochemical oxidation unit for the removal of carbamazepine [67], Figure 9 shows an electrooxidation unit treating tannery wastewater [72] and Figure 10 shows an electrooxidation of 5‐hydroxymethylfurfural [73]. Electrochemical oxidation has also been used in several studies to treat carwash wastewater [70-73]. However, due to its relatively high operating costs, it is often combined with other technologies such as biological remediation [67].
Gurung [67] investigated the effectiveness of electrochemical oxidation (EO) of carbamazepine synthetic solutions (CBZ) in membrane bioreactor (MBR) effluent using newly developed Ti/Ta2O5-SnO2 electrodes. The operating parameters included applied current density, initial CBZ concentration, pH, and temperature. The optimum
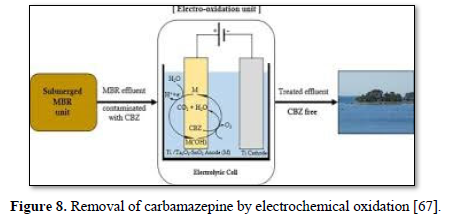



removals of carbamazepine (75.5%) (20 mg/ L) and TOC (71.1%) were achieved after 8 h of electrolysis under a current density of 9 mA/cm2, a pH of 6, a temperature of 30°C and 0.1 M Na2SO4 as supporting electrolyte. Increasing current density and temperature influenced the carbamazepine removal efficiency while the pH did not have any significant influence on carbamazepine removal efficiency. The performance of Ti/Ta2O5-SnO2 electrode was compared with the conventional Ti/PbO2 electrode and the results showed that under the same operating conditions, the carbamazepine removal efficiency of Ti/PbO2 electrode (77.9) was slightly higher than that of Ti/Ta2O5-SnO2 (71.7%). This newly developed electrode required the lowest energy consumption (60.3 kWh/m3) to achieve optimum carbamazepine removal and no heavy metals were leached (unlike the PbO2 electrode).
Luu [72] treated tannery wastewater using electrochemical oxidation by SnO2/Ti and PbO2/Ti anodes. The effects of current density, pH, stirring rate and reaction time on the pollutants removal efficiencies of tannery wastewater were studied. The results showed that SnO2/Ti and PbO2/Ti anodes can effectively remove over 80.0% of the color, chemical oxygen demand and total nitrogen in tannery wastewater after 90 min at a current density of 66.7 mA/cm2. The SnO2/Ti anode achieved higher pollutants removal efficiency in base liquid while the PbO2/Ti anode achieved higher pollutants removal efficiency in acidic liquid. The

current density and stirring rates significantly affected pollutant removal efficiencies, and the concentration of pollutants in the effluent decreased as the reaction time was increased. Nayir and Kara [73] treated container washing wastewater that contained many organic compounds using combined electrocoagulation (EC)-electrooxidation (EO) process. The wastewater was first treated by EC with iron (Fe) and aluminum (Al) electrodes. The maximum removal efficiencies of soluble chemical oxygen demand (82%) and color were (98%) obtained with Fe electrodes under 25 mA/cm2 current density, initial pH of 5 and 120-min operation time were obtained. Because of the relatively low SCOD removal efficiency, EO was used as post-treatment process using boron doped diamond electrode (BDD). The COD removal efficiency was increased to 89% while the color removal efficiencies decreased to 71% under 25 mA/cm2 current density, initial pH of 3 and 300-min operation time. This study showed that electrochemical processes caused new complex molecules formation in the wastewater which caused deterioration of the color and limited the process efficiency.
Panizza and Cerisola [61] investigated the anodic oxidation of a carwash wastewater using lead dioxide (PbO2) and boron-doped diamond (BDD) anodes in an electrolytic flow cell. The influences of the current (1- 3A), liquid flow rate (100-300 dm3/h) and temperature (25-40°C) on the performance of both systems with a stainless teel cathode were studied and the energy consumption was evaluated. Galvanostatic electrolysis led to complete COD removal due to the high amounts of effective hydroxyl radicals generated from water oxidation at each anode and the COD removal rate increases with rising applied current and liquid flow rate but was not affected by temperature. The performance of the BDD anode was always better than that of PbO2, requiring shorter electrolysis time to reach overall mineralization, thus leading to remarkably higher current efficiency and lower specific energy consumption (375 kWh/m3 and 770 kWh/m3, respectively].
Ganiyu [75] evaluated the effectiveness of electrochemical advanced oxidation processes including electrooxidation (EO), electrooxidation with hydrogen peroxide generation (EO-H2O2) and electro-Fenton process (EF) as alternative treatment techniques for complete removal of anionic surfactants and organic matters from carwash wastewater. The electrochemical processes were performed with acidified real carwash wastewater using boron doped anode and carbon felt cathode. In all cases, the chemical oxygen demand (COD) removal efficiency was always increased with the rise in applied current and complete organic matter decay was achieved at an applied current of 500 mA or above after 6 h of electrolysis. Faster and higher COD decay was observed with EF treatment compared to EO and EO-H2O2 treatments at all currents and electrolysis times. The major organic content of the wastewater could be degraded at all applied currents studied irrespective of the process used indicating the efficacy of processes for total remediation of carwash wastewater. Lower energy consumption and higher current efficiency were obtained with EF treatment compared to EO-H2O2.
Rubi-Juarez [76] treated carwash wastewater by a combined electrocoagulation and electrooxidation process. The electrooxidation process with BDD electrodes at 210 A/m2 for 120 min was effective in reducing the chemical oxygen demand by 82%, color by 81%, methylene blue active substances by 81%, biochemical oxygen demand by 73%, and chlorides by 72%. The electrocoagulation was effective at reducing organics when coupled with electrooxidation. The electrocoagulation with iron and aluminum produced similar results, but iron imparted color to the solution, so aluminum was used. Aluminum electrocoagulation at pH 7 with a current density of 150 A/m2 for 60 min reduced turbidity by 98%, color by 96%, oils by 92%, chemical oxygen demand by 76%, biochemical oxygen demand by 74%, and methylene blue active substances by 56%. The combined process was very effective in reducing oils by 100%, color by 99.3%, turbidity by 98.4%, chemical oxygen demand by 96%, biochemical oxygen demand by 93% and methylene blue active substances by 92%.
Davarnejad [77] treated carwash wastewater (CW) by an economic and eco-friendly method called Electro-Fenton (EF) technique. They investigate the effect of five variables (reaction time, current density, pH, H2O2/Fe2+ molar ratio and H2O2/carwash wastewater ratio in mL/L) on the quality characteristics of effluent including COD, BOD5, TOC, TSS, heavy metals, electric conductivity (EC), surfactants and hardness. The COD was selected as the main factor in a wastewater according to the environmental protocols. The results showed that the optimum removal of COD was 68.72% at reaction time of 75.80 min, current density of 58.81 mA/cm2, pH of 3.02, volume ratio of H2O2/CW of 1.62 mL/L, H2O2/Fe2+ molar ratio of 3.66.
FILTRATION TREATMENTS
Filtration is a process of removing particulate matter from water and wastewater by forcing the water through a porous media. The porous media can be natural as in the case of sand, gravel and clay or it can be a membrane made of various synthetic materials including cellulose acetate, cellulose nitrate (collodion), polyamide (nylon), polycarbonate, polypropylene, and polytetrafluoroethylene (Teflon) [78].
The membrane is a thin layer of semi-permeable material that separates substances when a driving force is applied across the membrane. Membrane processes are increasingly used for removal of microorganisms, particulates and natural organic material which can impart color, tastes and odors to water, and react with disinfectants to form disinfection by-products. As advancements are made in membrane production and module design, capital and operating costs of membrane filtration continue to decline [79].
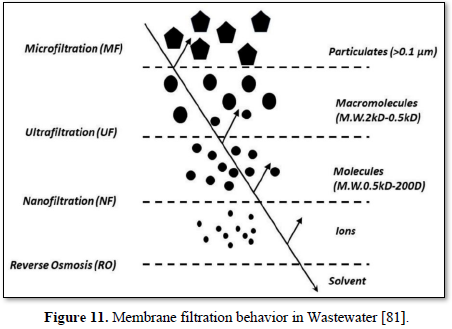
The size of materials that can be removed from the water depend upon the size of the membrane pores. Based on pore size, membrane filtration processes for water and wastewater are divided into four classes: microfiltration, ultrafiltration, reverse osmosis and nanofiltration [80]. Figure 11 shows the behavior of various membranes filtration in Wastewater [81].
In the carwash industry, wastewater must be treated and recycled to meet the present water shortage and the environmental laws. Treating and recycling carwash wastewater with filtration is economically and environmentally sustainable. Granular filtration and membrane filtration processes such as microfiltration, ultrafiltration, nanofiltration and reverse osmosis are technically and economically promising techniques for recycling carwash wastewater [82-83].

Granular Filtration
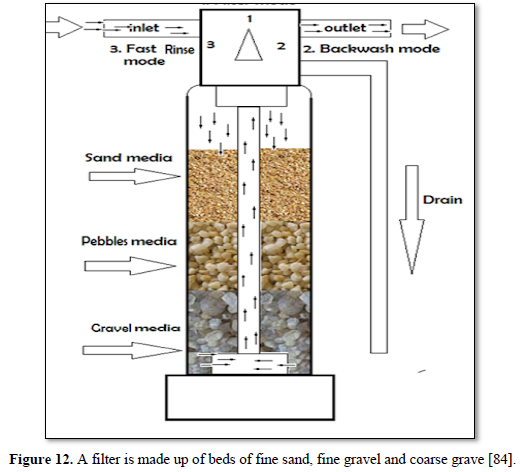
Granular filtration is a process where water flows through granular material while suspended solids (sand, clay, organic particles and iron and aluminum flocs) are retained and pathogenic microorganisms (bacteria, algae and protozoa) are removed from water and wastewater. The granular media could be made of sand, fine and course gravels, pebbles, synthetic polymers, diatomaceous earth, coal, sponge, charcoal, and cotton. Figures 12 and 13 show 2 types of granular filters made from sand and gravels [84,85].
Granular filters are used in combination with sedimentation and other chemical treatments such as coagulation. Reduction efficiencies of granular filters are within the range of 90-99%. With pre-treatment (typically coagulation), pathogen reductions are typically >99% whereas with no pretreatment, 90-99% reductions of larger pathogens (helminth ova and larger protozoans) and solids-associated pathogens can be achieved, but only <90% reductions of viruses and free bacteria are achieved [86].
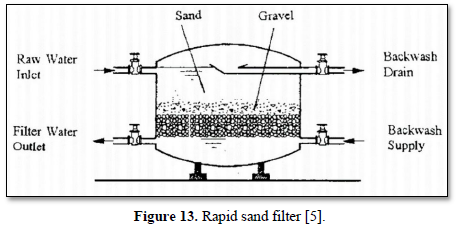


Moazzem [39] evaluated the performance of granular filtration (sand) and membrane filtration (ceramic ultrafiltration and reverse osmosis) systems combined with coagulation/flocculation and sedimentation for treating carwash wastewater for the purpose of reuse. Overall, 99.9% of turbidity, 100% of suspended solids and 96% of COD were removed from the carwash wastewater after treating by coagulation/flocculation, sedimentation, sand filtration, ceramic ultrafiltration and reverse osmosis. The treated water met the standards required for Class A Recycled Water in Australia.
Zaneti [40] investigated the treatment of wastewater from a typical carwash station in Brazil by flocculation-column flotation (FCF) plus sand filtration and chlorination. The results revealed that the chloride and TDS concentrations in the reclaimed water were stabilized below 350 and 900 mg/ L, respectively. The cost-benefit analysis showed that water reclamation using this technology was highly competitive and the payback period might be as short as one year.
Zaneti [88] employed a new flocculation-column flotation (FCF) with sand filtration and final chlorination technique for carwash wastewater reclamation. Water usage and savings audits for 20 weeks showed that almost 70% reclamation was possible. However, monitoring the physicochemical and biological parameters of wastewater and reclaimed water showed a high count of fecal and total coliforms in the wastewater and in the treated water, making final disinfection necessary. The cost-benefit analysis showed that for a carwash wastewater reclamation system in Brazil, at least 8 months were needed for the equipment amortization depending on water prices and daily wash demand.
Zaneti [88] employed a new flocculation-column flotation (FCF) with sand filtration and final chlorination technique for carwash wastewater reclamation. Water usage and savings audits for 20 weeks showed that almost 70% reclamation was possible. However, monitoring the physicochemical and biological parameters of wastewater and reclaimed water showed a high count of fecal and total coliforms in the wastewater and in the treated water, making final disinfection necessary. The cost-benefit analysis showed that for a carwash wastewater reclamation system in Brazil, at least 8 months were needed for the equipment amortization depending on water prices and daily wash demand.
Jamil [89] conducted a carwash wastewater treatment and recycling study using a unit consisting of coagulation-flocculation followed by sand and gravel filtration. The treatment and recycling process was designed for 16.2 m3/day carwash wastewater. The final design selected included an underground 1 m3 coagulation flocculation tank, a sand and gravel filter with 2.5 m2 surface area located at a height of 6 m above the ground level, a 0.5 m3 coagulant storage tank and a 20 m3 treated water storage tank. The coagulant storage tank and the treated water storage tank were located at the ground level. The system was effective in removing the pollutant from carwash wastewater and the treated water was used in the same car washing operation.
Microfiltration
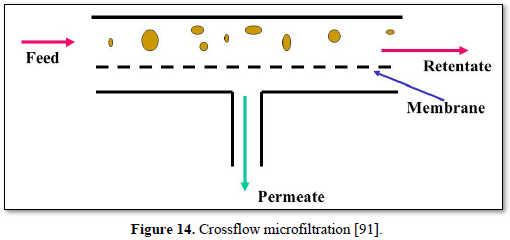
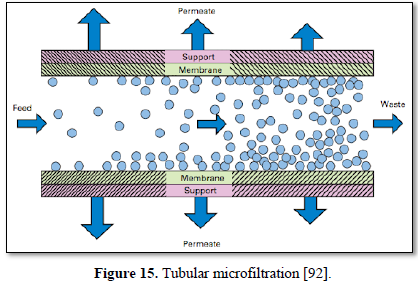
Microfiltration is a low pressure (100-400 kPa) physical separation process where a contaminated fluid is passed through a special pore-sized membrane to separate microorganisms (Giardia lamblia and Crypotosporidium cysts, algae, and some bacterial species except virus) and suspended particles from liquid stream but it does not remove dissolved contaminants. Microfiltration filters can be made with both organic materials (polymer-based membranes) and inorganic materials (ceramic or stainless steel) with membrane porosity between 0.1 to 10 μm. Microfiltration has been used in water treatment, industrial wastewater treatment and in the dairy and food processing industry [90]. The advantages of microfiltration are limiting the concentrations and number of chemicals that are applied during water treatment and removal of natural synthetic organic matter which reduces fouling potential [80].
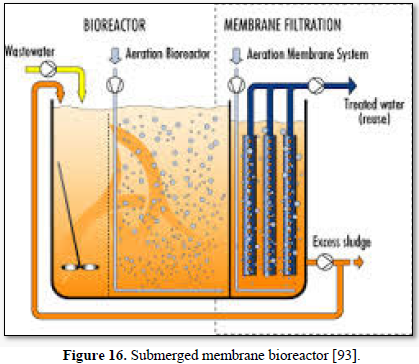
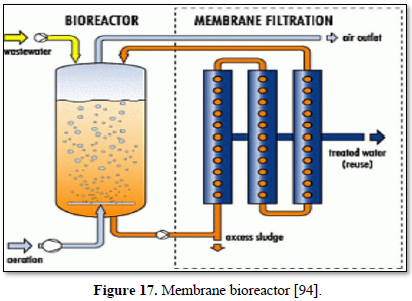
Microfiltration can be used alone as shown in Figures 14 and 15 [91,92] or in combination with biological process (membrane bioreactor) as shown in Figures 16 and 17 [93,94]. In the case of membrane bioreactor, the membranes are either submerged directly in the bioreactor (Figure 16) or kept outside the bioreactor (Figure 17). The advantages of membrane bioreactor are: it is economically attractive, compact, trouble-free operation, options for water reuse and fast delivery time [95].
Moazzem [95] evaluated the performance of an enhanced membrane bioreactor (eMBR) in treating carwash wastewater for the purpose of reuse. The eMBR consisted of an anaerobic tank, an anoxic tank, an aerobic membrane bioreactor (AMBR) and a UV disinfection unit. The eMBR produced high quality recyclable water (0.5-10.2 mg/L COD, 0.18-0.83 NTU turbidity and 0 E. Coli/100 mL) meeting Class A Recycle Water Standards. Decreases in the mixed liquor suspended solids concentration in the AMBR from 294 to 117 mg/L reduced the fouling of the membrane which increased the permeate flux from 5.9 to 6.7 L/m2h.
Boluarte [96] evaluated a membrane bioreactor (MBR) for treating carwash wastewater that contained significant concentrations of organics, particulate matter, sand, oil, grease, diesel and detergents. The results indicated that once the MBR system was acclimatized, 100% of suspended solids, 99.2% of COD, 97.3% of TOC and 41% of ammonia were removed. This study demonstrates that MBR is a potentially promising treatment system for recycling carwash wastewater for reuse in the same carwash station.



Pinto [97] evaluated the effectiveness of microfiltration hydrophilic membranes for carwash wastewater reclamation. The effects of geometry as well as pressure difference across the membrane and feed flow rate on permeate flux and quality of water for reuse were investigated. The effluent had initial turbidity of 85 NTU, total organics of 4.1 mg/L and inorganic carbon of 58 mg/L. Testing of flat cellulose commercial membranes revealed that microfiltration showed good retention of solids and organic matter as observed in turbidity and chemical oxygen demand reductions. Testing of commercial hollow fiber polyetherimide membranes showed initial flux of 440 L/m2h with a final permeate recovery rate of 80%. The rejection was 98.6% and the total organic and inorganic carbon in the effluent were 2.7 and 35.4 mg/L, respectively.
Ucar [98] investigated alternative treatments of carwash effluents including settling and membrane filtration processes. During settling, total solid concentration decreased rapidly within the first 2 h and then remained constant. However, chemical oxygen demand and conductivity decreased only by 10% and 4%, respectively. After settling, wastewater was filtered throughout a 100 μm filter and the microfiltration had a negligible effect on COD removal, probably due to high dissolved materials.
Hsu [99] presented a hybrid system that combined bio-carriers and non-woven membranes filtration that can remove both suspended solids and organic pollutants from carwash wastewater. The non-woven membrane served as microfiltration system to separate suspended solids from wastewater at a lower operating pressure and the microorganisms that grow on the surfaces. The porous bio-carriers made of polyurethane resin achieved higher organic removal. During 6 months of testing in a carwash facility in northern Taiwan, the influent COD and SS concentrations of 67 mg/L and 230 mg/L were reduced to less than 20 mg/L and 10 mg/L, respectively.
Daneshvar and Ghaedi [100] used Taguchi Method to evaluate treatment of carwash effluent using microfiltration polyvinylidene fluoride membrane with pore size of 0.13 micron. Taguchi Method was applied to investigate the effects of feed pressure at 3 levels (40, 70 and 100 kPa), feed flow rate at 3 levels (30, 40 and 50 L/h) and feed temperature at 3 levels (25, 35 and 45°C) on the permeation flux. The results showed that the most influential factor was feed pressure followed by the feed temperature. Feed flow rate had a low effect on permeation flux. At optimum conditions (100 kPa, 50 L/h, and 45°C), the Taguchi Model predicted the value of the permeation flux at 19.76 kg/m2.h which was in a good agreement with the experimental results.
Ultrafiltration
Ultrafiltration is a pressure-driven process in which a hydrostatic pressure forces a liquid against a semi permeable membrane to produce water with very high purity. An ultrafiltration membrane has a pore size of about 0.01-0.02 micron which can remove large particles, most microorganisms (bacteria, protozoa, algae and virus) and some natural minerals such as divalent ions. However, ultrafiltration cannot remove dissolved substances unless they are adsorbed with activated carbon or coagulated with alum or iron salts [78,83,97].
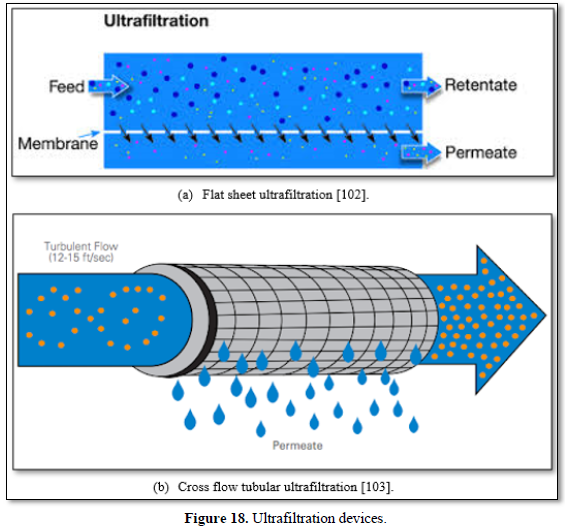
Most ultrafiltration membranes use polymeric materials (polysulfone, polypropylene, polyvinylidene fluoride, polyacrylonitrile, cellulose acetate, polylactic acid). However, ceramic membranes are used for high temperature applications. The primary advantages of low-pressure ultrafiltration membrane processes are: there is no need for chemicals (coagulants, flocculants, disinfectants, pH adjustment), constant quality of the treated water in terms of particles and microbial removal, compactness of process and plant and simplicity of automation [97,98]. However, fouling can cause difficulties in membrane technology for water and wastewater treatment [80-83]. Ultrafiltration is frequently used to pre-treat surface water, seawater, carwash wastewater and biologically treated municipal water upstream of the reverse osmosis unit. Figure 18 shows flat and tubular ultrafiltration units [102,103].
Lau [7] evaluated three types of commercial ultrafiltration membranes [UF PVDF100 (MWCO 100 kDa), UF PES30 (MWCO 30 kDa) and NF270] for the treatment of carwash wastewater effluent with respect to permeate flux, rejection of conductivity, total dissolved solid, chemical oxygen demand and turbidity. The results revealed that NF270 membrane exhibited greater flux stability and higher flux recovery during the treatment process compared to PVDF100 and PES30 membranes, indicating its higher resistance to fouling. With respect to turbidity removal, it was found that minimum rejection of 92% could be achieved irrespective of membrane properties and effluent characteristics. However, the performance of membrane in COD reduction was dependent on its properties and NF270 membrane showed the highest retention rate (70.9-91.5%) followed by PES30 membrane (54.9-83.9%) and PVDF100 membrane (56.1-82.4%). The NF270 membrane showed 60% separation rate in conductivity and 60 % total dissolved solid removal while the other two membranes (PVDF100 and PES30) were ineffective in reducing conductivity and total dissolved solid of effluent (only 13.6-35.4% separation rate in conductivity and total dissolved solid removal).
Pinto [97] evaluated the effectiveness of ultrafiltration membranes for carwash wastewater reclamation. The carwash effluent had initial turbidity of 85 NTU, organic carbon of 4.1 mg/L and inorganic carbon of 58 mg/L. The effects of geometry, pressure difference across the membrane and feed flow rate on permeate flux and quality of treated water were investigated. The results showed good retention of solids and organic matter. The final permeate recovery rate was 80% and the total organic and inorganic carbon in the effluent were 2.7 and 35.4 mg/L, respectively.
Ucar [98] investigated a sequential treatment process of carwash effluents which included settling followed by ultrafiltration. During settling, the total solid concentration decreased rapidly within the first 2 h but the chemical oxygen demand and conductivity decreased by 10% and 4%, respectively. The wastewater was then filtered by four ultrafiltration membranes of varying molecular weight cut off (1, 5, 10 and 50 kDa). The permeate COD concentrations varied from 64.5 to 85.5 4.3 mg/L, depending on UF pore size.

Boussu [102] investigated the economic and technical aspects of ultrafiltration for treating carwash wastewater for recycle in the main carwash operation. They indicated that the implementation of ultrafiltration process in the carwash wastewater purification systems was economically feasible, especially when expensive tap water was used for car washing. They emphasized the need to compromise between a high permeate permeability on one hand and a high permeate purity on the other hand.
Hamada and Miyazaki [103] proposed a system made of a cellulose acetate - hollow-fiber-type ultrafiltration membrane with the aid of flocculation and activated carbon for the treatment and reuse of carwash wastewater. First, the multi-blended flocculating agent containing bentonite, Al2(SO4)3, sodium alginic acid and a cationic polyacrylamide showed higher removals of COD and turbidity for carwash wastewater compared with using Al2(SO4)3 alone. Then, the effect of pure water permeability of the membrane on permeation flux in pretreated carwash wastewater by this agent was examined using three kinds of the cellulose acetate membranes whose molecular weight cut-offs were 150,000 Dalton. Permeation flux showed a higher value in the case of the membrane with higher pure water permeability. Full scale experiments with membrane areas of 32 m2 and 48 m2 were conducted under a membrane pressure of 20 kPa. When carwash wastewater was pretreated with 50 mg/L of this multi-blended flocculating agent, permeation flux through the cellulose acetate membrane with pure water permeability of 0.78 m3/(m2/h) at 100 kPa showed 1.0 m3/(m2/d) for more than 6 months. The COD, BOD, and the extract n-hexane values of reuse water were 3.7-15.7 mg/L, 2.5-14.0 mg/L and below 0.5 mg/L, respectively.
Nanofiltration
These membranes, which fall into a transition region between pure reverse osmosis membranes and pure ultrafiltration membranes (Figure 19) are called nanofiltration membranes. Nanofiltration has a pore size of 0.001 micron and can remove most of the organic molecules, all viruses, cysts, bacteria and wide range of salts and humic materials. Pushing water through these smaller membrane pores requires higher operation pressure of 600-1000 kPa. Because nanofiltration membranes remove alkalinity, blending raw water and product water or adding alkalinity may be needed to reduce corrosivity [104].
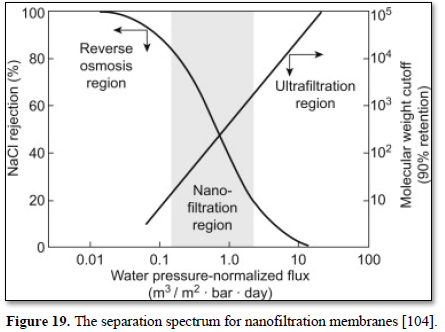

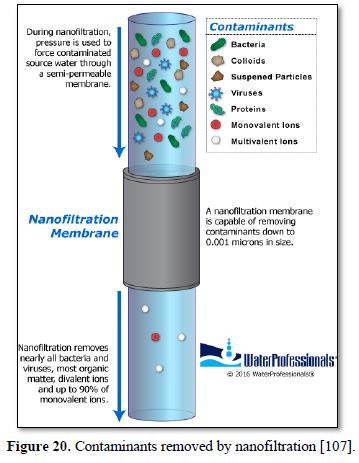
Nanofiltration is used to remove dissolved solids, most organic molecules and all viruses from surface and ground water [105,106] as well as various types of wastewater [68,107] as shown in Figure 20 [107]. Nanofiltration as a membrane liquid-separation technology shares many characteristics with reverse osmosis. However, unlike reverse osmosis which has high rejection of virtually all dissolved solutes, nanofiltration provides high rejection of multivalent ions such as calcium and low rejection of monovalent ions such as chloride. Nanofiltration provides a much more energy-efficient process compared with reverse osmosis. The nanofiltration membrane rejects various salts in proportion to their molecular size, so the order of rejection is Na2SO4 > CaCl2 > NaCl [104,106].
Koyuncu [108] studied the removal of several hormones and antibiotics from mixed solutions by nanofiltration membranes. They investigated the effects of solution chemistry, organic matter, and salinity on the rejection of tetracyclines, sulfanamides and selected hormones and their adsorption on membranes. The results showed that tetracyclines have a high adsorptive affinity for the membrane. Almost 80% of chlorotetracycline was adsorbed on the membrane surface compared with 50% for doxycycline. The adsorption rates for hormones were lower than those obtained for tetracyclines. Addition of calcium, organic matter and salinity influenced the rejections. Rejection of sulfanamides was low comparable to hormones and tetracyclines. Addition of antibiotics to hormone solution increased the hormone rejections while almost complete rejections were observed for tetracyclines.
Hilal [109] employed nanofiltration membranes as a pre-treatment unit operation in thermal membrane seawater desalination processes and as a partial demineralization to seawater. Two commercial nanofiltration membranes (NF90 and NF270) were evaluated for their performance in filtering the salt mixture of synthetic and real seawater in a crossflow nanofiltration membrane process at a pressure ranging from 400 to 900 kPa. The results showed that the rejection increased with pressure for the NF90 membrane and slightly increased with pressure for the NF270 membrane. Also, the NF90 membrane was able to reject both monovalent and divalent of all investigated mixtures and seawater but at a relatively low flux. It reduced the salinity of the seawater from 38 to 25.5 g/L using one stage of the nanofiltration membrane at 900kPa. This makes the NF90 membrane more suitable for application in the pre-treatment of desalination processes. The NF270 membrane had low rejection values of monovalent ions and reasonable rejection values for divalent ions. It reduced the seawater salinity from 38 to 33.6 g/L, but at a very high permeate flux.
Archer [110] studied the separation of an anionic surfactant from the alkyl-polyether-sulfate family by nanofiltration. The critical micellar concentration (CMC) of surfactant was 300 mg/L. They evaluated a negatively charged strong hydrophilic nanofiltration membrane with an active layer made of a proprietary polymer at various feed surfactant

concentrations (up to 20 × CMC), temperatures, and crossflow velocities. The results showed that the separation depended on the physical-chemical properties of the surfactant and the electrostatic interactions between the membrane and the ionic species in the aqueous solution. High values of the permeate flux (204 L/m2·h) and rejection (99.5%) were obtained. The results indicated that applications of the nanofiltration process appears to be suitable for the pre-treatment of industrial effluents with a significant concentration of anionic surfactants. Van der Bruggen [111] measured the water flux for two nanofiltration membranes (UTC-20 and NF70) using aqueous solutions of 11 organic compounds of different concentrations. The flux of aqueous solutions declined by more than 50% for solutions containing less than 1 g/L of some organic compounds as compared to the pure water flux. The flux declined as a function of the concentration of the organic compound and was related to adsorption on the membrane material. A clear correlation was found between the octanol-water partition coefficient and adsorption. This showed that both the surface charge and hydrophobicity of the membrane can play a role in the adsorption.
Van der Bruggen and Vandeccasteele [112] studied different mechanisms of flux decline for nanofiltration of aqueous solutions containing organic compounds. The focus in their research was on pore blocking and adsorption inside the membrane pores. The nanofiltration membranes used were one Dow membrane (NF70), two Toray Industries membranes (UTC-20 and UTC-60), and one Nitto-Denko membrane (NTR 7450). Experiments with different organic components in aqueous solution showed that adsorption resulted in a strong decrease of the water flux and the flux decline was a function of the concentration. The components that showed the largest effect had the highest polarity which indicated that adsorption is favored by the polarity of the components in solution. Moreover, the molecules with a size similar than the pore size had a stronger effect on the water flux than other molecules which can be explained as due to blocking of the pores by adsorbed compounds.
Lau [7] evaluated a commercial nanofiltration membranes (NF270) for treating carwash effluent with respect to permeate flux, rejection of conductivity, total dissolved solid, chemical oxygen demand and turbidity. The results revealed that the NF270 membrane exhibited greater flux stability and higher flux recovery during the treatment process indicating its higher resistance against fouling. It was found that a 92% reduction in turbidity could be achieved irrespective of effluent characteristics. The average chemical oxygen demand and total dissolved solids reductions were 81% and 60%, respectively.
Ucar [98] investigated a combined treatment of carwash effluents that included settling and nanofiltration processes. During settling, total solid concentration decreased rapidly within the first 2 h but chemical oxygen demand and conductivity decreased by 10% and 4%, respectively. When the wastewater effluent was then filtered by a nanofiltration membrane (NF270), the permeate COD reduction was 97%.
Boussu [102] evaluated the economic and technical aspects of nanofiltration for use to treat carwash wastewater. The results indicated that using nanofiltration to recycle wastewater in the rinsing step of carwash operations would be the optimal choice. The authors concluded that implementation of nanofiltration in the wastewater purification installation is economically feasible giving the fact that using tap water directly for car washing is very expensive.
Panpanit [113] evaluated the use of nanofiltration membrane for separation of oil water emulsion generated from car washing operations for recycling and reducing freshwater usage. The parameters studied were membrane type, emulsifier type, pressure and competing compounds. Both an-ionic and non-ionic emulsifiers were used in the experiments. The Ca2+ and Mg2+ were used as the main competitive ions. The results indicated that a polysulfone membrane caused more flux reduction than the cellulose acetate and thin film polyamide membranes. Higher concentrations of emulsifier presented negative flux decline. However, the presence of non-ionic emulsifier in oil emulsion caused significantly more flux reduction than an anionic emulsifier. Increased the competitive Ca2+ and Mg2+ ions resulted in significant positive nanofiltration flux and TOC removal.
Reverse Osmosis
Reverse osmosis is the tightest possible membrane process in liquid/liquid separation. Water is separated from dissolved salts in solution by filtering through a semipermeable membrane at a pressure greater than osmotic pressure as shown in Figure 21 [114]. Reverse osmosis membrane has a pore size around 0.0001 micron which removes all organic molecules, pesticides, cysts, bacteria, all virus and all minerals including monovalent ions. Reverse osmosis allows removal of particles as small as dissolved individual ions (sodium, chlorine, calcium, and magnesium), metal ions, minerals and organics and produces water that meets the most demanding specifications [115].
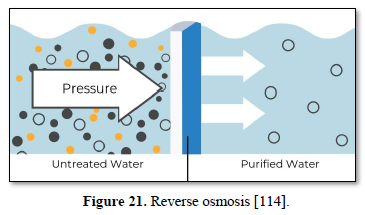

Some of the advantages of reverse osmosis are: removes nearly all contaminant ions and most dissolved non-ions, relatively insensitive to flow and total dissolved solids level, suitable for small systems with a high degree of seasonal fluctuation in water demand, operates immediately without break-in period, low effluent concentration possible, bacteria and particles are also removed, and operational simplicity and automation allow for less operator attention and make it suitable for small system applications. Some of the limitations of reverse osmosis are high capital and operating costs, managing the effluent (brine solution) is a potential problem, high level of pre-treatment is required in some cases, membranes are prone to fouling and the most water produced for use is between 25-50 percent of the feed [79,82,104].
Janik and Kupiec [4] stated that all carwash stations can use a reverse osmosis (RO) system for freshwater purification and wastewater desalination. Fresh water contains various amounts of dissolved impurities that are left on the car as spots when the water evaporates. The dissolved impurity level is characterized by total dissolved solids. The more total dissolved solids the rinse water contains, the more visible the spots on the car are.
The total dissolved solids in tap water vary within the range of 50-1,200 ppm, with an average of about 300 ppm for most waters. Reverse osmosis is used in the carwash industry for purification of fresh water to receive spot-free rinse water. Spot-free water should have total dissolved solids less than 30 ppm. Cars rinsed with spot-free water are air-dried and do not have to be wiped off, which eliminates the need for towels and additional personnel to dry cars at the end of the process [116,117].
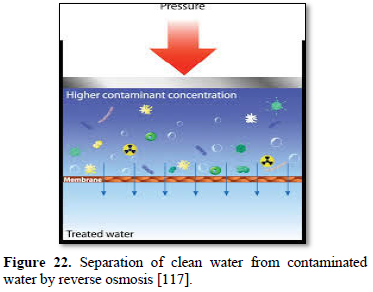
In the reverse osmosis process of car washing, pressurized feed water is pushed through the center of the membrane as shown in Figure 22 [117]. As water is squeezed out through the membrane, the membrane captures the solids in the water and the spot-free rinse water is produced. Reverse osmosis is particularly sensitive to feed water temperature with the optimum being 25°C. A typical membrane may lose 1-2% of its flow rate for every degree below that value. A preheating or a larger membrane may be required to achieve the desired level of performance. Thus, preheating is an important part of reverse osmosis performance to prevent fouling and premature membrane failure. A 5 μm filter is mostly recommended, and where chlorine is present (chlorine can wipe out some membrane), carbon filters may be required to protect certain types of membranes [114].

Salt (sodium chloride) is commonly used to make winter roads passable. About 30-85 kg of salt is spread per kilometer in some countries [118]. Salt accumulates on vehicles, causing (and accelerating the already existing) corrosion. High saline water loads are deposited into the carwash wastewater reclamation system during winter season and at the beginning of spring. Salt in wash water may also cause some problems in the carwash equipment and limit water reuse applications [116]. Therefore, Application of reverse osmosis treatment for carwash wastewater will captures the salt [117,118].
Madwar and Tarazi [119] stated that wastewater is considered a major resource of the water budget in many countries around the world which has necessitated the expansion of the applications of reverse osmosis to treat wastewaters for reuse. They presented a feasibility study for 10,000 m3/d wastewater and seawater desalination plants in the UAE, based on reverse osmosis membrane technology and the associated pre-treatment units. Desalination of wastewater produced water quality to suit many industrial uses such as power generation, textile, pulp and paper, and construction industries. They demonstrated the economic advantage of wastewater desalination, which is attributable to low salt content compared to seawater desalination. The study showed that the cost of desalting 1 m3 of wastewater is US$ 0.47, compared to US$ 1.06 for seawater.
Moazzem [39] evaluated the performance of filtration systems with coagulation/flocculation and sedimentation in treating carwash wastewater for the purpose of reuse. Overall, 99.9% of turbidity, 100% of suspended solids and 96% of COD were removed from the carwash wastewater after treating by coagulation, flocculation, sedimentation, sand filtration, ceramic ultrafiltration and reverse osmosis. The treated water met the standards required for Class A Recycled Water in Australia and standards imposed in Belgium and China and can be reused. However, optimization is required to reduce the sludge produced by this system.
Shete and Simkar [120] investigated the performance of ultrafiltration and reverse osmosis for carwash wastewater treatment for reuse. The results showed that using ultrafiltration can reduce total dissolved solids by 82.20 %, total suspended solids by 81.08 %, COD by 67.50 %, and oil and grease by 74.97 %. The authors believed that the treated wastewater was safe to release in any nearby waterbodies without causing any harm to society. However, using reverse osmosis, reduced total dissolved solids by 82.21 %, total suspended solids by 91.95 %, COD by 81.03 %, and oil and grease by 90.03 %. This wastewater was safe to reuse as water in any productive activity such as car washing.
DiPaolo [121] stated that reverse osmosis (RO) systems use a pump to increase the pressure on the feed side of the equipment and forces the water across and through a semipermeable membrane. This process results in approximately 96 - 99 % total dissolved solids removal from the carwash wastewater making it suitable for reuse. The author stated that when applied and functioning correctly, RO equipment can effectively reduce levels of salt, hardness and silica minerals that contribute to carwash related spotting. Adding a RO system to a carwash operation provides a final rinse of pure mineral-free water to each vehicle, resulting in glass, chrome and all painted surfaces to dry spot-free.
BIOLOGICAL TREATMENTS
Biological wastewater treatments are complex processes at the intersection of biology and biochemistry. They rely on bacteria and other organisms to break down and assimilate organic wastes using normal cellular processes. The goal of biological wastewater treatment is to create a system in which the result of decomposition is easily collected water with minimum pollutants for proper disposal and/or utilization. Biological treatments have evolved and are becoming more effective, efficient, and can achieve compliance with environmental discharge quality regulations [122].
Biological treatments of wastewaters are used worldwide because they are effective and more economical than many physical, mechanical, and chemical processes. However, biological treatments are often supplemented with additional disinfection treatments including chlorination and UV treatment, as well as a range of filtration options including granular filtration, carbon filtration and microfiltration. Biological treatments are usually divided into two processes: aerobic process in which oxygen is present and anaerobic process in which oxygen is absent. Both processes can be controlled and refined to achieve the optimal removal of organic substances from wastewater [122-126].
Aerobic wastewater treatment processes include simple aerobic tanks, oxidation ditches, surface and spray aeration, activated sludge, trickling filters, aerated ponds and lagoons, and constructed wetlands as well as various types of biofiltration. Diffused aeration systems are used to maximize oxygen transfer and minimize odors during treatment of wastewater. Aeration provides oxygen to the bacteria and other organisms as they decompose organic substances in the wastewater. Aerobic treatment is well suited for treating waste streams high in biodegradable organic content and is often used to treat municipal wastewater, wastewater generated by pulp and paper industry, waste generated from food processing industry and industrial waste streams containing carbon molecules [26,122,124,125] as well as carwash wastewater [127-130].
In contrast, anaerobic treatment uses bacteria to decompose organic materials in an oxygen-free environment. Lagoons, septic tanks, and anaerobic digesters are best-known anaerobic treatments which are used for treating effluent from food and beverage manufacturing, municipal wastewater, chemical effluent, and agricultural wastewater. Anaerobic digestion drives one of the most robust areas of resource recovery (energy recovery). In this form of energy recovery (known as bioenergy), anaerobic digestion is used to produce biogas, which is composed primarily of methane and small amount of carbon dioxide. Operators can use biogas to help fuel operations on the way to become energy net zero, or even turn waste streams into revenue streams [122,124,131].
Biofilters
A biofilter is a bed of media on which microorganisms attach and grow to form a biological layer called biofilm. Biofiltration is thus usually referred to as a fixed-film process used for air pollution control, water treatment and wastewater treatment. Generally, the biofilm is formed by a community of different microorganisms (bacteria, fungi and protozoa) and extracellular polymeric substances. The water to be treated can be applied intermittently or continuously over the media, via up-flow or downflow. Typically, a biofilter has two or three phases, depending on the feeding strategy (percolating or submerged biofilter): a solid phase (media), a liquid phase (water) and a gaseous phase (air). Most biofilters use media such as sand, crushed rock, gravel, and plastic or ceramic material shaped as small beads and rings [132].
Chaudhary [133] reported that biofiltration was first introduced in England in 1893 as a trickling filter (Figure 23) for wastewater treatment and has since been successfully used for the treatment of different types of water including surface water for drinking purpose, wastewater treatment, aquaculture water recycling, greywater recycling and carwash water recycling as a way to minimizing water replacement while increasing water quality. There are several configurations of biofilters produced by several technology companies. Figure 24 shows up-flow biofilter used for treatment of wastewater [134]. Figure 25 shows a compact biofilter for wastewater treatment [135]. Figure 26 shows a biofilter used in a commercial prawn hatchery [136].
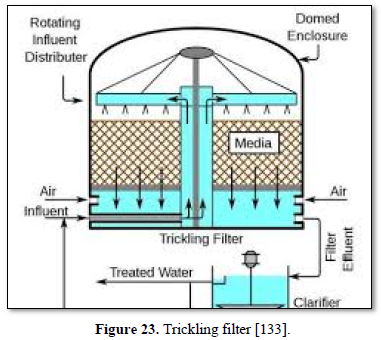
Organic matter and other water components diffuse into the biofilm where the treatment occurs by biodegradation process under aerobic condition, which means that microorganisms require oxygen for their metabolism. Oxygen can be supplied to the biofilm, either concurrently or counter currently with water flow. Aeration occurs passively by the natural flow of air through the process or by forced air supplied by blowers. The main factors influencing the efficiency of biofilter are the water composition, the biofilter hydraulic loading, the type of media, the feeding strategy (percolation or submerged media), the age of the biofilm, temperature, and aeration. Biological filters internal hydrodynamics and the microbial biology and ecology are complex and variable, characteristics that confer robustness to the process and give it the capacity to maintain its performance or rapidly return to initial levels following a period of no flow, intense use, toxic shocks or media backwash [137].
The structure of the biofilm protects microorganisms from difficult environmental conditions and retains the biomass inside the process, even when conditions are not optimal for its growth. Other advantages of biofiltration processes include: biofiltration allows the development of microorganisms with relatively low specific growth rates because microorganisms are retained within the biofilm, biofilters are less subject to variable or intermittent loading and to hydraulic shock, operational costs are usually low, final treatment result is less influenced by biomass separation since the biomass concentration at the effluent is much lower than that in suspended biomass and attached biomass becomes more specialized (higher concentration of relevant organisms). However, because filtration and growth of biomass leads to an accumulation of matter in the filtering media, this type of fixed-film process is subject to bio-clogging and flow channeling. Depending on the type of application and the media used for microbial growth, bio-clogging can be controlled using physical and/or chemical methods such as backwash using air and/or water to disrupt the bio-mat and recover flow or using oxidizing chemicals (Peroxide and ozone) or biocide agents [138-139].
Malimen [127] examined the efficiency of a biological treatment process in purifying carwash wastewaters from two finish automatic car washing stations. Both were using rotating bed biofilm reactors for wastewater treatment and
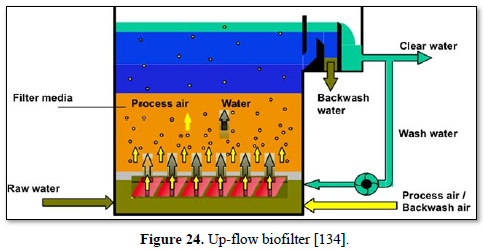
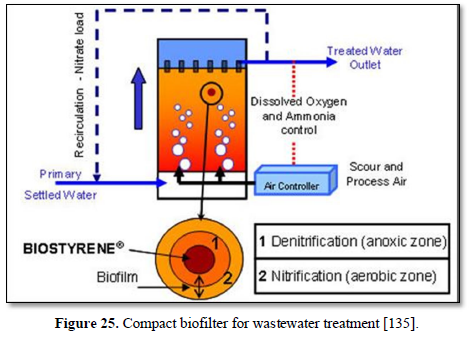
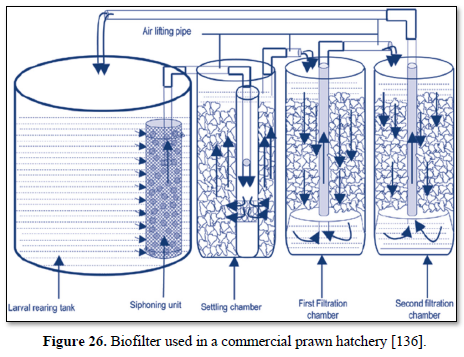



used 87 % of recycled water per carwash. Several samples were taken from the purified water on a biweekly basis and analyzed. Outdoor temperature seemed not to have any significant effect on the purification efficiency. The reductions of surfactants and chemical oxygen demand were 95 % and 87-95 %, respectively. Other water quality parameters such as conductivity, pH, oxygen concentration, total solids, and biological oxygen demand were comparable to values reported in the literature. The wastewater was characterized by relatively low organic and high phosphorus contents. The operational parameters examined in this study were hydraulic retention time, organics, suspended solid and total nitrogen loading rates. The factors affecting phosphorus removal in the biological filter appeared to be influent COD, COD/TP, BOD/COD, nitrogen, and SS/TP.
Söderlundh [140] investigated the treatment efficiency of wastewater from two car washes using a biofilter of peat and carbon-containing ash. The treatment included an oil separator and a peat/ash biofilter. The main function of the oil separator was to reduce the amount of oil in the wastewater. The peat/ash biofilter was used as a second step to treat mainly heavy metals. A comparison with the guiding values for wastewater from car washes in the municipality of Kristianstad showed that this type of filter worked well.
Bioreactors
A bioreactor is a special vessel that sustains and supports the growth of microorganisms and their activities (biochemical reactions). Bioreactors are used in several biological processes including cells and tissue culture, biomedical industrial processes to produce pharmaceuticals, vaccines, or antibodies, food and fermentation industries for production of organic acids, alcohols, wine, and various food products and wastewater treatment [122,126].
Bioreactors provide a homogeneous environment by constantly stirring the contents to maintain proper contact between the substrate, microorganisms and nutrient required for their growth and activities. They also maintain a controlled environment conditions for the biological reactions including temperature, pH, and oxygen [125]. Bioreactors are divided into 2 types: aerobic in which oxygen is present and anaerobic in which oxygen is absent. Both processes can be controlled and refined to achieve the optimal removal of organic substances from wastewater. Figure 27 shows an aerobic bioreactor [124] and an anaerobic bioreactor [131].
Mallick and Chakraborty [129] treated wastewater from automobile service station in a sequential reactor system consisting of anoxic reactor(A1) and aerobic reactor (A2) for reuse in car washing. The collected wastewater contained phenol (37 mg/L), hydrocarbons (475 mg/L), COD (506 mg/L), NH4+-N (170 mg/L), NO3−-N (135 mg/L), phosphate (20 mg/L) and metals. The results showed 99 % removals of phenol and hydrocarbons in reactor A1 at an HRT of 18 h. Residual NH4+-N was oxidized in reactor A2 with more than 99% efficiency at an HRT 6 h. The effluent COD reduction was 94% at combined hydraulic retention time of 24 h. The organisms Pseudomonas aeruginosa identified in anoxic reactor biomass were capable of degrading phenol and hydrocarbons utilizing NO3−-N as electron acceptor while the organisms Lysinibacillus sp., Stenotrophomonas sp., Pseudomonas eruginosa identified in the aerobic reactor biomass showed potential NH4+-N utilization.
Mazumber and Mukherje [130] explored the potential treatment of automobile service station wastewater by coagulation and activated sludge process. The oily wastewater (600 mg/L) was firstly treated using the coagulants alum, FeSO4 and CaCl2. The results showed that removal of oil concentration using the alum dose of 100 - 400 mg/L, alum + bentonite dose of 20 - 250 mg/L and FeSO4 dose of 50 - 200 mg/L was 300 mg/L (50% reduction). Subsequently, treatment of the wastewater with acclimated suspended biomass (activated sludge) resulted in a final 68% removal efficiency under a batch operation of 30 h.
Shabbazi [141] indicated the importance of bioremediation of sodium dodecyl sulfate (SDS) which is one of the main surfactant components in detergents used in high amounts in car washing. They isolated SDS - degrading bacteria (P. aeruginosa KGS) from a carwash wastewater in Tehran and studied the bacterial alkylsulfatase enzyme activity. They identified the coding gene of alkylsulfatase enzyme that hydrolyses sulfate -ester bonds to give inorganic sulfate and alcohol. The results indicated that SDS-degrading bacterium isolated from carwash wastewater showed valuable biodegrading potentials. A maximum degradation of SDS (84%) was obtained in a basal salt medium containing 1.5mM SDS at a pH of 7.1, a temperature of 30°C, agitation at 150 rpm and 4 d incubation.
Hosseini [142] reported that the anionic surfactant sodium dodecyl sulphate, (SDS) that widely used all over the world as a detergent component eventually end-up and accumulate in household or industrial sewage systems causing numerous problems in sewage treatment facilities due to their high foaming capabilities as well as direct toxic effects on many organisms the in ecosystem. They isolated two different bacteria from Tehran municipal activated sludge and determined their 16S rRNA gene sequencing. The two isolates were found to be Acinetobacter johnsoni and Pseudomonas beteli. After optimizing the environmental conditions for their growth (pH and temperature), the two bacterial isolates were tested for their ability to degrade SDS using it as a sole carbon source. Both Pseudomonas beteli and Acinetobacter johnsoni were able to degrade 97.2% and 96.4% of the SDS after 10 d of occupation, respectively. A mixed culture of the two isolates did not significantly increase SDS utilization (97.6%).
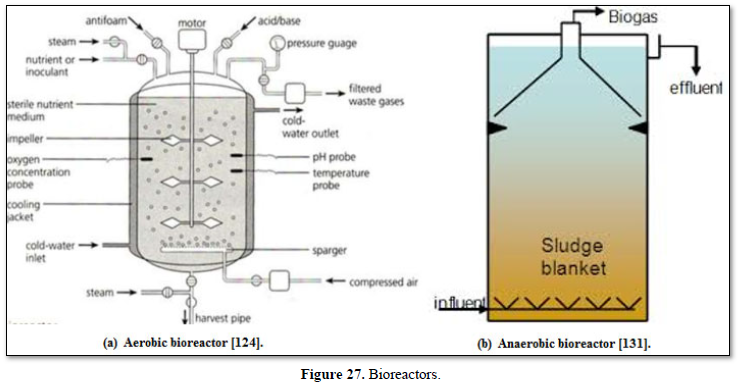

Triton X-100, SDS and rhamnolipid with glucose by Pseudomonas aeruginosa, Bacillus subtilis and compost microorganisms in liquid culture media. The results showed that CTAB was recalcitrant to degrade by the three microorganisms and it also inhibited microorganisms from utilizing readily degradable carbon source. The non-ionic surfactant Triton X-100 could also hardly be degraded, but it was not toxic to microorganisms and did not inhibit their growth. The anion surfactant SDS had no toxicity to microorganisms and could be co-degraded as carbon source with glucose.
Wetlands
A wetland is a distinct ecosystem that is flooded by water, either permanently or seasonally, where oxygen-free processes prevail. The primary factor that distinguishes it from other landforms or water bodies is the characteristic vegetation of aquatic plants adapted to the unique hydric soil. Wetlands are areas where water covers the soil or is present either at or near the surface of the soil all year or for varying periods of time during the year. Wetlands support both aquatic and terrestrial species. There are 4 main types of freshwater wetlands in North America: ponds, marshes, swamps, and peat bogs. Wetlands provide many societal benefits including food and habitat for fish and wildlife including threatened and endangered species, water quality improvement, flood storage, shoreline erosion control and some, economically beneficial [144, 145].
Constructed wetlands Constructed wetlands have been designed for treatment of various wastewaters including municipal wastewater, industrial effluent and storm runoff. The main three broad types of constructed wetlands are (a) subsurface flow constructed wetland which can be either with vertical flow (the effluent moves vertically, from the planted layer down through the substrate and out) or with horizontal flow (the effluent moves horizontally, parallel to the surface), (b) surface flow constructed wetland which has horizontal flow [146].
A constructed wetland is an engineered sequence of water bodies planted with different vegetation designed to filter and treat pollutants found in wastewater. Vegetation in a wetland also provides a substrate (roots, stems, and leaves) upon which microorganisms (periphyton) can grow as they break down organic materials. The periphyton and natural chemical processes are responsible for approximately 90 % of pollutant removal and waste breakdown. The plants remove about 10 % of pollutants, and act as a carbon source for the microbes when they decay. Different species of aquatic plants have different rates of heavy metal uptake, a consideration for plant selection in a constructed wetland used for wastewater treatment. Physical, chemical, and biological processes combine in wetlands to remove contaminants from flowing wastewater. Therefore, an understanding of these processes is fundamental not only to designing wetland systems but to understanding the fate of chemicals once they enter the wetland [147].
Although, constructed wetlands are not typically been designed for pathogen removal, but instead have been designed to remove other water quality constituents such as suspended solids, organic matter (BOD/COD) and nutrients (nitrogen and phosphorus), they are considered a sanitation system as all types of pathogens are expected to be removed in constructed wetlands. In a free water surface flow wetland, 1-2 log10 reduction of pathogens can be achieved. However, bacteria and virus reduction may be less than 1 log10 in systems that are heavily planted with vegetation, because vegetation (which assists in removing other pollutants such as nitrogen and phosphorus) minimize bacterial and virus exposure to sunlight in these systems [148].
Skrzypiecbcef [149] stated that constructed wetlands are characterized by specific conditions enabling various physical and biochemical processes simultaneously as a result of specific environment for the growth of microorganisms and aquatic and semiaquatic plants which are capable of living in aerobic, anaerobic and facultative-anaerobic conditions. Their interaction contributes to the intensification of oxidation and reduction responsible for the removal and retention of pollutants. These processes are supported by sorption, sedimentation and assimilation. Due to their advantages of low operational costs and high removal efficiency, there is growing interest in the use of constructed wetlands for the treatment or pre-treatment of various types of industrial wastewater. The authors analyzed current use of subsurface horizontal flow beds for the treatment of industrial wastewater, among others from crude oil processing, paper production, food industry including wineries and distillery, olive oil production, coffee processing. sewage and sludge from milk processing. In all cases, constructed wetlands provided an appropriate level of treatment and in addition the so-called ecosystem service (Figure 28).
Bakacs [150] investigated whether rain garden mesocosms are an appropriate management practice for reducing carwash pollutants. The concentrations of total phosphorus, total suspended solids, and surfactants were measured in carwash runoff before and after treatment in three rain garden mesocosms. The total suspended solids and surfactant showed reductions ranging from 84 to 95% and surfactant reductions ranging from 89 to 96%. However, the removal efficiencies for surfactants were not enough to reduce final concentrations below the reported values for aquatic toxicity.
Peng [151] studied the concentrations of heavy metals in the leaves of two aquatic plants Potamogeton pectinatus L. and Potamogeton malaianus Miq., and the corresponding water and sediment samples from the Donghe River in Jishou City of Hunan Province, China to determine metal contamination from the intensive industrial activities in the surrounding area. The results showed that the concentrations of heavy metals in the sediments, especially Cd, Mn and Pb, were much higher than the eco-toxic threshold values developed by the USEPA [19]. Between the two plant species, P. pectinatus showed higher capacity in metal accumulation. The highest concentrations of Cd, Pb, Cu, Zn and Mn were found in the leaves of P. pectinatus, reaching 596, 318, 62.4, 6590 and 16,000 mg/kg (DW), respectively. Significantly positive relationships were observed among the concentrations of Zn, Cu and Mn in the leaves of both aquatic plants and those in water, indicating the potential use of the two plants for pollution monitoring of these metals. In addition, a laboratory experiment was conducted to investigate the ability of P. pectinatus and P. malaianus to remove heavy metals from contaminated river water. The average removal efficiencies by P. pectinatus and P. malaianus for Cd, Pb, Mn, Zn and Cu were 92%, 79%, 86%, 67% and 70%, respectively. The results indicated that P. pectinatus and P. malaianus had high capabilities to remove heavy metals directly from the contaminated water.
Torrens [152] treated carwash wastewater with wetland. The carwash wastewater contained several pollutants such as sand, dust, surfactants, organic matter, fat, oil/water emulsions, asphalt remnants and salts as well as E. Coli. They designed and constructed three pilot plants at a car wash facility in Montfullà, Girona, Catalonia, Spain: (a) a vertical flow constructed wetland (VFCW), (b) a horizontal flow constructed wetland (HFCW) and (c) an infiltration‐percolation filter (IP). The purpose of the study was to evaluate the viability and performance of the different technologies to treat the effluent from the carwash facility for internal recycling. The three systems performed very efficiently with regard to turbidity, organic matter, hydrocarbon, suspended solids, detergents, fat and oil. E. coli was reduced to acceptable level for recycling. The study showed that constructed wetland technology was effective in treating carwash wastewater for reuse.
Tamiazzo [153] used an innovative constructed wetland arranged in a "cascade" to simulate a wall system (WCCW)
to treat carwash wastewater containing anionic surfactants (AS). Three plant species were tested at different AS inlet concentrations (10, 50, and 100 mg/L) with two hydraulic retention times (3 and 6 d). The plant species Rribbon grass (Typhoides arundinacea L.), Moench (Phalaris arundinacea L), water mint (Mentha aquatica L), and divided sedge (Carex divisa Hudson Cd) grew constantly over the experimental period, showing a capacity to tolerate even the highest AS concentration. When the HRT of 6 d was used, raising the AS concentration in inlet increased the AS concentration in outlet. Inlet concentrations of 10, 50, and 100 mg/L resulted in final outlet concentrations of 0.13-0.15, 0.47-0.78, and 1.19-1.46 mg/L, respectively.
ADSORPTION TREATMENT
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. Adsorption differs from absorption in which a fluid (the absorbate) is dissolved by a liquid or solid (the absorbent). Adsorption is a surface phenomenon, while absorption involves the whole volume of the material (Figure 29). The term sorption encompasses both adsorption and absorption processes, while the term desorption is the reverse of it [154-156].
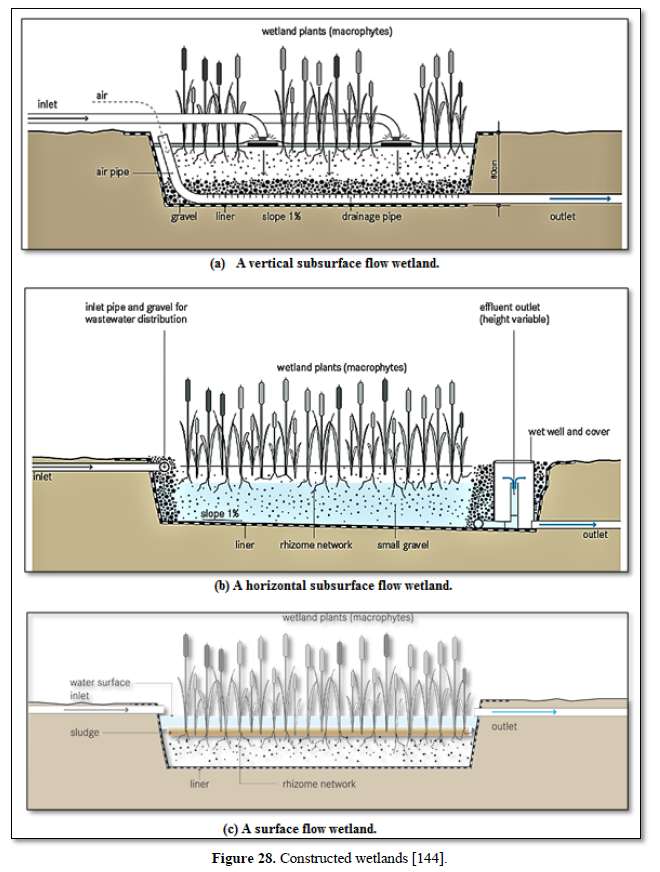

Adsorption is a consequence of surface energy. In a bulk material (ionic, covalent or metallic), all the bonding requirements of the constituent atoms of the material are filled by other atoms in the material. However, atoms on the surface of the adsorbent are not wholly surrounded by other adsorbent atoms and therefore can attract adsorbates. The exact nature of the bonding depends on the details of the species involved, but the adsorption process is generally classified as physisorption or chemisorption. It may also occur due to electrostatic attraction [157,158].
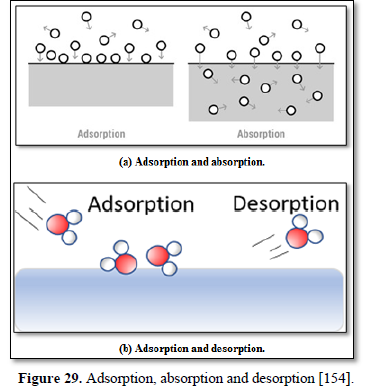

Adsorption is present in many natural, physical, biological and chemical systems and is widely used in industrial applications such as heterogenous catalysts, activated charcoal capturing and using waste heat to provide cold water for air conditioning and other process requirements such as adsorption chiller and synthetic resins, increasing storage capacity of carbide derived carbons, ion exchange, chromatography, water purification and pharmaceutical industry applications, which use adsorption as a means to prolong neurological exposure to specific drugs [159,160]. Figure 30 shows a n adsorption reactor [161].

Baddor [162] described a carwash wastewater treatment by adsorption to acceptable level so that it can be reused in same car washing operation. Bentonite was used to remove dissolved solids, surface active substances and oils and grease from wastewater. Bentonite granular are used for adsorption of particles less than 0.2 mm in size. The advantages of using bentonite are good efficiency, low cost and no effect on water pH.
Abdel-Magid and Olabi [163] research work confirmed the negative effects of carwash stations in Aleppo city on natural environment and contamination of air, water and soil. Their research work treated wastewater discharged from carwash operations to an acceptable level that enables its recycling and reuse in the same application. First, laboratory tests were conducted on samples taken from carwash stations to determine optimal conditions for removal of all surface-active substances, total dissolved solids and residual oils and grease from the carwash wastewater. Locally available granular clay (bentonite) was used for removal of pollutants and the effects of clay dose, pH and temperature on percent removal were studied. The work centered on determining optimum conditions for carwash wastewater treatment using Aleppo bentonite. The results showed that the adsorption process using bentonite was effective in removing surface-active substances, total dissolved solids and oil and grease from carwash wastewater, without the need for expensive equipment or chemicals. The optimal bentonite granular diameter for effective adsorption was smaller or equal to 0.2 mm, and best treatment efficiency occurred at a pH of 4, a temperature of 20ºC and mixing for 30 min. The study showed environmental and economic benefits including reduction of water pollution and preservation of water resources through recycling of water.
Kowsalya [164] stated that untreated carwash wastewater is a major source of pollution to many waterbodies. They treated carwash wastewater by adsorption using low cost and easily available adsorbent materials such as the leaves of Prosopis juliflora and Casuarina equisetifolia. They also used waste cotton cloth, which is cellulosic fiber rich in carbon content, to prepare activated carbon. The adsorption system treated the wastewater to acceptable reduction levels of various pollutants. The removal levels were 94.5% for TSS, 95.4% for BOD, 96.6% for COD, 88.89% for methylene blue anionic substances and 99.5% for Oil and Grease.
Bintizayadi [165] developed and optimized a preparation of powdered and granular sugarcane bagasse activated carbon and determined the optimum conditions for powdered and granular sugarcane bagasse activated carbon of adsorption. The carwash wastewater had chemical oxygen demand (COD), oil and grease (O&G), and surfactant as methylene blue absorbing substances (MBAS) of 461 ± 3 mg/L, 83 ± 5 mg/L, and 78 ± 47 mg/L respectively. The activated carbon optimum preparation conditions were 20 % impregnation, 500 °C temperature for 2 h. It has microporous structure with iodine number of 749 mg/g and ash content of 12 %. About 81 % of carbon, 17 % oxides. About 95 % ethylene comprising of aromatics, hydroxyls and alcohol groups are responsible to adsorb pollutants. The powder size of 0.063 mm attained maximum removals of 95% of COD, 94% of O&G and 100% of MBAS at a pH of 8, a dosage of 2 g/150 ml for 3 h contact time. On the other hand, the granular size of 1.18 mm had removals of 93 %, 85 %, and 90 % for COD, O&G and MBAS, respectively. This study highlighted the importance of using sugarcane bagasse activated carbon as an alternative adsorbent in removing the pollutants of COD, O&G and MBAS from carwash wastewater.
CONCLUSIONS
Professional carwash wastewater treatment and reclamation technology has been in use for three decades and is growing in sophistication. Reclamation has attracted more attention in the past several years from regulators and manufacturers as means of water conservation, pollution reduction and environmental quality control. Most wastewater reclaim systems that have been installed meet the needs of the individual professional carwash operator, whether that be to reduce sewer discharge or freshwater consumption, control water and sewer hookup costs, meet regulatory demands or some combination of these factors. The circumstances faced by the professional carwash operator and the desire to conserve water or reduce discharges will dictate the choice of approach and reclaim equipment installed. Where the reclaim water is intended for reuse in the professional carwash will dictate the level of treatment the water receives.
This study described the electrochemical, physical and biological treatment options for carwash wastewaters for recycling to achieve pollution reduction, water conservation and economic benefits for car wash operators. The general categories of carwash wastewater treatments include chemical coagulation-flocculation, electrocoagulation, electrooxidation, filtration (including granular filtration, microfiltration, ultrafiltration, nanofiltration and reverse osmosis), biological treatments (including biofilters, bioreactors and wetlands) and adsorption.
The environmentally friendly, modern carwash requires a good washing technology, proper water recycling system followed by advanced water treatment methods, and compatible washing chemicals. Professional carwash reclaiming systems use water treated by one or more of these methods, although technology may differ from installation to installation. Therefore, it is important to note that choosing the wrong combination of cleaning solutions or treatment processes can create more problems than it solves. It is imperative for the professional carwash operator to understand each element of the reclaim system and its intended use.
ACKNOWLEDGEMENTS
The authors appreciate the assistance provided by the Ms. D. M. El Nakib, the Manager of the Bioengineering Laboratory and staff of the Department of Agricultural Engineering, Faculty of Agriculture, Cairo University.
COMPETING INTERESTS
The authors have declared that no competing interests exist.
AUTHORS’ CONTRIBUTIONS
This work was carried out in collaboration among all authors. All authors read and approved the final manuscript.
-
Barnes LL (2016) Best management practices for commercial car washes. Great Lakes Pollution Prevention Round Table, United States Environmental Protection Agency, Chicago, Illinois, USA. Available online at: https://www.ideals.illinois.edu/bitstream/handle/2142/98573/BMPs-commercial-car-washes.pdf?sequence=2&isAllowed=y
-
Department of Ecology (2012) Vehicle and equipment wastewater discharge: Best management practices manual. Publication Number WQ-R-95-056, Department of Ecology, Olympia, State of Washington, USA. Available online at: https://apps.ecology.wa.gov/publications/documents/95056.pdf
-
Monney I, Donkor EA, Buamah A (2020) Clean vehicles, polluted waters: Empirical estimates of water consumption and pollution loads of the carwash industry. Heliyon 6(5): e03952.
-
Janik HZ, Kupiec A (2007) Trends in modern car washing. Pol J Environ Stud 16(6): 927-931.
-
Aikins S, Boakye DO (2015) Carwash wastewater characterization and effect on surface water quality: A case study of washing bays sited on Oda and Daban streams in Kumasi, Ghana. ARPN J Sci Technol 5(4): 190-197.
-
Fall C, Lopez-Vazquez CM, Jimenez-Moleon MC, Ba KM, Diaz-Delgado C, et al. (2007) Carwash wastewaters: Characteristics, volumes and treatability by gravity oil separation. Rev Mex Ing Quim 6(2): 175-184.
-
Lau WJ, Ismail AF, Firdaus S (2013) Car wash industry in Malaysia: Treatment of car wash effluent using ultrafiltration and nanofiltration membranes. Sep Purif Technol 104(1): 26-31.
-
United States Environmental Protection Agency (USEPA) (2008) Managing vehicle washing to prevent contamination of drinking water. Water Protection Practices Bulletin No. EPA816-F-01-024, Office of Water, United States Environmental Protection Agency, Washington, DC, USA. Available online at: https://www.oregon.gov/deq/FilterDocs/EPASWPPracticesBulletin_VehicleWashing.pdf
-
Hashim NH, Zayadi N (2016) Pollutants characterization of carwash wastewater. MATEC Web Conf 47: 05008.
-
Brown C (2002) Water effluent and solid waste characteristics in the professional carwash industry. Report for International Car Wash Association, Washington DC, USA. Available online at: https://www.mcacarwash.org/assets/documents/watereffluentsolidwastecharacteristics.pdf
-
Gorsuch AM, Denit J, Stigall CE, Martin EE (1982) Guidance document for effluent discharges from the auto and other laundries point source category, Effluent Guidelines Division, Office of Water and Wastewater Management, United States Environmental Protection Agency, Washington, DC, USA.
-
Nguegang B, Sibanda T, Tekere M (2019) Cultivable bacterial diversity, physiochemical profile and toxicity determination of carwash effluents. Environ Monit Assess 191(8): 478-487.
-
Sibanda T, Selvarajan R, Tekere M (2019) Targeted 165 rRNA amplicon analysis reveals the diversity of bacterial communities in carwash effluents. Int Microbiol 22(2): 181-189.
-
Odeyemi OE, Adedeji AA, Odeyemi OJ (2018) Effects of discharge from carwash on the physico-chemical parameters and zooplanktonic abundance of Odo-Ebo River, Ile-Ife, Nigeria. Agric Environ 10: 83-96.
-
El-Ashtoukhy EZ, Amin NK, Fouad YO (2015) Treatment of real wastewater produced from Mobil carwash station using electrocoagulation technique. Environ Monit Assess 187(10): 628-638.
-
Rai R, Sharma S, Gurung DB, Sitaula BK, Shah RDT (2019) Assessing the impacts of vehicle wash wastewater on surface water quality through physio-chemical and benthic macroinvertebrates analyses. Water Sci 40(1): 39-49.
-
Danha C, Utete B, Soropa G, Rufasha SB (2014) Potential impact of wash bay effluent on the water quality of a subtropical river. J Water Resource Prot 6: 1045-1050.
-
Rai R, Sharma S, Gurung DB, Sitaula BK, Raut N (2018) Assessment of environmental impacts of vehicle wash centres at Olakha, Thimphu Bhutan. Int Res J Environ Sci 7: 1-10.
-
New Hampshire Department of Environmental Services (NHDES) (2010) Wastewater discharges from vehicle washing. Environmental fact sheet, New Hampshire Department of Environmental Services, Concord, New Hampshire, USA. Available online at: https://www.plaistow.com/sites/g/files/vyhlif1071/f/uploads/dwgb-22-10.pdf
-
RSCP (2007) Environmental regulations and best management practices. Regional Source Control Program, Environmental Services, Capital Regional District, Victoria, British Columbia, Canada.
-
SEOA (2007) Pollution prevention guidelines: Vehicle washing and cleaning. Scottish Environmental Protection Agency, Glasgow, England. Available online at: https://www.exeter.ac.uk/media/universityofexeter/campusservices/sustainability/pdf/PPG13_Vehicle_Washing.pdf
-
SAEPA (2016) Storm water management for wash bays. Environmental Protection Authority-South Australia, Adelaide, Australia. Available online at: https://www.epa.sa.gov.au/files/7593_water_wash.pdf
-
Zaneti RN, Etchepare R, Rubio J (2013) Carwash wastewater treatment and water reuse: A case study. Water Sci Technol 67(1): 82-88.
-
Morel A, Diener S (2006) Greywater management in low and middle-income countries: Review of different treatment systems for households or neighborhoods. Sandec Report No. 14/06, Department of Water and Sanitation in Developing Countries, Swiss Federal Institute of Aquatic Science and Technology, Zurich, Germany. Available online at: https://sswm.info/sites/default/files/reference_attachments/MOREL%20and%20DIENER%202006%20Greywater%20Management.pdf
-
Brown C (2002) Water Use in the professional car wash industry. International Carwash Association. Chicago, Illinois, USA. Available online at: https://www.carwash.org/docs/default-document-library/Water-Use-in-the-Professional-Car-Wash-Industry.pdf
-
Angelakis AN, Rose JB (2014) Evolution of sanitation and wastewater technology through the centuries. The International Water Association, London, England.
-
Bratby J (2006) Coagulation and Flocculation in wastewater treatment. IWA Publishing, London, England. Available online at: https://www.iwapublishing.com/news/coagulation-and-flocculation-water-and-wastewater-treatment
-
Aquarden Technologies (2020) Coagulation and flocculation in wastewater treatment. Aquarden Technologies, Skaeving, Denmark. Accessed on: January 12, 2021. Available online at: https://aquarden.com/technologies/coagulation-flocculation/
-
Ives KJ (1978) The scientific basis of flocculation. Springer, New York, New York, USA.
-
Hudson HE (1981) Water clarification processes. Practical design and evaluation. Van Nostrand Reinhold, New York, New York, USA.
-
van Loosdrecht MCM, Keller J, Nielsen PH, Lopez-Vazquez CM, Brdjanovic D (2014) Experimental Methods in wastewater treatment. IWA Publishing, London, England. Available online at: https://experimentalmethods.org/wp-content/uploads/2018/01/Experimental-Methods-in-Wastewater-Treatment.pdf
-
AES (2020) Coagulation/flocculation packages. AES Arabia Limited Water and Wastewater Treatment Systems. Riyadh, Kingdom of Saudi Arabia. Accessed on: January 12, 2020. Available online at: http://www.aesarabia.com/coagulation-flocculation-packages/
-
Teh CY, Budiman PM, Shak KPY, Wu TY (2016) Recent advancement of coagulation-flocculation and its application in wastewater treatment. Ind Eng Chem Res 55(16): 4363-4389.
-
Bolto BA, Dixon DR, Gray SR, Ha C, Harbour PJ, et al. (1996) The use of soluble organic polymer in waste treatment. Water Sci Technol 43(9): 117-124.
-
Ødegaard H (1998) Optimized particle separation in the primary step of wastewater treatment. Water Sci Technol 37(10): 43-53.
-
Jekel MR, Heinzmann B (1989) Residual aluminum in drinking-water treatment. J Water Supply Res Technol-Aqua 38: 281-288.
-
Monney I, Buamah R, Donkor EA, Etuaful R, Nota HK, et al. (2019) Treating waste with waste: The potential of synthesized alum from bauxite waste for treating carwash wastewater for reuse. Environ Sci Pollut Res Int 26(13): 12755-12764.
-
Li T, Xue-Jun T, Fu-Yi C, Qi Z (2007) Reuse of carwash wastewater with hollow filter membrane aided by enhanced coagulation and activated carbon treatments. Water Sci Technol 56(12): 111-118.
-
Moazzem S, Wills J, Fan L, Roddick F, Jegatheesan V (2018) Performance of ceramic ultrafiltration and reverse osmosis membranes in treating carwash wastewater for reuse. Environ Sci Pollut Res Int 25(9): 8654-8668.
-
Zaneti R, Etchepare R, Rubio J (2012) More environmentally friendly vehicle washes: Water reclamation. J Clean Prod 37: 115-124.
-
Al-Gheethi A, Mohamed RMSR, Rahman MAA, Johari MR, Kassim AHM (2016) Treatment of wastewater from carwashes using natural coagulation and filtration system. Mater Sci Eng 136: 1-7.
-
Boluarte IAR, Andersen M, Pramanik BK, Chang CY, Bagshaw S, et al. (2016) Reuse of car wash wastewater by chemical coagulation and membrane bioreactor treatment processes. Int Biodeterior Biodegrad 113: 44-48.
-
Rubio J, Zaneti RN (2009) Treatment of wash-rack wastewater with water recycling by advanced flocculation-column flotation. Desalin Water Treat 8(1-3): 146-153.
-
Aboulhassan MA, Souabi S, Yaacoubi A, Baudu M (2006) Removal of surfactant from industrial wastewaters by coagulation flocculation process. Int J Environ Sci Technol 3(4): 327-332.
-
Etchepare R, Zaneti R, Azevedo A, Rubio J (2014) Application of flocculation-flotation followed by ozonation in vehicle wash wastewater treatment/disinfection and water reclamation. Desalin Water Treat 56(7): 1728-1736.
-
Mohamed RMSR, Ibrahim Kutty NMA, Kassim AHM (2014) Efficiency of using commercial and natural coagulants in treating carwash wastewater. Aust J Basic Appl Sci 8: 227-234.
-
Posavčić H, Halkijević I, Vuković Z (2019) Application of electrocoagulation for water conditioning. Environ Eng-Inzenjersrvo Okolisa 6(2): 59-70.
-
Rodriguez J, Stopić S, Krause G, Friedrich B (2007) Feasibility assessment of electrocoagulation towards a new sustainable wastewater treatment. Environ Sci Pollut Res 14(7): 477-482.
-
An C, Huang G, Yao Y, Zhao S (2017) Emerging usage of electrocoagulation technology for oil removal from wastewater: A review. Sci Total Environ 579: 537-556.
-
Lai CL, Lin SH (2003) Treatment of chemical mechanical polishing wastewater by electrocoagulation: System performance and sludge settling characteristics. Chemosphere 54(3): 235-242.
-
Al-Shannag M, Al-Qodah Z, Bani-Melhem K, Qtaishat MR, Alkasrawi M (2015) Heavy metal ions removal from metal plating wastewater using electrocoagulation: Kinetic study and process performance. Chem Eng J 260: 749-756.
-
Woytowich DL, Dalrymple CW, Britton MG (1993) Electrocoagulation treatment of ship bilgewater for the US Coast Guard in Alaska. Mar Technol Soc J 27(1): 92.
-
Mohamud AA, Çalışkan Y, Bektaş N, Yatmaz HC (2018) Investigation of shipyard wastewater treatment using electrocoagulation process with Al electrodes. Separ Sci Technol 53(15): 2468-2475.
-
de Santana MM, Zanoelo EF, Benincá C, Freire FB (2018) Electrochemical treatment of wastewater from a bakery industry: Experimental and modeling study. Process Saf Environ Prot 116: 685-692.
-
Zhang H, Luo Z, Zhang Z, Liu Y, Chen Y (2020) Electro-flocculation pre-treatment experiments of shale gas drilling wastewater. Nat Gas Ind B 7(4): 309-316.
-
Chu JY, Li YR, Li N, Haung WH (2012) Treatment of car-washing wastewater by electrocoagulation-ultrasound technique for reuse. Adv Mat Res 433-440: 227-232.
-
Gonder ZB, Balcioglu G, Vergili I, Kaya Y (2007) Electrochemical treatment of carwash wastewater using Fe and and Al electrode: Techno-economic analysis and sludge characterization. J Environ Manage 200: 380-390.
-
Moulood I, Abdul-Majeed BA (2019) Treatment of simulated carwash wastewater by electrocoagulation with sonic energy. J Eng 25(9): 30-40.
-
Atiyah IM, Abdul-Majeed BA (2019) Carwash wastewater treatment by electrocoagulation using aluminum foil electrodes. J Eng 25(10): 50-60.
-
Takdastan A, Azimi A, Salari Z (2011) The use of electrocoagulation process for removal of turbidity, COD, detergent and phosphorous from carwash effluent. J Water Wastewater 22(3): 19-25.
-
Panizza M, Cerisola G (2010) Applicability of electrochemical methods to carwash wastewater for reuse-Part 2: Electrocoagulation and anodic oxidation integrated process. J Electroanal Chem 638(2): 236-240.
-
Priya M, Jevanthi J (2019) Removal of COD, oil and grease from automobile wash water effluent using electrocoagulation technique. Microchem J 150: 104070.
-
Sirés I, Brillas E, Oturan MA, Rodrigo MA, Panizza M (2014) Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ Sci Pollut Res Int 21(14): 8336-8367.
-
Anglada Á, Urtiaga A, Ortiz I (2009) Contributions of electrochemical oxidation to waste-water treatment: Fundamentals and review of applications. J Chem Technol Biotechnol 84(12): 1747-1755.
-
Särkkä H, Bhatnagar A, Sillanpää M (2015) Recent developments of electro-oxidation in water treatment - A review. J Electroanal Chem 754: 46-56.
-
Robles-Molina J, de Vidales MJM, García-Reyes JF, Cañizares P, Sáez C, et al. (2012) Conductive-diamond electrochemical oxidation of chlorpyrifos in wastewater and identification of its main degradation products by LC-TOFMS. Chemosphere 89(10): 1169-1176.
-
Gurung K, Ncibi MC, Shestakova M, Sillanpaa M (2018) Removal of carbamazepine by electrochemical oxidation using a Ti/Ta2O5-SnO5 electrode. Appl Catal B: Environ 221: 329-338.
-
Basile A, Cassano A, Rastog NK (2015) Advances in membrane Technology for wastewater treatment: Materials, processes and application. Woodhead Publishing, Cambridge, England.
-
Chu Y-Y, Wang W, Wang M (2010) Anodic oxidation process for the degradation of 2, 4-dichlorophenol in aqueous solution and the enhancement of biodegradability. J Hazard Mater 180(1-3): 247-252.
-
Bogdanowicz R, Fabiańska A, Golunski L, Sobaszek M, Gnyba M, et al. (2013) Influence of the boron doping level on the electrochemical oxidation of the azo dyes at Si/BDD thin film electrodes. Diam Relat Mater 39: 82-88.
-
Ramírez C, Saldaña A, Hernández B, Acero R, Guerra R, et al. (2013) Electrochemical oxidation of methyl orange azo dye at pilot flow plant using BDD technology. J Ind Eng Chem 19(2): 571-579.
-
Luu TL (2020) Tannery wastewater treatment after activated sludge pre-treatment using electro-oxidation on inactive anodes. Clean Technol Environ 22: 1701-1713.
-
Nayir TY, Kara S (2017) Container washing wastewater treatment by combined electrocoagulation-electrooxidation. Separ Sci Technol 53(8): 1-12.
-
Liu Y-J, Hu C-J, Lo S-L (2018) Direct and indirect electrochemical oxidation of amine-containing pharmaceuticals using graphite electrodes. J Hazard Mater 366: 592-605.
-
Ganiyu SO, dos Santos EV, Costa ECTDA, Martínez-Huitle CA (2018) Electrochemical advanced oxidation processes (EAOPs) as alternative treatment techniques for carwash wastewater reclamation. Chemosphere 211: 998-1006.
-
Rubi-Juarez H, Barrera-Diaz CE, Linares-Hernández I, Fall C, Bilyeu B (2015) A combined electrocoagulation-electrooxidation process for carwash wastewater reclamation. Int J Electrochem Sci 10(8): 6754-6767.
-
Davarnejad R, Sarvmeili K, Sabzehe M (2020) Car wash wastewater treatment using an advanced oxidation process: A rapid technique for the COD reduction of water pollutant sources. J Mex Chem Soc 63(4): 164-175.
-
Ersahin ME, Ozgun H, Dereli RK, Ozturk I, Roest K, et al. (2012) A review on dynamic membrane filtration: Materials, applications and future perspectives. Bioresour Technol 112: 196-206.
-
SDWF (2020) Ultrafiltration, nanofiltration and reverse osmoses. Safe Drinking Water Foundation, Saskatoon, Saskatchewan, Canada. Accessed on: January 10, 2020. Available online at: https://www.safewater.org/fact-sheets-1/2017/1/23/ultrafiltrationnanoandro
-
Hakim MW, Alkhudhiri A, Al-Batty S, Zacharof M-P, Maddy J, et al. (2020) Ceramic microfiltration membranes in wastewater treatment: Filtration behavior, fouling and prevention. Membranes 10(9): 1-34.
-
AMTA (2020) Membrane filtration for water reuse. American Membrane Technology Association, Stuart, Florida, USA, Accessed on: January 10, 2020. Available online at: https://www.amtaorg.com/Membrane_Filtration_for_Water_Reuse.html
-
WWD (2000) Membrane filtration for water and wastewater. Water and Wastewater Digest, Arlington Heights, Illinois, USA. Accessed on: January 10, 2020. Available online at: https://www.wwdmag.com/desalination/membrane-filtration-water-and-wastewater
-
Boussu K, Eelen D, Vanassche S, Vandecasteele C, Van der Bruggen B, et al. (2008) Technical and economic evaluation of water recycling in the carwash industry with membrane processes. Water Sci Technol 57(7): 1131-1135.
-
BSS (2020) Sand filter. Benbell Softener Systems, Nolda, Uttar Pradesh, India. Accessed on: January 28, 2020. Available online at: http://www.benbellsoftenersystems.in/sand-filter/
-
SSWMT (2020) Rapid sand filtration. Sustainable Sanitation and Water Management Toolbox, Willisau, Switzerland. Accessed on: January 28, 2020. Available online at: https://sswm.info/sswm-university-course/module-6-disaster-situations-planning-and-preparedness/further-resources-0/rapid-sand-filtration
-
WHO (2000) Guidelines for the safe use of wastewater, excreta and greywater, Volume 2: wastewater use in agriculture. World Health Organization, Geneva, Switzerland. Accessed on: October 12, 2020. Available online at: https://www.who.int/water_sanitation_health/publications/gsuweg2/en/
-
Abdelmoez W, Barakat NAM, Moaz A (2013) Treatment of wastewater contaminated with detergents and mineral oils using effective and scalable technology. Water Sci Technol 68(50): 974-981.
-
Zaneti R, Etchepare R, Rubio J (2011) Car wash wastewater reclamation: Full-scale application and upcoming features. Resour Conserv Recycl 55(11): 953-959.
-
Jamil A, Umer M, Karim SS, Amaduddin M, Akhtar K, et al. (2017) Design of a car wash wastewater treatment process for local car wash stations. J Pakistan Inst Chem Eng 45(2): 83-95.
-
Skrzypek M, Burger M (2010) Isoflux ® ceramic membranes - Practical experiences in dairy industry. Desalination 250: 1095-1100.
-
EMIS (2020) Microfiltration. Bat Knowledge Center, Brussels, Belgium. Accessed on: January 25, 2020. Available online at: https://emis.vito.be/en/bat/tools-overview/sheets/microfiltration
-
PFG (2020) Tubular membrane filter. Porex Filtration Group, Fairburn, Georgia, USA. Accessed on: January 28, 2020. Available online at: http://www.porexfiltration.com/learning-center/technology/what-is-microfiltration/
-
Lenntech (2020) Submerged membrane bioreactor. Lenntech Water Treatment and Purification, South Miami, Florida, USA. Accessed on: December 20, 2020. Available online at: https://www.lenntech.com/processes/submerged-mbr.htm
-
Hydroblue (2012) How membrane bioreactor is important for wastewater treatment plan, Hydro Blue Membrane Technology Company, JiangNan Industry Park, NanChun, China. Accessed on: December 20, 2020. Available online at: https://www.hydrobluemem.com/how-membrane-bioreactor-is-important-for-wastewater-treatment-plant/
-
Moazzem S, Ravishankar H, Fan L, Roddick F, Jegatheesan V (2020) Application of enhanced membrane bioreactor for the reuse of carwash wastewater. J Environ Manage 254: 109780.
-
Boluarte IAR, Andersen M, Pramanik BK, Chang C-Y, Bagshaw S, et al. (2016) Reuse of car wash wastewater by chemical coagulation and membrane bioreactor treatment processes. Int Biodeterior Biodegrad 113: 44-48.
-
Pinto ACS, Grossi LDB, de Melo RAC, de Assis TM, Ribeiro VM, et al. (2017) Carwash wastewater treatment by micro and ultrafiltration membranes: Effects of geometry, pore size, pressure difference and feed flow rate in transport properties. J Water Process 17: 143-148.
-
Ucar D (2017) Membrane processes for the reuse of car washing wastewater. J Water Reuse Desalination 8(2): 169-175.
-
Hsu S-K, Chen C-H, Chang W-K (2011) Reclamation of car washing wastewater by a hybrid system combining bio-carriers and non-woven membranes filtration. Desalin Water Treat 34(3): 349-353.
-
Daneshvar A, Ghaedi M (2015) Application of microfiltration membrane for treatment of car wash effluent by Taguchi method prediction. Iranian J Environ Technol 1(2): 1-10.
-
Alfa Laval (2020) Membrane filtration systems. Alfa Laval, Lund, Sweden. Accessed on: January 28, 2020. Available online at: https://www.alfalaval.com/products/separation/membranes/membrane-filtration-systems/
-
Boussu K, Kindts C, Vandecasteele C, Van der Bruggen B (2007) Applicability of nanofiltration in the carwash industry. Sep Purif Technol 54(2): 139-146.
-
Hamada T, Miyazaki Y (2004) Reuse of carwash water with a cellulose acetate ultrafiltration membrane aided by flocculation and activated carbon treatments. Desalination 169(169): 257-267.
-
Baker RW (2004) Membrane technology and applications. Membrane Technology and Research, Inc. Menlo Park, California, USA.
-
Cakmakci M, Basoinar AB, Balaban U, Uyak V, Koyumcu I, et al. (2009) Comparison of nanofiltration and adsorption techniques to remove arsenic from drinking water. Desalin Water Treat 9(1-3): 149-154.
-
Smbekke HDM, Voorhoeve DK, Hiemstra P (1997) Environmental impact assessment of groundwater treatment with nanofiltration. Desalination 113(2-3): 293-296.
-
Water Professional (2020) Nanofiltration. Water Professionals, North Carolina, Raleigh, USA. Accessed on: December 2020. Available online at: https://www.waterprofessionals.com/learning-center/nanofiltration/
-
Koyuncu I, Arikan OA, Wiesner MR, Rice C (2008) Removal of hormones and antibiotics by nanofiltration membranes. J Membr Sci 309(1-2): 94-101.
-
Hilal N, Al-Zoubi H, Darwish NA, Mohamed AW (2007) Performance of nanofiltration membranes in the treatment of synthetic and real seawater. Sep Sci Technol 42(3): 493-515.
-
Archer AC, Mendes AM, Boaventura RAR (1999) Separation of an anionic surfactant by nanofiltration. Environ Sci Technol 33(6): 2758-2764.
-
Van der Bruggen B, Braeken L, Vandecasteele C (2002) Flux decline in nanofiltration due to adsorption of organic compounds. Sep Purif Technol 29(1): 23-31.
-
Van der Bruggen B, Vandeccasteele C (2001) Flux decline during nanofiltration of organic components in aqueous solution. Environ Sci Technol 35(17): 3535-3540.
-
Panpanit S, Visvanathan C, Muttamara S (2000) Separation of oil-water emulsion from car washes. Water Sci Technol 41(10): 109-116.
-
RS (2020) Reverse osmosis. Rodi Water Purification Systems, Aztec, New Mexico, USA. Accessed on: December 20, 2020. Available online at: https://www.rodisystems.com/how-does-reverse-osmosis-work.html
-
Wagner J (2001) Membrane filtration handbook. Practical tips and hint. Osmonics Inc, Minnetonka, Belgium. Available online at: https://dpyxfisjd0mft.cloudfront.net/filtertechnics/Membrane%20Filtration%20Handbook%20Filter-Technics.pdf?1600418482&w=0&h=0
-
Sayers D (2002) Coming clean at the carwash customers value a spot-free wash. Mod Car Care 9(1): 1.
-
KAY (2020) Reverse osmosis water and clean water management. Kay Plumbing Services, Lexington and Columbia Plumbers, Lexington, North Carolina, USA. Accessed on: December 25, 2020. Available online at: https://kayplumbing.com/plumbing-blog/reverse-osmosis-water/
-
WTIC (1996) Using salt and sand for winter road maintenance. The Wisconsin Transportation Information Center, Wisconsin Transportation Bulletin. Volume 6: 1.
-
Madwar K, Tarazi H (2003) Desalination techniques for industrial wastewater reuse. Desalination 152(1-3): 325-332.
-
Shete BS, Simkar NB (2014) Use of membrane to treat car wash wastewater. Int J Res Sci Adv Technol 1(3): 13-19.
-
DiPaolo R (2016) A carwash’s guide to reverse osmosis. Professional Car washing and Detailing magazine. Akron, Ohio, USA.
-
Wiesmann U, Choi I, Dombrowsk EM (2007) Fundamentals of biological wastewater treatment. Wiley Publisher, Hoboken, New Jersey, USA.
-
Deal R (2019) Biological Wastewater Treatment for Industrial Applications. NASF Surface Technology White Papers 83(12): 8-13.
-
Marcos VS (2007) Basic principle of wastewater treatments. AWA Publisher, New York, New York, USA.
-
Tchobanglous G, Burton FL (1991) Wastewater engineering: Treatment, disposal and reuse. McGraw-Holl Publishing Company, London, England.
-
Wang LK, Shammas NK, Hung YT (2009) Advanced biological treatment process. Humana Press, Totowa, New Jersey, USA.
-
Malimen E, Nico I, Valotmen S, Hakala J, Mononen T, et al. (2012) Biological treatment of car wash wastewaters - A reduction survey. Linnaeus ECO-TECH, Kalmar, Sweden.
-
Pak D, Chang W (2000) Factors affecting phosphorous removal in two biofilter systems treating wastewater from car washing facility. Water Sci Technol 41(4-5): 487-492.
-
Mallick SK, Chakraborty S (2019) Bioremediation of wastewater from automobile service station in anoxic-aerobic sequential reactors and microbial analysis. Chem Eng J 361: 982-989.
-
Mazumber D, Mukherjee S (2011) Treatment of automobile service station wastewater by coagulation and activated sludge process. Int J Environ Sci Dev 2: 64-69.
-
EMIS (2021) Anaerobic wastewater treatment. Flemish knowledge Centre for Best Available Techniques, Vito, Belgium. Accessed on: February 8, 2021. Available online at: https://emis.vito.be/en/bat/tools-overview/sheets/anaerobic-biological-wastewater-treatment
-
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8(9): 623-633.
-
Chaudhary DS, Vigneswara S, Ngo H-H, Shim WG, Moon H (2003) Biofilter in water and wastewater treatment. Korean J Chem Eng 20(6): 1054-1065.
-
AQUABIOTEC (2021) Biofiltration advanced treatment. Aquabiotec Engineering Sarl, Paris, France. Accessed on: March 10, 2021. Available online at: https://www.pulpandpaper-technology.com/products/aquabiotec-engineering-sarl/biofiltration-advanced-treatment
-
ENVIROPRO (2021) Compact biofilter for wastewater treatment. Veolia Water Technology, High Wycombe Buckinghamshire, England. Accessed on: March 10, 2021. Available online at: https://www.enviropro.co.uk/entry/34041/Veolia-Water-Technologies-UK/Biostyr-compact-biofilter-for-wastewater-treatment/
-
Hossain MS, Das N, Choudhury IA, Bhattacharjee H (2009) Biofilter preparation by using indigenous materials for freshwater shrimp hatchery operations in Bangladesh. World Aquac 40(1): 5-11.
-
Curds CR, Hawkes HA (1983) Ecological aspects of used water treatment: The processes and their ecology, Academic Press, London, England.
-
Odegaard H (2006) Innovations in wastewater treatment: The moving bed biofilm process. Water Sci Technol 53(9): 17-33.
-
Talbot P, Bélanger G, Pelletier M, Laliberté G, Arcand Y (1996) Development of a biofilter using an organic medium for on-site wastewater treatment. Water Sci Technol 34(3-4): 435-441.
-
Söderlundh B, Svensson M, Mårtensson L (2010) Treatment of wastewater from car washes using a biofilter. Linnaeus Eco- Tech Proceeding of the International Conference on Natural Sciences and Technologies for Waste and Wastewater Treatment Remediation Emissions Related to Climate Environmental and Economic Effects, Kalmar, Sweden.
-
Shabbazi R, Kasra-kemashahi R, Gharavi S, Moosavi-Nejad Z, Borzooee F (2013) Screening of SDS-degrading bacteria from car wash wastewater and study of the alkylsulfatase enzyme activity. Iran J Microbiol 5(2): 153-158.
-
Hosseini F, Malekzadeh F, Amirmozafari N, Ghaemi N (2007) Biodegradation of anionic surfactant by isolated bacteria from activated sludge. Int J Environ Sci Technol 4(1): 127-132.
-
Guangming Z, Haiyan F, Hua Z, Xingzhong Y, Muxing F, et al. (2007) Co-degradation with glucose of four surfactants, CTAB, Triton X-100, SDS and Rhamnolipid in liquid culture media and compost matrix. Biodegradation 18(3): 303-310.
-
Mitsch JW, Gosselink JG (1986) Wetlands. Van Nostrand Reinhold Publishing Company, New York, USA.
-
Dotro G, Langergraber G, Molle P, Nivala J, Juárez JP, et al. (2017) Treatment wetlands. IWA Publishing, London, England.
-
Hammer DA (1989) Constructed wetlands for wastewater treatment: Municipal, Industrial and Agricultural. Lewis’s publishers, Chelsea, Michigan, USA.
-
Davies TH, Hart BT (1990) Use of aeration to promote nitrification in reed beds treating wastewater. In Proceeding of the International on the use of Constructed Wetlands in Water Pollution Control, Cambridge, England.
-
Keddy P (2010) Wetland Ecology: Principals and conservation. Cambridge University Press, Cambridge, England.
-
Skrzypiecbcef K, Gajewska MH (2017) The use of constructed wetlands for the treatment of industrial wastewater. J Water Land Dev 34 (VII-IX): 233-240.
-
Bakacs ME, Yergeau SE, Obropta CC (2013) Assessment of carwash runoff treatment using bioretention mesocosms. J Environ Eng 139(8): 1132-1136.
-
Peng K, Luo C, Luo L, Li X, Shen Z (2008) Bioaccumulation of heavy metal by aquatic plants Potamogeton pectinatus L. and Potamogeton alaianus Miq and their potential use for contamination indicator in wastewater treatment. Sci Total Environ 392: 22-29.
-
Torrens A, Folch S, Salgot M, Aulinas M (2018) Recycling of carwash effluents treated with subsurface flow constructed wetlands. In Constructed Wetlands for Industrial Wastewater Treatment (J. Taberham, Editor), John Wiley & Sons Ltd, New York, USA.
-
Tamiazzo J, Breschigliaro S, Salvato M, Borin M (2015) Performance of a wall cascade constructed wetland treating surfactant-polluted water. Environ Sci Pollut Res Int 17: 12816-12828.
-
Tien C (2019) Introduction to adsorption: Basics, analysis, and applications. Wiley and Son Inc., New York, USA.
-
Toth J (2002) Adsorption: Theory, modelling and analysis. Marcel Dekker Inc, New York, USA.
-
Atkins PW, Paula JD, Keeler J (2018) Atkin’s physical chemistry (Eleventh ed.). Oxford, United Kingdom.
-
Calvert JG (1990) Glossary of atmospheric chemistry terms. Pure Appl Chem 62(11): 2167-2219.
-
Ferrari L, Kaufmann J, Winnefeld F, Plank J (2010) Interaction of cement model systems with superplasticizers investigated by atomic force microscopy, zeta potential, and adsorption measurements. J Colloidal Interface Sci 347(1): 15-24.
-
Czelej K, Cwieka K, Kurzydlowski KJ (2016) CO2 stability on the Ni low-index surfaces: Van der Waals corrected DFT analysis. Catal Commun 80(5): 33-38.
-
Czelej K, Cwieka K, Colmenares JC, Kurzydlowski KJ (2016) Insight on the interaction of methanol-selective oxidation intermediates with Au- or/and Pd-containing monometallic and bimetallic Core@Shell Catalysts. Langmuir 32(30): 7493-7502.
-
CECE (2021) Separation. Visual Encyclopedia of Chemical Engineering, Department of Chemical Engineering, College of Engineering, University of Michigan, Illinois, USA.
-
Baddor IM, Farhoud N, Abdel-Magid IM, Alshami S, Ahmad FH, et al. (2014) Study of carwash wastewater treatment by adsorption. A paper presented at the International Conference of Engineering, Information Technology and Science, Infrastructure University Kuala Lumpur, Selangor Darul Ehsan, Malaysia. Accessed on: August 20, 2020. Available online at: https://www.academia.edu/22502156/Study_of_Car_Wash_Wastewater_Treatment_by_Adsorption
-
Abdel-Magid IM, Olabi A (2014) Study of carwash wastewater treatment by adsorption. International Conference of Engineering, Information Technology, and Science (ICEITS 2014), Infrastructure University Kuala Lumpur, Selangor Darul Ehsan, Malaysia. Accessed on: February 10, 2021. Available online at: https://www.researchgate.net/publication/271704061_Study_of_Car_Wash_Wastewater_Treatment_by_Adsorption
-
Kowsalya E, Subhashini S, Sharmila S, Rebecca LJ (2020) Treatment of carwash wastewater and its reuse to manage water. Int J Recent Technol Eng 8(5): 3605-3610.
-
Bintizayadi N (2017) Performance of powdered and granular sugarcane bagasse activated carbon in removing pollutants of carwash wastewater, MSC Thesis, Faculty of Civil and Environmental Engineering, University Tun Hussein Onn, Johor, Malaysia.

















