Review Article
Bismuth Level in the Prostate of the Normal Human: A Systematic Review
3783
Views & Citations2783
Likes & Shares
Knowledge of the etiology and pathogenesis of most prostate malfunctions and pathologies is very limited. Despite advances in medicine, the differential diagnosis of benign hypertrophic and carcinogenic prostate has steadily increased in complexity and controversy. It has been suggested that the prostate bismuth (Bi) level may help solve these problems related to prostate disorders, especially as an indicator of prostate cancer risk, as an elevated Bi level in the prostate may be a sign of prostate cancer in the future. These suggestions promoted more detailed studies of the Bi level in the prostate of healthy men. In present review we analyze data published concerning Bi prostatic levels in healthy persons. In all 2249 items in the literature of the years dating back to 1921 were identified in the following databases: PubMed, Scopus, Web of Science, the Cochrane Library, and ELSEVIER-EMBASE. This data was subject to an analysis employing both the “range” and “median” of means. In this way the disparate nature of published Bi content of normal prostates was evaluated. Of the articles examined, 15 were selected for objective analysis of data from 760 healthy subjects. The contents of prostatic Bi (on a wet mass basis) spanned the interval from 0.00066 mg/kg to ≤0.04 mg/kg with 0.0046 mg/kg as median for their means. The data included a wide range of values and the samples were small, hence it is advisable that further studies with strong quality control of results be performed.
Keywords: Bismuth, Human prostate gland, Normal prostatic tissue, Biomarkers
Abbreviations
Bi: Bismuth; PCa: Prostate cancer; BPH: Benign prostatic hyperplasia; TE: Trace element; AES: Atomic emission spectrometry; ICP-MS: Inductively coupled plasma mass spectrometry; ICP-OES: Inductively coupled plasma optical emission spectrometry; M: Mean; SD: Standard deviation; WHO: World Health Organization
INTRODUCTION
Amongst the many pathological prostatic conditions, prostatic carcinoma (PCa), chronic prostatitis and benign prostatic hyperplasia (BPH) are very frequently encountered, especially in the elderly [1-3]. Their causes and pathogenesis are poorly understood. Moreover, despite biomedical advances, the differential diagnosis of prostate diseases has become progressively more complex and controversial. An improvement of this situation, especially recognition of relevant risk factors and the disorders’ etiologies can allow great reduction in the incidence of these prostatic disorders.
In our previous studies the involvement of trace elements (TEs) in the function of the prostate gland was indicated. [4-15]. It was also found that content of TEs in prostatic tissue, including bismuth (Bi), can play a significant role in etiology of PCa [16-21]. Furthermore, it was demonstrated that the changes of some TE levels and Zn/Bi ratios in prostate tissue can be useful as biomarkers [22-28].
The first data of Bi content in human prostatic tissue (0.05 mg/kg of wet tissue) were published almost 60 years ago in the early 60s [29,39]. Zakutinsky [29] and Tipton and Cook [30] indicated that the content of Bi in the human prostate equals or below than 0.04 and 0.02 mg/kg of wet tissue, respectively. This finding allowed conclude that the prostate can accumulate Bi, because the level of metal in glands was almost four orders of magnitude higher the blood level (0.000002 mg/L) [31]. Moreover, recent experimental results identified that some Bi compounds should be considered as genotoxic carcinogens [32,33]. These findings promoted more extensive considerations of the Bi content of prostatic tissue of healthy persons, as well as of patients with different prostatic disorders, including BPH and PCa.
The effects of TEs, including Bi, are related to their level in tissues and fluids. Recorded observations range from a deficiency state, through normal function as biologically essential components, to an imbalance, when excess of one element interferes with the function of another, to pharmacologically active levels, and finally to toxic and even life-threatening concentrations [34-36]. In this context, until now there are no data on any biological function of Bi in organisms, but a lot of publications testify to adverse health effects in different organs or tissues of exposure to this metal and its compounds [37-42]. However, it still remains unclear what precise mechanism is responsible for Bi genotoxicity [32,33].
By now, a few publications have reported the level of Bi content in tissue of “normal” and affected glands. However, subsequent research works has been considered necessary to provide a practical reference data of Bi contents in prostate norm and disorders, because the findings of various investigations indicate some discrepancies.
The present study deals with the importance of Bi contents in prostate tissue as a biomarker of gland condition. Therefore, we systematically reviewed all relevant literature and performed a statistical analysis of the Bi level in "normal" gland tissue, which may provide insight into the etiology and diagnosis of prostate diseases as a higher Bi rate than these normal rates may be an indication of the possibility of pathological development in the prostate.
MATERIALS AND METHODS
Data Sources and Search Strategy
Aiming at finding the most relevant articles for this review, a thorough comprehensive web search was conducted by consulting the PubMed, Scopus, Web of Science, the Cochrane Library, and ELSEVIER-EMBASE databases, as well as from the personal archive of the author collected between 1966 to December 2020, using the key words: prostatic trace elements, prostatic Bi content, prostatic tissue, and their combinations. For example, the search terms for Bi content were: “Bi mass fraction”, “Bi content”, “Bi level”, “prostatic tissue Bi” and “Bi of prostatic tissue”. The language of the article was not restricted. The titles from the search results were evaluated closely and determined to be acceptable for potential inclusion criteria. Also, references from the selected articles were examined as further search tools. Relevant studies noted for each selected article were also evaluated for inclusion.
ELIGIBILITY CRITERIA
Inclusion Criteria
Only papers with quantitative data of Bi prostatic content were accepted for further evaluation. Studies were included if the control groups were healthy human males with no history or evidence of urological or other andrological disease and Bi levels were measured in samples of prostatic tissue.
Exclusion Criteria
Studies were excluded if they were case reports. Studies involving persons from Bi contaminated area and subjects that were Bi occupational exposed were also excluded.
DATA EXTRACTION
A standard extraction of data was applied, and the following available variables were extracted from each paper: method of Bi determination, number and ages of healthy persons, sample preparation, mean and median of Bi levels, standard deviations of mean, and range of Bi levels. Abstracts and complete articles were reviewed independently, and if the results were different, the texts were checked once again until the differences were resolved.
STATISTICAL ANALYSIS
Studies were combined based on means of Bi levels in prostatic tissue. The articles were analyzed and “Median of Means” and “Range of Means” were used to examine heterogeneity of Bi contents. The objective analysis was performed on data from the 15 studies, with 760 subjects.
RESULTS
Information about Bi levels in prostatic tissue in different prostatic diseases is of obvious interest, not only to understand the etiology and pathogenesis of prostatic diseases more profoundly, but also for their diagnosis, particularly for PCa diagnosis and PCa risk prognosis [28,34]. Thus, it dictates a need for reliable values of the Bi levels in the prostatic tissue of apparently healthy subjects, ranging from young adult males to elderly persons.
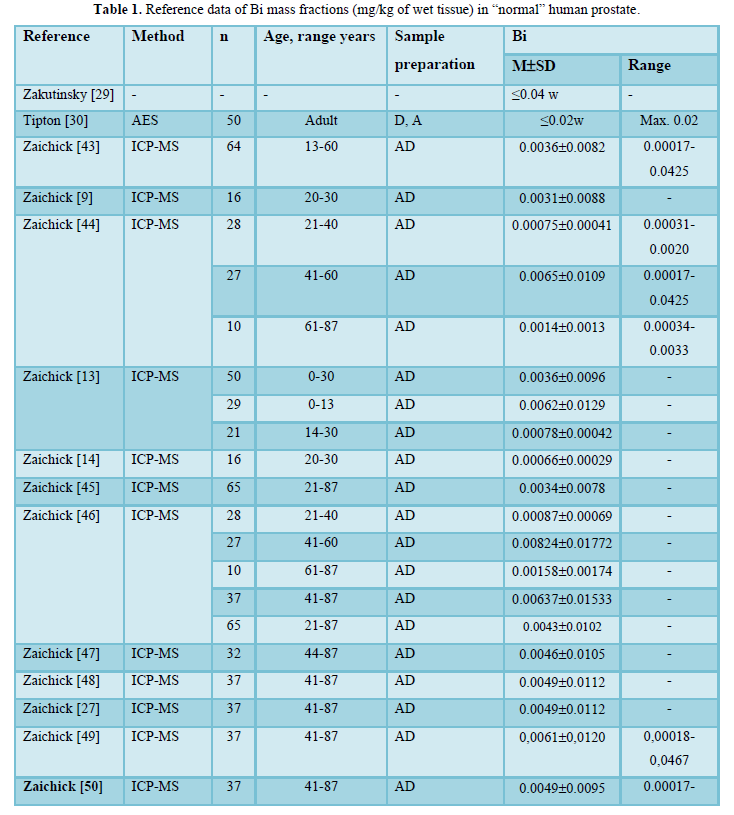
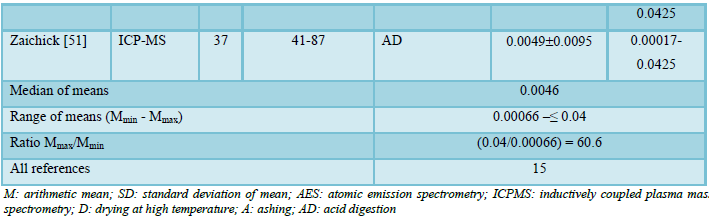
Possible publications relevant to the keywords were retrieved and screened. A total of 2249 publications were primarily obtained, of which 2234 irrelevant papers were excluded. Thus, 15 studies were ultimately selected according to eligibility criteria that investigated Bi levels in tissue of normal prostates (Table 1) and these 15 papers [9,13,14,27,29,30,43-51] comprised the material on which the review was based. A number of values for Bi mass fractions were not expressed on a wet mass basis by the authors of the cited references. However, we calculated these values using the medians of published data for water - 83% [52-55] and ash - 1% (on a wet mass basis) contents in normal prostates of adult men [30,54,56,57].
Table 1 summarizes general data from the 15 studies. The retrieved studies involved 760 subjects. The ages of subjects were available for 13 studies and ranged from 0–87 years. Information about the analytical method and sample preparation used was available for 14 studies. All fourteen studies determined Bi levels by destructive (require high temperature drying, ashing or acid digestion of tissue samples) analytical methods (Table 1): one using atomic emission spectrometry (AES), and thirteen - inductively coupled plasma mass spectrometry (ICPMS).
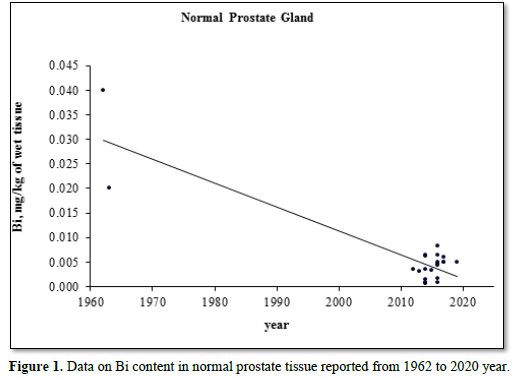
Figure 1 illustrates the data set of Bi measurements in 15 studies during the period from 1962 to 2020.



DISCUSSION
The range of means of Bi mass fractions reported in the literature for “normal” prostatic tissue varies widely from 0.00066 mg/kg [14] to ≤0.04 mg/kg [29] with median of means 0.0046 mg/kg wet tissue (Table 1). Thus, the maximal value of mean Bi mass fraction reported [29] was 60.6 times higher the minimal value of mean [14]. This variability of reported mean values can be explained by a dependence of Bi content on many factors, including analytical method imperfections, differences in “normal” prostate definitions, possible non-homogeneous distribution of Bi levels throughout the prostate gland volume, age, ethnicity, diet, smoking, alcohol intake, consuming supplemental Zn and Se, and others. Not all these factors were strictly controlled in the cited studies. For example, in some studies the “normal” prostate means a gland of an apparently healthy man who had died suddenly, but without any morphological confirmation of “normality” of his prostatic tissue. In other studies, the “normal” prostate means a non-cancerous prostate (but hyperplastic and inflamed glands were included) and even a visually normal prostatic tissue adjacent to a prostatic malignant tumor. Some researchers used as the “normal” prostate the glands of patients who died from acute and chronic non-prostatic diseases including subjects who had suffered from prolonged wasting illnesses. In some studies, whole glands were used for the investigation while in others the Bi content was measured in pieces of the prostate. Therefore, published data allowed us to estimate the effect of only a few factors on Bi content in “normal” prostate tissue.
ANALYTICAL METHOD
The trend line of Bi content data in “normal” prostate (Figure 1) showed that an improvement of analytical technologies during last almost 60 years impacted significantly on the means and variability of reported values. Thus, in our opinion, the leading cause of inter-observer variability was insufficient quality control of results in studies published in the early 60s [29,30]. In all reported papers destructive analytical methods were used. These methods require drying, ashing or acid digestion of the samples at a high temperature. There is evidence that use of this treatment causes some quantities of TEs to be lost [34,58,59]. On the other hand, the Bi content of chemicals used for acid digestion can contaminate the prostate samples. Thus, when using destructive analytical methods, it is necessary to allow for the losses of TEs, for example when there is complete acid digestion of the sample. Then there are contaminations by TEs during sample decomposition, which require addition of some chemicals. It is possible to avoid these problems by using non-destructive methods, but up to now there are no analytical methods which allow quantify Bi content in “normal” prostate without ashing or acid digestion of the samples at a high temperature. It is, therefore, reasonable to conclude that the strong quality control of results is very important factor for using the Bi content in prostatic tissue as biomarkers.
AGE
In a few studies a significant increase in Bi content with increasing of age was shown by the comparison of different age groups or the Pearson’s coefficient of correlation between age and Bi content in prostate tissue [44,46]. The most detailed investigations of age-dependence of prostatic Bi were done by Zaichick and Zaichick [46]. For example, a strongly pronounced tendency for an age-related increase of Bi mass fraction was observed in the prostate for the third to sixth decades [46]. In prostates of 41–60-year-old men, the mean Bi mass fraction was almost one order of magnitude higher than that in the prostates of 20–39-year-old males. Thus, the accumulated information, studied by us from reported data, allowed a conclusion that there is a significant increase in Bi mass fraction in “normal” prostate from age 21 years to the sixth decades.
ANDROGEN-INDEPENDENCE OF PROSTATIC BI LEVELS
There was not found any difference between Bi levels in prostates of teenagers before puberty and of post pubertal teenagers and young adults [9,13,14]. These findings allowed us to conclude that the Bi content in “normal” prostates does not depend on the level of androgens, and vice versa.
DIETARY BI INTAKE
Bi exposure occurs through various ways like food and water consumption, inhalation, and skin contact. Food and drinking water are the main sources of Bi exposure [60,61]. Most people receive the largest portion of their daily Bi intake via food and Bi is contained in all kinds of food. Data on Bi dietary intakes are very limited and vary widely from 0.0004 mg/day in the United Kingdom (UK) [60-63] to 1.58 mg/day in the Canary Islands [64]. In the UK study the highest contents of Bi was found in dairy products (0.0064 mg/kg), sugar (0.005 mg/kg), and milk (0.002 mg/kg) [62]. In the Canary Islands study the highest contents of Bi was found in viscera (38.1 mg/kg), while the lowest in yogurts (0.184 mg/kg). There were no found significant differences between Bi content in the different types of food including cold meat and sausages, red meat, milk, vegetables, potatoes, nuts, pastries, sweets, eggs, soft drinks, and alcoholic beverages [64]. In oils and waters Bi concentrations were under the detection limit (0.02 mg/L) of ICP-OES method used in the study [64]. In accord with this study the Bi concentration in milk (Spain, Burgos) was 0.305±0.426 mg/L (M, mean ± SD, standard deviation), while Bi content in the milk samples in Turkey was indicated at least one order of magnitude lower and ranged from 0.0065 to 0.0143 mg/L [65].
It was found that population dietary exposures in the UK have increased by 5-fold for 10 years [62]. In spite of this fact, till now there are no health-based guidance values for Bi dietary intake. Moreover, no data available on levels of Bi in drinking water in different countries, and a World Health Organization (WHO) drinking water guideline value has not been set [62].
It was shown that a strong link exists between Bi intake and this metal level in key organs, such liver and kidney [66]. From this it was hypothesized that dietary Bi intake affects the metal’s levels in the prostate.
PROSTATIC BI CONTENT IN COMPARISON WITH OTHER BODY ORGANS, TISSUES AND FLUIDS
The determination of Bi in human organs, tissues and fluids is subject to large variation and older data should be approached with caution. In recent publication data on Bi content in human organs were not found, but the liver and kidney have been shown to be the target organs of Bi [39]. It is known also that the content of Bi in the kidney approximately one order of magnitude higher than in other organs (e.g., liver, lung, skeletal muscles, brain, bones) [67].
Reported concentrations of this metal in blood serum and urine of non-exposed persons varied very widely. For example, in old publications blood Bi levels were between 0.001 mg/L and 0.015±0.012 mg/L (M ± SD) [68], while in the study of Vanhoe [69] published in 1993 values of Bi concentrations in human serum of healthy adults (n=19) gave a range from
Because the median of prostatic Bi content means obtained in the present review (0.0046 mg/kg of wet tissue) is approximately one order of magnitude higher the reference serum value (
It is known that Bi is deposited in many organs [38], but is retained longest in the kidney [66]. The half-life of Bi in blood varies from 3.5 minutes to 17–22 years [39]. Thus, in spite of the possible changes in Bi intake, humans are in a state of positive Bi balance, because there is a component of Bi metabolism with very long half-life. This may well explain the increase of Bi content in kidney and some other key organs, including prostate, with the increase of age (see paragraph “Age”).
Bi occurs naturally as a free metal or minerals, such as bismite (bismite oxide) and bismuthite (bismuth sulfide), which is commonly associated with sulfide ores of lead, copper and tin dioxide [70]. All-natural chemical elements of the Periodic System, including Bi, present in all subjects of biosphere [34,71,72]. During the long evolutional period intakes of Bi in organisms were more or less stable and organisms were adopted for such environmental conditions. Moreover, organisms, including human body, involved low doses of this metal in their functions [73].
The chemical behavior of Bi is similar to that of As, Pb, and Sb [76]. For centuries Bi minerals has been used in medicine and cosmetics, as well as pigments [39,70,74,75]. Since the 19th century Bi-contained compounds has been used in plenty of applications for the treatment of a wide range of diseases including syphilis, amebiasis, colitis, and other bacterial and parasite infections. However, Bi compounds use slowed down in the middle of the 20th century after the reversible Bi encephalopathy occurred in France and Australia [74]. For example, in France between 1973 and 1980 approximately 1000 cases of Bi related neurotoxicity and over 70 deaths were reported [74]. Thus, today Bi salts are primarily used for the treatment of peptic ulcers, functional dyspepsia, chronic gastritis, and other gastrointestinal disturbances [39].
In spite of a long story of Bi using in medicine and cosmetics, a really drastically increase of environmental Bi pollution links with the industrial revolution, with appearing various sources of this metal exposure. Bi has many important properties like softness, low-melting point, high relative density, resistance to corrosion, low thermal conductivity, and extreme diamagnetism. This metal and its compounds are widely used in non-ferrous metallurgy, as well as in arm, atomic, electronic, chemical, ceramic, pharmaceutical, and cosmetic industry, Bi compounds have a multitude of uses in industrial processes and products, such as the manufacture and reprocessing of nuclear fuel rods, battery cathodes, semiconductors, numerous readily fusible alloys, quenching baths for steel production, catalysts in the chemical industry, flame retardants, mirrors, as well as for anti- Helicobacter pylori therapy and dental health [41,70]. Bi is heavier and the relatively non-toxic metal in comparison with lead. As such, there is an increased use of Bi as a replacement for lead in manufacturing of lead-free shot bullets, malleable steels, lubricating greases, fire sprinkler systems, ceramic glazes, fishing sinkers, food processing equipment, free-machining brasses for plumbing applications, crystal ware, thermoelectric materials, solders, pearlescent pigments, cosmetics, medicines, etc. [70,75]. By now there are 39 Bi compounds, 17 of which are employed in the pharmaceutical industry and in many cosmetic products [76]. Furthermore, Bi in the nanomaterial forms has great potential for computed tomography imaging and thermotherapy [41,74,77].
Environmental Bi pollution occurs mainly through a combination of land (through atmospheric emissions originating from residues from coal, oil, and gas combustion, urban refuse, mine tailings and smelter slag, and also from waste, fertilizers and sludge application), water (through irrigation and industrial liquid waste), and air (through atmospheric industrial emissions and vehicle exhaust) contamination and is subsequently introduced into the food chain and drinking water [78-80]. In contrast to organic pollutants the non-biodegradable nature of Bi, as all other metals, is the prime reason for its prolonged persistence in the environment. Due to its non-biodegradable nature and continuous use, Bi concentration accumulates in the environment with increasing hazards [81]. Furthermore, in the environment, inorganic Bi can be bio transformed into highly mobile, membrane-permeable and therefore toxic trimethyl bismuth by methanobacteria [32,33]. Moreover, it was found that intestinal microbiota of human body exhibit highly productive mechanisms for the formation of this toxic volatile derivative trimethyl bismuth [82], which induces cyto- and genotoxic effects in human cells [32]. These findings should be considered in the medical application of Bi, as well as in environmental and occupational medicine, since the formation of methylated Bi derivatives in the human gut may damage mammalian cells, including cells of prostate.
Bi is an important product in the world industry. For example, the world production of Bi in 2017 was estimated to be about 17 thousand tons [83]. The world's largest producers are China and Laos. Other countries as Japan, Mexico, Kazakhstan, and Canada continue to increase this metal production [83]. Since the use of Ba is linked to the rapidly developing modern technologies, we can conclude that the need of industry in this metal increased for decades and would for continue to increase in the future. Age-dependent increase of Bi mass fractions in the ‘normal” prostate tissue, which was indicated in the present review, indirectly confirm this conclusion. As was mentioned above, elevated Bi level is a poisonous factor affecting every organ in the body and the prostate gland is not the exclusion.
Thus, according our study for not polluted areas no one influencing factor could explain the variability of published means for prostatic Bi levels from 0.00066 mg/kg to ≤ 0.04 mg/kg of wet tissue. Moreover, prostate tissue Bi contents showed large variations among individuals (values ±SD for means in Table 1), but sources of the variation remain unknown. It is, therefore, reasonable to assume from data of our study that inaccuracy of analytical technologies employed caused so great variability of published means for prostatic Bi levels. This conclusion was supported the fact that the Certified Reference Materials for quality control of results were used only in a very few reported studies.
There are some limitations in our study, which need to be taken into consideration when interpreting the results of this review. The sample size of each study was sometimes relatively small (from 10 to 65), and a total of 760 normal controls were investigated from all 15 studies. As such, it is hard to draw definite conclusions about the reference value of the Bi content in “normal” prostate as well as about the clinical value of the Bi levels in “normal” prostates as a biomarker.
CONCLUSION
The present study is a comprehensive study regarding the determination of Bi content in “normal” human prostates. With this knowledge Bi levels may then be considered as a biomarker for the recognition of prostate disorders. The study has demonstrated that level of Bi in “normal” prostates depends on many factors such as age, dietary Bi intake, and others. Because of the uncertainties we have outlined, we recommend other studies on Bi content in “normal” human prostate with the strong quality control of results be performed.
- Nickel JC (2011) Prostatitis. Can Urol Assoc J 5: 306-315.
- Lim KB (2017) Epidemiology of clinical benign prostatic hyperplasia. Asian J Urol 4: 148-151.
- Rawla P (2019) Epidemiology of prostate cancer. World J Oncol 10(2): 63-89.
- Avisyn AP, Dunchik VN, Zhavoronkov AA, Zaichick VE, Sviridova TV (1981) Histological structure of the prostate and content of zinc in it during various age period. Arkh Anat Gistol Embriol 81(11): 76-83.
- Zaichick V (2004) INAA and EDXRF applications in the age dynamics assessment of Zn content and distribution in the normal human prostate. J Radioanal Nucl Chem 262: 229-234.
- Zaichick V, Zaichick S (2013) The effect of age on Br, Ca, Cl, K, Mg, Mn, and Na mass fraction in pediatric and young adult prostate glands investigated by neutron activation analysis. Appl Radiat Isot 82: 145-151.
- Zaichick V, Zaichick S (2013) INAA application in the assessment of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn mass fraction in pediatric and young adult prostate glands. J Radioanal Nucl Chem 298:1559-1566.
- Zaichick V, Zaichick S (2013) NAA-SLR and ICP-AES application in the assessment of mass fraction of 19 chemical elements in pediatric and young adult prostate glands. Biol Trace Elem Res 156: 357-366.
- Zaichick V, Zaichick S (2013) Use of neutron activation analysis and inductively coupled plasma mass spectrometry for the determination of trace elements in pediatric and young adult prostate. Am J Analyt Chem 4: 696-706.
- Zaichick V, Zaichick S (2014) Relations of bromine, iron, rubidium, strontium, and zinc content to morphometric parameters in pediatric and nonhyperplastic young adult prostate glands. Biol Trace Elem Res 157: 195-204.
- Zaichick V, Zaichick S (2014) Relations of the neutron activation analysis data to morphometric parameters in pediatric and nonhyperplastic young adult prostate glands. Adv Biomed Sci Eng 1: 26-42.
- Zaichick V, Zaichick S (2014) Relations of the Al, B, Ba, Br, Ca, Cl, Cu, Fe, K, Li, Mg, Mn, Na, P, S, Si, Sr, and Zn mass fractions to morphometric parameters in pediatric and nonhyperplastic young adult prostate glands. BioMetals 27: 333-348.
- Zaichick V, Zaichick S (2014) The distribution of 54 trace elements including zinc in pediatric and nonhyperplastic young adult prostate gland tissues. J Clin Lab Invest Updates 2(1): 1-15.
- Zaichick V, Zaichick S (2014) Androgen-dependent chemical elements of prostate gland. Androl Gynecol: Curr Res 2: 2.
- Zaichick V, Zaichick S (2015) Differences and relationships between morphometric parameters and zinc content in nonhyperplastic and hyperplastic prostate glands. Br J Med Med Res 8: 692-706.
- Schwartz MK (1975) Role of trace elements in cancer. Cancer Res 35: 3481-3487.
- Zaichick V, Zaichick S (1999) Role of zinc in prostate cancerogenesis. In: Mengen und Spurenelemente. 19. Arbeitstagung. Jena: Friedrich-Schiller-Universitat; pp: 104-115.
- Zaichick V, Zaichick S, Wynchank S (2016) Intracellular zinc excess as one of the main factors in the etiology of prostate cancer. J Anal Oncol 5: 124-131.
- Zaichick V, Zaichick S, Rossmann M (2016) Intracellular calcium excess as one of the main factors in the etiology of prostate cancer. AIMS Mol Sci 3: 635-647.
- Fukuda H, Ebara M, Yamada H, Arimoto M, Okabe S, et al. (2004) Trace elements and cancer. Jpn Med Assoc J 47(8): 391-395.
- Chen QY, DesMarais T, Costa M (2019) Metals and mechanisms of carcinogenesis. Annu Rev Pharmacol Toxicol 59: 537-554.
- Dunchik V, Zherbin E, Zaichick V, Leonov A, Sviridova T (1980) Method for differential diagnostics of prostate malignant and benign tumours. Russian patent (Author’s Certificate No 764660, priority of invention 27.10.1977). Discoveries, Inventions, Commercial Models, Trade Marks 35: 13.
- Zaichick V, Sviridova T, Zaichick S (1997) Zinc in the human prostate gland: Normal, hyperplastic and cancerous. Int Urol Nephrol 29: 565-574.
- Zaichick V, Sviridova T, Zaichick S (1997) Zinc in human prostate gland: Normal, hyperplastic and cancerous. J Radioanal Nucl Chem 217: 157-161.
- Zaichick S, Zaichick V (2012) Trace elements of normal, benign hypertrophic and cancerous tissues of the human prostate gland investigated by neutron activation analysis. Appl Radiat Isot 70: 81-
- Zaichick V, Zaichick S (2016) Ratios of selected chemical element contents in prostatic tissue as markers of malignancy. Hematol Med Oncol 1(2): 1-8.
- Zaichick V, Zaichick S (2017) Trace element levels in prostate gland as carcinoma’s markers. J Cancer Ther 8: 131-145.
- Zaichick V, Zaichick S (2017) Ratios of Zn/trace element contents in prostate gland as carcinoma’s markers. Cancer Rep Rev 1(1): 1-7.
- Zakutinsky DI, Parfyenov Yu D, Selivanova LN (1962) Data book on the radioactive isotopes toxicology. Moscow: State Publishing House of Medical Literature.
- Tipton IH, Cook MJ (1963) Trace elements in human tissue. Part II. Adult subjects from the United States. Health Phys 9: 103-145.
- Serfontein WJ, Mekel R, BankS, Barbezat G, Novis B (1979) Bismuth toxicity in man - I. Bismuth blood and urine levels in patients after administration of a bismuth protein complex (Bicitropeptide). Res Commun Chem Pathol Pharmacol 26(2): 383-389.
- Von Recklinghausen U, Hartmann IM, Rabieh S, Hippler J, Hirner AV, et al. (2008) Methylated bismuth, but not bismuth citrate or bismuth glutathione, induces cyto- and genotoxic effects in human cells in vitro. Chem Res Toxicol.21(6): 1219-1228.
- Thomas F, Bialek B, Hensel R (2011) Medical use of bismuth: The two sides of the coin. J Clinic Toxicol S3: 004.
- Zaichick V (2006) Medical elementology as a new scientific discipline. J Radioanal Nucl Chem 269: 303-309.
- Hunter P (2008) A toxic brew we cannot live without. Micronutrients give insights into the interplay between geochemistry and evolutionary biology. EMBO Rep 9(1): 15-18.
- López-Alonso M (2012) Trace minerals and livestock: Not too much not too little. ISRN Vet Sci 2012: 704825.
- Bradley B, Singleton M, Po ALW (1989) Bismuth toxicity--a reassessment. J Clin Pharm Ther 14(6): 423-441.
- Cengiz N, Uslu Y, Gök F, Anarat A (2005) Acute renal failure after overdose of colloidal bismuth subcitrate. Pediatr Nephrol 20(9):1355-1358.
- Erden A, Karahan S, Bulut K, Basak M, Aslan T, et al. (2013) A case of bismuth intoxication with irreversible renal damage. Int J Nephrol Renovasc Dis 6: 241-243.
- Ece A, Tünay Z (2018) Successful management of acute bismuth intoxication complicated with acute renal failure, seizures and acute pancreatitis in a child. J Clin Exp Invest (9): 131-134.
- Liu Y, Shen C, Zhang X, Yu H, Wang F, et al. (2018) Exposure and nephrotoxicity concern of bismuth with the occurrence of autophagy. Toxicol Ind Health 34(3): 188-199.
- Borbinha C, Serrazina F, Salavisa M, Viana-Baptista M (2019) Bismuth encephalopathy- a rare complication of long-standing use of bismuth subsalicylate. BMC Neurol 19(1): 212.
- Zaichick S, Zaichick V, Nosenko S, Moskvina I (2012) Mass fractions of 52 trace elements and zinc trace element content ratios in intact human prostates investigated by inductively coupled plasma mass spectrometry. Biol Trace Elem Res 149: 171-183.
- Zaichick V, Zaichick S (2014) Use of INAA and ICP-MS for the assessment of trace element mass fractions in adult and geriatric prostate. J Radioanal Nucl Chem 301: 383-397.
- Zaichick V (2015) The variation with age of 67 Macro- and microelement contents in nonhyperplastic prostate glands of adult and elderly males investigated by nuclear analytical and related methods. Biol Trace Elem Res 168: 44-60.
- Zaichick V, Zaichick S (2016) Age-related changes in concentration and histological distribution of 54 trace elements in nonhyperplastic prostate of adults. Int Arch Urol Complic 2(2): 019.
- Zaichick S, Zaichick V (2016) Prostatic tissue levels of 43 trace elements in patients with BPH. Br J Med Med Res 15(2): 1-12.
- Zaichick V, Zaichick S (2016) Prostatic tissue levels of 43 trace elements in patients with prostate adenocarcinoma. Cancer and Clinical Oncology 5(1): 79-94.
- Zaichick V, Zaichick S (2017) Chemical element contents in normal and benign hyperplastic prostate. Ann Mens Health Wellness 1(2): 1006.
- Zaichick V (2017) Differences between 66 chemical element contents in normal and cancerous prostate. J Anal Oncol 6: 37-56.
- Zaichick V, Zaichick S (2019) Comparison of 66 chemical element contents in normal and benign hyperplastic prostate. Asian J Urol 6, 275-289.
- Isaacs JT (1983) Prostatic structure and function in relation to the etiology of prostatic cancer. Prostate 4(4): 351-366.
- Leissner KM, Fielkegard B, Tisell LE (1980) Concentration and content of zinc in human prostate. Invest Urol 18: 32-35.
- Woodard HQ, White DR (1986) The composition of body tissues. Br J Radiol 59: 1209-1218.
- Arnold WN, Thrasher JB (2003) Selenium concentration in the prostate. Biol Trace Elem Res 91(3): 277-280.
- Schroeder HA, Nason AP, Tipton IH, Balassa JJ (1967) Essential trace metals in man: Zinc. Relation to environmental cadmium. Chron Dis 20: 179-210.
- Saltzman BE, Gross SB, Yeager DW, Meiners BG, Gartside PS (1990) Total body burdens and tissue concentrations of lead, cadmium, copper, zinc, and ash in 55 human cadavers. Environ Res 52: 126-145.
- Zaichick V (1997) Sampling, sample storage and preparation of biomaterials for INAA in clinical medicine, occupational and environmental health. In: Harmonization of Health-Related Environmental Measurements Using Nuclear and Isotopic Techniques. Vienna: IAEA. pp: 123-133.
- Zaichick V (2004) Losses of chemical elements in biological samples under the dry ashing process. Trace Elem Med (Moscow) 5(3): 17-22.
- Hamilton EI, Minski MJ, Cleary JJ (1972/1973) The concentration and distribution of some stable elements in healthy human tissues from the United Kingdom. Sci Total Environ 1: 341-374.
- Woolrich PF (1973) Occurrence of trace metals in the environment. An overview. Am Ind Hyg Assoc J 34(5): 217-226.
- Rose M, Baxter M, Brereton N, Baskaran C (2010) Dietary exposure to metals and other elements in the 2006 UK Total Diet Study and some trends over the last 30 years. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 27(10): 1380-1404.
- Fowler BA, Sexton MJ (2007) Bismuth. In: Fowler BA, Nordberg M, Friberg L, Nordberg G (Eds.), Handbook on the Toxicology of Metals, 3rd ed. USA: Academic Press. pp: 433-443.
- González-Weller D, Rubio C, Gutiérrez AJ, González GL, Mesa JMC, et al. (2013) Dietary intake of barium, bismuth, chromium, lithium, and strontium in a Spanish population (Canary Islands, Spain). Food Chem Toxicol 62: 856-858.
- Kilinc E (2015) Determination of trace Bi by ICP-OES after magnetic solid phase extraction with fullerene C60 modified γ-Fe2O3 superparamagnetic iron oxide nanoparticles. Anal Methods 7: 24.
- Johnson AL, Blaine ET, Lewis AD (2015) Renal pigmentation due to chronic bismuth administration in a rhesus macaque (Macaca mulatta). Vet Pathol 52(3): 576-579.
- Moskalev YuI (1985) Mineral metabolism. Moscow: Meditsina.
- Slikkerveer A, De Wolff FA (1989) Pharmacokinetics and toxicity of bismuth compounds. Med Toxicol Adv Drug Exp 4(5): 303-323.
- Vanhoe H, Versieck J, Vanballenberghe L, Dams R (1993) Bismuth in human serum: Reference interval and concentrations after intake of a therapeutic dose of colloidal bismuth subcitrate. Clin Chim Acta 219(1-2): 79-91.
- Poddalgoda D, Hays SM, Nong A (2020) Derivation of biomonitoring equivalents (BE values) for bismuth. Regul Toxicol Pharmacol 114: 104672.
- Vernadsky VI (1978) Living matter. Moscow: Nauka.
- Zaichick V, Ermidou-Pollet S, Pollet S (2007) Medical elementology: A new scientific discipline. Trace Elem Electroly 24(2): 69-74.
- Dolara P (2014) Occurrence, exposure, effects, recommended intake and possible dietary use of selected trace compounds (aluminium, bismuth, cobalt, gold, lithium, nickel, silver). Int J Food Sci Nutr 65(8): 911-924.
- Bartoli M, Jagdale P, Tagliaferro A (2020) A short review on biomedical applications of nanostructured bismuth oxide and related nanomaterials. Materials (Basel) 13(22): 5234.
- Wang R, Li H, Sun H (2011) Bismuth: Environmental Pollution and Health Effects. Encyclopedia of Environmental Health. Elsevier. pp: 414-420.
- Schramel P, Wendler I (1999) Antimony, lead, cadmium, platinum, mercury, tellurium, thallium, bismuth, tungsten, tin [Biomonitoring Methods, 1999]. In: Analyses of Hazardous Substances in Biological Materials 6: 80-109.
- Badrigilan S, Heydarpanahi F, Choupani J, Jaymand M, Samadian H, et al. (2020) A review on the biodistribution, pharmacokinetics and toxicity of bismuth-based nanomaterials. Int J Nanomed 15: 7079-7096.
- Fahey NSC, Karagatzides JD, Jayasinghe R, Tsuji LJS (2008) Wetland soil and vegetation bismuth content following experimental deposition of bismuth pellets. J Environ Monit 10(8): 951-954.
- Xiong Q, Zhao W, Guo X, Shu T, Chen F, et al. (2015) Dustfall heavy metal pollution during winter in North China. Bull Environ Contam Toxicol 95(4): 548-554.
- Klöckner P, Reemtsma T, Wagner S (2021) The diverse metal composition of plastic items and its implications. Sci Total Environ 764: 142870.
- Müller J, Ruppert H, Muramatsu Y, Schneider J (2000) Reservoir sediments - A witness of mining and industrial development (Malter Reservoir, eastern Erzgebirge, Germany). Environ Geol 39(12): 1341-1351.
- Michalke K, Schmidt A, Huber B, Meyer J, Sulkowski M, et al. (2008) Role of intestinal microbiota in transformation of bismuth and other metals and metalloids into volatile methyl and hydride derivatives in humans and mice. Appl Environ Microbiol 74(10): 3069-3075.
- USGS (U.S. Geological Survey) (2019) Mineral Commodity Summaries: Bismuth. Reston, VA, USA: USGS. pp: 34-35.



