Case Report
Galassi III Arachnoidcyst: Surgical Treatment: - Microscopy X Endoscopy
4240
Views & Citations3240
Likes & Shares
INTRODUCTION
Arachnoid cyst is a developmental anomaly, with duplication or split of the arachnoid membrane, or in rarer cases, caused by head trauma or hemorrhage of prematurity. Most are stable, showing no growth [1-12]. Considering the entire population, the arachnoid cyst may increase in approximately 2.5% of the cases [3-7], however, when we consider the pediatric population, this incidence may reach close to 10% of the cases and 1-1.5% of then become symptomatic. When this occurs, it is believed to be due to a cleft valve mechanism, osmosis or CSF production by the cyst wall [4]. They are commonly an incidental finding, being the majority asymptomatic (about 85%) and reserving surgical intervention only for cysts that cause symptoms, mass effect or hydrocephalus. It is not known for sure why the cysts increase, nor if it is possible to predict in which cysts this will occur. Main symptoms, when they occur, are: headache, macro crania, seizure, developmental delay. The incidence is about 2.5% in children. In this population, constant monitoring is necessary, as they have a higher growth rate, especially in those with a location susceptible to the development of hydrocephalus. In this study, we report two surgical cases that aimed at fenestration and communication with cisterns and arachnoid space to create or expand CSF communication and reduce intracranial hypertension in the Galassi III cyst. The shunt placement option in our service is reserved for cases of fenestration failure, due to the disadvantages of its use and lower long-term success rate [12].
CASE 1
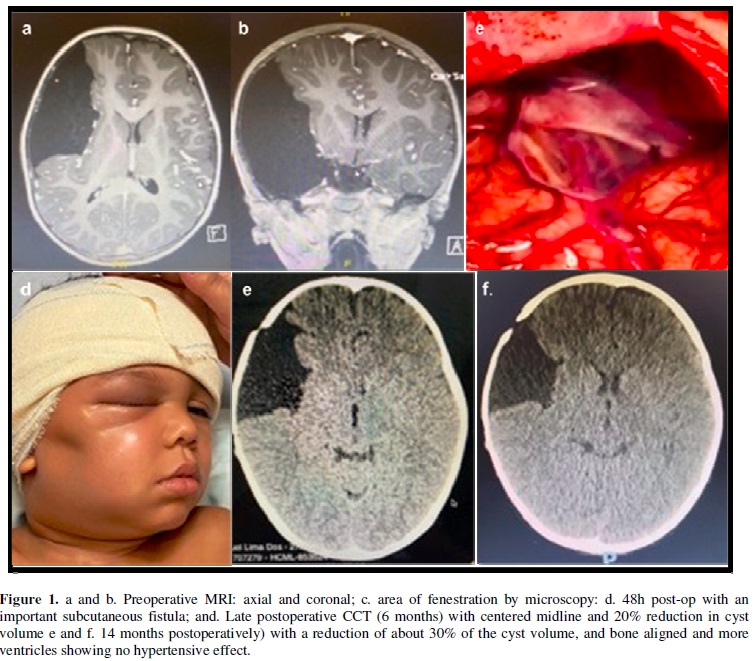
4-year-old schoolboy, monitored by a pediatrician regularly since birth. He presented bone bulging in the temporal region to the D, however never investigated. He had mild head trauma, evolving 48 hours later, with a progressive decline in his general condition, headache and drowsiness. He was taken to the emergency room, where a cranial CT was performed, which showed a large middle fossa and Galassi III toroidal cyst with signs of bleeding and midplane deviation. We opted for urgent surgery, with cyst fenestration by microscopy and communication of base cisterns.
Procedure performed uneventfully and immediately postoperatively with complete resolution of symptoms. However, after 36 hours, the child developed signs of an important subcutaneous fistula and initial signs of intracranial hypertension (headache, nausea and drowsiness). A lumbar puncture was performed and continuous CSF drainage was placed for 48 hours (liquogard) with complete resolution of the fistula without recurrence and neurological improvement. Boy evolved very well, being discharged from hospital at the 6th. postoperative day. Outpatient follow-up and 2-year follow-up, demonstrated improvement in school performance and excellent cognitive and psychological development. Current images show about 40% reduction in cyst volume. Figure 1.
CASE 2
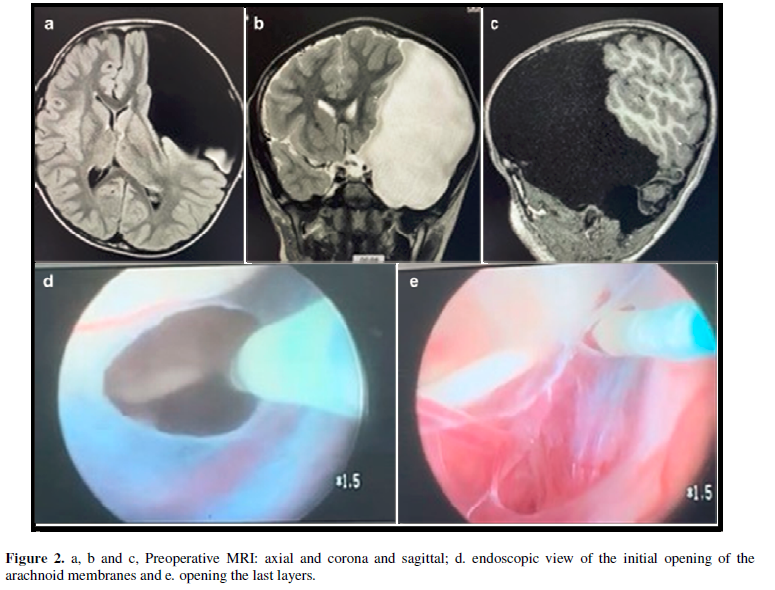
A 5-year-old student, also regularly monitored by more than one pediatrician since birth. He presented an important bone bulging in the E fronto temporo parietal region and macro crania, percentile above 95%, also never investigated. He presented a picture of progressive visual difficulty, and complaint of diplopia. He was taken to the hospital unit where MRI of the brain with and without contrast was performed, which showed a gigantic middle fossa cyst, occupying the entire temporal fossa, and compression and displacement of the temporal, parietal and occipital lobes to the left and addition, we verified a midline deviation of about 1 cm, associated to brainstem deviation and bone deformity with migraine. Admitted on GCS 15, with paresis of the III and IV nerves on left, in addition to nausea.
Due to the limitations of the unit, which does not have a surgical microscope available, we opted for endoscopic fenestration of the cyst. Surgery performed uneventfully and gradually improving the clinical and neurological status. There was no CSF fistula and the patient progressed satisfactorily in the first 30 days. However, after this period, he complained again of nausea and vomiting, in addition to bulging of the surgical wound, indicating a small subcutaneous fistula. Observed for 48 hours in order to confirm a suggestive picture of occlusion of endoscopic fenestration. A peritoneal cyst drainage was then performed, using a self-regulating flow shunt. It evolved with immediate improvement of symptoms and remains so to this day, with a 2-month follow-up. Figure 2.

DISCUSSION
Fenestration of the cyst involves craniotomy and then use of microscopy or endoscopy. In the first one, a small circular craniotomy is chosen, with opening of the dura mater and a greater possibility of access such as arachnoid layers and control of adjacent structures [3,8 and 13]. Endoscopic approach is less invasive but requires more surgical experience and fenestration is limited. Complications caused by both types of surgery include hemorrhage, dural rupture after surgery, and subdural hygroma or hematoma and CSF leak. In both options, surgery is not 100% effective, because cyst recurrence is another possible complication [9]. Endoscopic decompression allows initial decompression in the normal CSF pathways in cisterns, while craniotomy involves immediate decompression of the superficial cortical portion, before establishing the CSF flow. Therefore, cysts amenable to an endoscopic approach with normal cisternal tract communication may be better treated endoscopically [1].

CONCLUSION
The predominant surgical intervention techniques are microsurgical cyst excision, endoscopic fenestration, and peritoneal cyst shunt placement. The goal of all techniques is to relieve the cyst-induced pressure on the specific structure and/or eliminate the obstruction of cerebrospinal fluid (CSF) flow caused by the cyst. All three techniques have advantages and disadvantages, and the right choice is not always easy. Literature does not present clear evidence of which is the most effective. In symptomatic cases, the choice of method depends on the location and classification of the cyst, as well as the experience and technical availability of the neurosurgery service. The suprasellar and quadrigeminal regions are most amenable to neuroendoscopy. Interhemispheric cysts should be treated by microsurgical fenestration, in most of cases [11-13].
- Di Rocco F, James RS, Roujeau T, Puget S, Sainte-Rose C, et al. (2010) Limits of endoscopic treatment of sylvian arachnoid cysts in children. Childs Nerv Syst 26: 155-162.
- Ali ZS, Lang SS, Bakar D, Storm PB, Stein SC (2014) Pediatric intracranial arachnoid cysts: Comparative effectiveness of surgical treatment options. Childs Nerv Syst 30:461-464.
- Madeline JH, Stephanie CT, Russell CT, James AK (2018) A Review on the Effectiveness of Surgical Intervention for Symptomatic Intracranial Arachnoid Cysts in Adults. World Neurosurg 123: e259-e272.
- Sameer HH, Safain MG, Heilman CB (2013) Arachnoid cyst slit valves: The mechanism for arachnoid cyst enlargement. J Neurosurg Pediatric 12: 62-66.
- Marquez IB, Barbosa JV (2014) Arachnoid Cyst Spontaneous Rupture. Med Port 27(1): 137-141.
- Kai L, Kong DS, Zhang J, Wang SX, Xun Y, et al. (2019) Association between ELP4 rs986527 polymorphism and the occurrence and development of intracranial arachnoid cyst. Brain Behav 9: e01480.
- Zhen T, Yongxin L, Fengjun Z, Dongdong Z, Cailei Z, et al. (2015) Children with Intracranial Arachnoid Cysts - Classification and Treatment. Med J 94(44): e1749.
- Nimer A, Aman D, Christoph JG, Mortazavi MM, Watanabe K, Loukas M, et al. (2013) The intracranial arachnoid mater: A comprehensive review of its history, anatomy, imaging, and pathology. Childs Nerv Syst 29: 17-33.
- Katrin R, Högfeldt MJ, Roberto DM, Magnus T (2016) Surgery for intracranial arachnoid cysts in children-A prospective long-term study. Childs Nerv Syst 32(7): 1257-1263.
- Katrin R, Daniel J, Thomas M, Christer J, Ingmar S, et al. (2016) Prevalence and symptoms of intracranial arachnoid cysts: a population-based study. J Neurol 263(4): 689-694.
- Subodh R, Renuka S, Sharma, Srilata M, Jaleel M (2014) Neuroendoscopy for Intracranial Arachnoid Cysts in Infants: Therapeutic Considerations. J Neurol Surg 77(4): 333-343.
- Samuel H, Alex S, Owen S, Nijuguna M, Ryan W, et al. (2019) Natural History of Intracranial Arachnoid Cysts. World Neurosurg 126: e1315-e1320.
- Fatima M, Sanaullah B, Aneela D (2018) Management of Arachnoid Cysts: A Comprehensive Review of Arachnoid Cysts. Cures 10(4): e2458.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- Journal of Pathology and Toxicology Research
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Advance Research on Alzheimers and Parkinsons Disease
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)




