Review Article
Correlation between Angiographic Parenchymal Heterogeneity and Cerebral Infarction Following Aneurysmal Subarachnoid Hemorrhage
3704
Views & Citations2704
Likes & Shares
Background: Aneurysmal subarachnoid hemorrhage (aSAH) is highly lethal due to development of delayed cerebral ischemia resulting from cerebral vasospasm. Our study therefore aims to determine the relationship between area of parenchymal heterogeneity on angiography, proposed to represent vasospasm, and delayed cerebral infarction.
Methods: All patients who were diagnosed with ruptured aneurysmal subarachnoid hemorrhage by cerebral angiography in Songklanagarind hospital from January 2016 to December 2018 WERe enrolled. Patient demographic data and correlation between angiographic parenchymal heterogeneity by digital subtraction angiography and cerebral infarction by follow-up non-contrast computed tomography were analyzed using descriptive statistics.
Results: Amongst 35 patients with 147 areas of angiographic parenchymal heterogeneity, 16 (10.9%) areas positively matched with presence of delayed cerebral infarction. Areas of angiographic parenchymal heterogeneity were mostly present in frontal high (20.4%), frontal low (17.0%), and upper anterior MCA cortex (12.9%). There was no statistically significant relationship between angiographic parenchymal heterogeneity and area of delayed infarct observed (p<0.17). Areas with a positive matched with angiographic parenchymal heterogeneity were frontal low (31.3%), frontal high (25.0%), frontal parasagittal (12.5%), and lower mid MCA cortex (12.5%). 131 areas showed angiographic parenchymal heterogeneity without corresponding cerebral infarction in the same area upon follow-up, and 71 areas of cerebral infarction showed no prior angiographic parenchymal heterogeneity. Only one area showed high positive correlation; angiographic parenchymal heterogeneity in left frontal parasagittal and cerebral infarct in left lower anterior MCA cortex.
Conclusions: There was no significant relationship between angiographic parenchymal heterogeneity and the development of cerebral infarction.
Keywords: Aneurysmal subarachnoid hemorrhage, Vasospasm, Cerebral infarction, Delayed cerebral ischemia, Digital subtraction angiography
ABBREVIATIONS
aSAH: Aneurysmal subarachnoid hemorrhage; DCI: Delayed cerebral ischemia; DSA: Digital subtraction angiography; HIS: Hospital Medical Information System; CT: Computerized tomography; PACS: Picture Archiving and Communication System; ASPECTS: Alberta Stroke Program Early CT Score GCS: Glassgow Coma Scale; ICA: Internal carotid artery; ACA: Anterior cerebral artery; MCA: Middle cerebral artery; AcoA: Anterior communicating artery; PcoA: Posterior communicating artery; VA: Vertebral artery; PICA: Posterior inferior cerebellar artery; AICA: Anterior inferior cerebellar artery; CTP: Computerized tomography perfusion; MRP: Magnetic resonance perfusion
INTRODUCTION
Aneurysmal subarachnoid hemorrhage (aSAH) is a highly lethal neurological disease. In patients who survive the initial bleeding incident of a ruptured intracranial aneurysm, a crucial complication leading to poor outcome is delayed cerebral ischemia (DCI) defined as neurological deterioration presumed to be secondary to cerebral ischemia or the development of a new infarction [1-4]. However, the pathogenesis of DCI continues to be inadequately understood.
A proposed pathogenesis for the development of DCI is angiographic vasospasm which can occur in up to 70% of patients. Nevertheless, the correspondence between angiographic vasospasm and the clinical symptoms can be inconsistent [5,6]. As a result, most clinical trials in the last few decades aimed to predict clinical outcomes by determining the relationship between delayed parenchymal infarction and angiographic vasospasm. Some have found that the severity of vasospasm based on the degree of arterial narrowing is strongly correlated with regional cerebral infarction, while another study revealed no significant correlation between angiographic vasospasm and regional hypoperfusion in aSAH by means of cerebral blood flow [7,8]. In the presence of vasospasm, delayed blood flow to the brain parenchyma is expected which should result in parenchymal heterogeneity on imaging. We therefore infer that parenchymal heterogeneity may be able to predict cerebral infarction, although such correlation had never been proven thus far.
The purpose of this study is to determine prognostic relationship between delayed cerebral ischemia associated with angiographic vasospasm and parenchymal infarction following aSAH. We aimed to identify the association between angiographic parenchymal heterogeneity in parenchymal phase of DSA and brain infarction visualized in follow-up imaging.
MATERIALS & METHODS
Study design and source of data
This is a single-institution retrospective descriptive study conducted in Songklanagarind Hospital, Songkhla, Thailand. Patients with ruptured aneurysmal subarachnoid hemorrhage diagnosed via cerebral angiography between January 1, 2016 and December 31, 2018 WEre retrieved from the database in the computerized Hospital Medical Information System (HIS). The diagnosis and classification of ruptured aneurysmal subarachnoid hemorrhage were based on clinical evaluation by qualified neurosurgeons and confirmed with a computerized tomography (CT) scan and cerebral angiography.
All patients received enteral nimodipine. Euvolemia was maintained by daily adjustment of intravenous fluid infusion to keep fluid input and output balanced.
Prophylactic hypervolemia was employed in cases with neurological deterioration. Neurological deterioration was promptly evaluated and if no other cause was identified, patients underwent cerebral angiography and hemodynamic augmentation with vasopressors. The attending neurointerventionist and neurosurgeon jointly determined if endovascular interventions should be performed.
Patient data were collected in a case record form by the author. Date of birth and sex were obtained from the national identification card available in HIS. Age was determined based on date of first cerebral angiogram after the diagnosis of aneurysmal subarachnoid hemorrhage has been made. Initial systolic and diastolic blood pressure were obtained from HIS. Hunt and Hess classification was determined by clinical correlation. Date of cerebral angiogram and follow-up CT scans were recorded in the hospital’s Picture Archiving and Communication System (PACS).
Patients who did not undergo follow-up non-contrast CT scan after initial cerebral angiogram between one and eight weeks were excluded. CT images with regions containing hematoma, infarctions due to angiography or treatment complications (such as clipping complications from accidental clipping of other vessels or intentional clipping of non-target vessels to stop intraoperative hemorrhage; coiling complications including thrombosis, hemorrhage, or aneurysmal rupture during or after the procedure), previous infarctions were also excluded.
Definitions of terms
The brain parenchyma have been divided into 31 areas adapted from Alberta Stroke Program Early CT Score (ASPECTS) [9] and included other areas to include whole brain parenchyma as follows; bilateral frontal low, bilateral frontal high, bilateral frontal parasagittal, bilateral upper anterior MCA cortex (adjacent to the most superior margin of the ganglionic structures), bilateral upper mid MCA cortex (adjacent to the most superior margin of the ganglionic structures), bilateral upper posterior MCA cortex (adjacent to the most superior margin of the ganglionic structures), bilateral lower anterior MCA cortex (at the level of the thalamus and basal ganglion), bilateral lower mid MCA cortex (at the level of the thalamus and basal ganglion), bilateral lower posterior MCA cortex (at the level of the thalamus and basal ganglion), bilateral insular lobes, bilateral lentiform nucleus, bilateral caudate nucleus, bilateral internal capsules, bilateral occipital poles, bilateral occipital lobes, and posterior circulation.
The presence of parenchymal heterogeneity on cerebral angiogram, defined as areas of decreased parenchymal blood flow compared to the area of normal parenchymal flow, were assigned to 31 areas of the brain. The severity of parenchymal heterogeneity was further categorized as being severe or non-severe, where severe parenchymal heterogeneity is defined as persistent delayed blood flow for more than 2 Seconds [10].
Areas of cerebral infarction or parenchymal hypoattenuation was defined as a region of abnormally decreased attenuation of brain structures relative to attenuation of other parts of the same structures or of the contralateral hemisphere in non-contrast CT scan, assigned to 31 areas of the brain in the same fashion.
Matched angiographic parenchymal heterogeneity was defined as angiographic parenchymal heterogeneity and hypoattenuation on the follow-up non-contrast CT scans occurring in the same area of the brain.
Image analysis
31 areas of parenchymal heterogeneity were manually outlined on digital subtraction angiography (DSA) using a catherization system(bi-plane) operated by Philips Allura Xper FD 20 (9890000-85102, No.24633 m 15330).
All patients underwent non-contrast head CT imaging performed using a 64-multislice CT scanner operated by Philips Brilliance (No.10146) and a 160-slice CT scanner operated by Toshiba Aquilion prime (No.BKA 1522134). CT parameters were calculated based on body weight, ranging between 80 kVp and 120 kVp. Themilliampaere(mA) was selected based on body weight and using a dose-modulated technique in Philips and Toshiba scanner. The slice thickness was 3 mm, with a 2 mm thickness interval, and the reconstruction into the sagittal and coronal view also had a slice thickness of 3 mm and a 2 mm thickness interval. 31 areas of parenchymal infarction were manually outlined on baseline and follow-up non-contrast CT images while blinded to the knowledge of the location of aneurysm and area of prior vasospasm.
Data collection
One neurointerventionist, blinded to the patient’s clinical details, reviewed the images of the cerebral angiogram for the anatomical distribution of angiographic parenchymal heterogeneity and finally the follow-up CT scan for the anatomical distribution of cerebral infarction. Angiographic parenchymal heterogeneity was defined as area of decreased parenchymal blood flow on parenchymal phase digital subtraction angiography not attributable to atherosclerosis, catheter-induced vasospasm, or vessel hypoplasia as determined by the neurointerventionist. Hypodensities seen only on the follow-up CT scan and not otherwise explained (e.g., ventriculostomy tract, hematoma reabsorption, or surgical intervention) were considered infarctions from DCI and termed “delayed infarctions”. Images were read in a darkened room, and were studied from near and far and even obliquely.
Baseline non-contrast CT scan was used to determine the area of previous infarction or gliosis which were then excluded from this study.
The database and patient charts were retrospectively reviewed to obtain clinical data including history of vascular risk factors, admission Glasgow Coma Scale (GCS) scores, Hunt and Hess scores and Modified Fisher grading scale. We also obtained information regarding aneurysm location and treatment (i.e., clipping or coiling).
STATISTICAL ANALYSIS
Sample size calculation
The sample size was calculated based on an infinite population proportion.
= 95% confidence level = 1.96
P = 0.42
D = Allowable error (10%) = 0.1
n = 94
Statistical methods
The statistical analysis was performed using the R software 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) with “epicalc” and “pheatmap” packages.
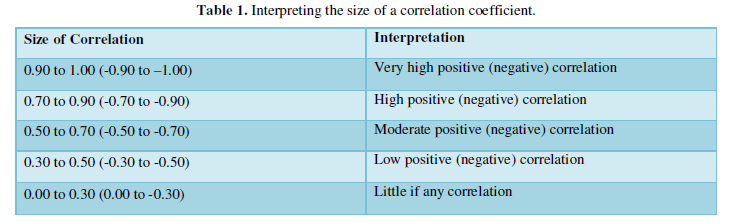
Baseline characteristics descriptive data was analyzed as frequency, percent, mean ± standard deviation (SD). Relationship between data was analyzed using Chi-squared test and Pearson’s product-moment correlation and was illustrated with heatmap correlation. The levels of correlation were interpreted based on the following ranges [11] (Table 1).

ETHICAL CONSIDERATIONS
This study was approved by our Human Research Ethics Committees (HRECs). Confidentiality of patient data was achieved by using coded number to substitute for patient name or hospital number.
RESULTS
Between January 1, 2016 and December 31, 2018, there were 205 cases of aneurysmal subarachnoid hemorrhage diagnosed by cerebral angiogram meeting the inclusion criteria. After excluding patients without follow-up CT scans in the targeted duration, 89 patients were included for review of cerebral angiogram. Only 36 patients showed angiographic parenchymal heterogeneity. After excluding one patient due to presence of treatment complication, 35 patients were included in the analysis.
Patient characteristics are shown in Table 1. Most patient were females (68.6%), aged 54.3±16.3 years, 19 (54.3%) patients were hypertensive with initial SBP 147.6±21.3 mmHg and DBP 85.8±13.3 mmHg. Hunt and Hess grade of 2 were found in 34.3% of the patients, and modified Fisher grade 4 were assigned in 60.0% of patients.
Patient characteristics are shown in Table 1. Most patient were females (68.6%), aged 54.3±16.3 years, 19 (54.3%) patients were hypertensive with initial SBP 147.6±21.3 mmHg and DBP 85.8±13.3 mmHg. Hunt and Hess grade of 2 were found in 34.3% of the patients, and modified Fisher grade 4 were assigned in 60.0% of patients.
Angiography showed that the most common aneurysmal rupture sites were the anterior communicating (ACoA) 40.0%, posterior communicating (PCoA) 28.6%, and internal carotid (ICA) 17.1%, while the incidence of other aneurysms in MCA, ACA, VA, PICA, and AICA were about 2.9% each.
60.0% of patients received coiling as treatment, while the rest underwent aneurysm clipping. The median duration between angiogram and follow-up CT were about 12 days (IQR 9-15).
By means of parenchymal location, each patient had 31 areas with a total of 1085 areas in 35 patients. 147 areas showed angiographic parenchymal heterogeneity. All patients demonstrated non-severe angiographic parenchymal heterogeneity.
Most areas of angiographic parenchymal heterogeneity were present in frontal high (20.4%), frontal low (17.0%), and upper anterior MCA cortex (12.9%), as shown in Table 3. No angiographic parenchymal heterogeneity was observed in insular, lentiform, caudate, internal capsule, and occipital pole area.
Positive matched between angiographic parenchymal heterogeneity and infarction were observed in 16 areas (10.9%) from 147 angiographic parenchymal heterogeneity findings. The positive matched areas were in frontal low (31.3%), frontal high (25.0%), frontal parasagittal (12.5%), and lower mid MCA cortex (12.5%) areas, as shown in Table 4. 131 areas showed angiographic parenchymal heterogeneity without corresponding cerebral infarction in the follow-up CT-scan, mainly involving the frontal high, frontal low, and upper anterior MCA cortex, about 19.8%, 15.3%, and 13.7%, respectively.
There was no statistically significant relationship between angiographic parenchymal heterogeneity and area of delayed infarction observed (p<0.17).
71 areas of cerebral infarct without prior angiographic parenchymal heterogeneity were found in frontal low, frontal parasagittal, and lower posterior MCA cortex, about 14.0%, 11.3%, and 9.9%, respectively.
Heatmap correlation showed no predictable pattern of angiographic parenchymal heterogeneity and cerebral infarct. Only one pair with high positive correlation was observed between angiographic parenchymal heterogeneity in the left frontal parasagittal area and cerebral infarct in left lower anterior MCA cortex (r=0.7, r range 95%CI=0.47-0.84, p<0.05). Other 52 pairs of low positive correlation (r 0.3-0.5) were observed but there was no overall predictable pattern observed.
DISCUSSION
It has long been known that an important consequence of aneurysmal subarachnoid hemorrhage is delayed cerebral ischemia. One of the proposed causes of delayed cerebral ischemia is vasospasm where subarachnoid hemorrhage may cause increased parenchymal arteriole constriction at physiological intravascular pressures, resulting in reduced blood flow [12,13]. Therefore, angiographic parenchymal heterogeneity, proposed to represent vasospasm, should be able to identify areas with lower cerebral blood flow prone to have further infarction.
In our study, we found that matched angiographic parenchymal heterogeneity, i.e., angiographic parenchymal heterogeneity and hypoattenuation in follow-up non-contrast CT scan within the same area, were positive in 16 areas out of 147 areas of angiographic parenchymal heterogeneity which was not statistically significant(p<0.17). Only one positive matched area showed high positive correlation, while 52 positive matched areas showed low positive correlation, but there was no overall predictable pattern observed. The majority of positive matched areas were frontal low, frontal high, and frontal parasagittal, which may result from the majority of ruptured aneurysm in ACoA, consistent with previous study which stated that frontal lobe infarction is associated with ruptured ACoA aneurysm [14]. Contrary to another study, the area of cerebral infarction may or may not related to the site of aneurysm rupture [15]. Posterior circulation aneurysms including PCoA, VA, AICA, and PICA were accounted for up to 40.0% of our patients, but we observed only one positive match between parenchymal heterogeneity and delayed posterior circulation infarct. This may be due to high tolerance of neurons in the posterior circulation against ischemia, resulting in fewer incidence of posterior circulation infarct, combined with low sensitivity of CT scans in detection of posterior circulation infarction [16]. For deep grey matter lesions, no angiographic parenchymal heterogeneity was observed. This may be due to the region being obscured by other areas of the brain, resulting in under detection of parenchymal heterogeneity in such areas. In addition, subsequent infarction in the deep grey matter may occur as a consequence of ACA pathologies and not from vasospasm localized in the deep grey matter itself.
Previous literature demonstrated that the mean clot thickness of aSAH correlates with subsequent vasospasm and resulting in cerebral infarction [17]. More than half of our patients (60.0%) showed modified Fisher grade 4, which is denoted by thick clots with intraventricular hemorrhage, yet the subsequent cerebral infarction observed in our study is less than expected compared with those observed in previous literature. Moreover, subarachnoid blood has been shown to cause vasospasm which may in turn affect the areas of angiographic parenchymal heterogeneity, but the extent and location of subarachnoid hemorrhage was not evaluated in this study [18].
The occurrence of angiographic vasospasm peaks between days 7 and 14 after aSAH [19]. The mean time between aSAH and cerebral angiogram in our patients was about four days which was likely too early in order to detect vasospasm. Prompt cerebral angiography and coiling for early diagnosis and treatment was employed at our institution, which could result in under detection of vasospasm due to inappropriate timing of angiogram acquisition for visualization of vasospasm.
We conducted this study using follow-up non-contrast CT scans because they are widely available, less time-consuming, and easier to perform in intensive care patients compared to other imaging modalities. Previous studies have suggested that the best time to acquire a follow-up imaging study in order to detect cerebral infarction is within six weeks after SAH, which is when DCI most likely would have occurred while brain edema from surgical or endovascular procedures would begin to resolve [20]. However, in our study we recruited patients with follow-up imaging between one and eight weeks from the date of the initial cerebral angiogram to maximize the population sample size in this study. In addition, the timing of follow-up CT scans is decided by our institution’s neurosurgeon based on the patient’s clinical condition which results in many follow-up scans being acquired outside the optimal timing. Median interval time between initial cerebral angiogram and follow-up CT scan was about two weeks which is within the appropriate timeframe of less than six weeks after initial SAH.
Many medications are used in prevention and treatment of vasospasm, however previous review of literature found that nimodipine was the only treatment that provided a significant benefit compared to other medications, such as fasudil, a calcium channel blocker, and magnesium, an endothelin receptor antagonist [21]. Oral administration of 60 mg nimodipine every 4 h over a period of 21 consecutive days is recommended by the current guidelines of the American Stroke Association [22]. In our study almost all patient received enteral nimodipine before their scheduled cerebral angiogram. Only one patient received nimodipine two days after cerebral angiogram was performed. Adequate administration of enteral nimodipine in our study population may contribute towards lowered incidence of vasospasm, and therefore fewer incidence of parenchymal heterogeneity in our study.
While previous angiographic assessment of vasospasm relies on the comparison of the size of cerebral arteries to an internal standard, we instead opted for the use of degree of parenchymal heterogeneity, regardless of degree of luminal narrowing. As patients with aneurysmal subarachnoid hemorrhage frequently undergo cerebral angiogram as part of their treatment using endovascular coiling, being able to detect vasospasm from cerebral angiogram will not only promptly guide management for clinicians but also predict disease prognosis for patients and their relatives. However, this method still needs further validation in terms of its usefulness in prediction of vasospasm. Other imaging modalities capable of detecting vasospasm are also available, including CT perfusion (CTP) and MR perfusion (MRP), where some studies have even advocated the use of early whole brain CTP in order to identify tissue at risk for DCI [23]. Compared to cerebral angiogram, perfusion imaging has been shown to be far more accurate in predicting the area of DCI [24]. Nevertheless, the ability to provide concurrent endovascular therapy for aSAH still makes cerebral angiogram a widely popular modality despite its questionable usefulness in prediction of cerebral infarction. Some articles have demonstrated that cerebral infarction can result from a combination of various causes other than vasospasm. Previous studies have shown that delayed infarcts following aSAH can occur in territories without angiographic vasospasm and may be caused by an interplay between a multitude of various etiologies. Other mechanisms that may contribute to the development of DCI include cerebral vascular dysfunction, micro thrombosis, cortical spreading depolarizations, and neuroinflammation [25]. This could be the reason why there was no obvious pattern of correlation between areas of angiographic parenchymal heterogeneity and areas of delayed infarction observed in our study. Our results show that the presence of angiographic parenchymal heterogeneity, potentially an indicator of vasospasm, is not very helpful in predicting DCI. This supports the findings from other investigators who also demonstrated that the positive predictive value of vasospasm for DCI is only 67% [26]. Vasospasm is likely not the sole cause of cerebral infarct, and using angiographic vasospasm as an indicator for cerebral infarct may cause many missed areas of DCI in patients with SAH.
There are several limitations in our study. Firstly, this is a retrospective study in a single institution, and therefore may be limited in terms of external validity of our results. Secondly, only one reviewer participated in the review of imaging studies, therefore interobserver variability was not addressed. Thirdly, all cases of angiographic parenchymal heterogeneity in this study were all non-severe type; therefore, the possibility of association between severe parenchymal heterogeneity and infarction may not be excluded. Severe angiographic parenchymal heterogeneity might be able to demonstrate a relationship with cerebral infarction, continuous record the data including only severe angiographic parenchymal heterogeneity might show the positive correlation. In addition, diagnostic accuracy of angiographic parenchymal heterogeneity in detecting vasospasm is still questionable, and further studies in order to validate its diagnostic value should be performed.
This study also did not take the patient’s clinical symptoms correlated with the area of cerebral infarction into account. Therefore, association with area of cerebral infarct, if any, may not translate directly to changes in plan of treatment and clinical outcome. Finally, if we can find the predictive findings from angiographic parenchymal heterogeneity which affect the area at risk of infarction and clinical outcomes, appropriate therapy and medical intervention should be performed promptly, such as balloon angioplasty, intra-arterial infusion of vasodilators, or aggressive triple H therapy, to help prevent further cerebral infarction.
CONCLUSION
In conclusion, our study found no significant relationship between angiographic parenchymal heterogeneity and delayed cerebral ischemia. This might be because angiographic parenchymal heterogeneity is unable to accurately represent cerebral vasospasm. A multitude of various other factors, not only vasospasm, may also contribute to development of delayed cerebral ischemia.
- Fisher CM, Kistler JP, Davis JM (1980) Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 6(1): 1-9.
- Dorsch NW, King MT (1994) A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage Part I: Incidence and effects. J Clin Neurosci 1(1): 19-26.
- Fisher CM, Roberson GH, Ojemann RG (1977) Cerebral vasospasm with ruptured saccular aneurysm-The clinical manifestations. Neurosurgery 1(3): 245-248.
- Saito I, Shigeno T, Aritake K, Tanishima T, Sano K (1979) Vasospasm assessed by angiography and computerized tomography. J Neurosurg 51(4): 466-475.
- Saito I, Ueda Y, Sano K (1977) Significance of vasospasm in the treatment of ruptured intracranial aneurysms. J Neurosurg 47(3): 412-429.
- Mahmoud AM (2015) Postaneurysmal subarachnoid hemorrhage vasospasm: A review of the incidence of radiographic and clinical vasospasm. Egypt J Neurol Psychiatr Neurosurg 52(3): 172-175.
- Siironen J, Porras M, Varis J, Poussa K, Hernesniemi J, et al. (2007) Early ischemic lesion on computed tomography: predictor of poor outcome among survivors of aneurysmal subarachnoid hemorrhage. J Neurosurg 107: 1074-1079.
- Brown RJ, Kumar A, Dhar R, Sampson TR, Diringer MN (2013) The relationship between delayed infarcts and angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery 72(5): 702-708.
- Mokin M, Primiani CT, Siddiqui AH, Turk AS (2017) ASPECTS (Alberta Stroke Program Early CT Score) measurement using hounsfield unit values when selecting patients for stroke thrombectomy. Stroke 48(6): 1574-1579.
- Dhar R, Scalfani MT, Blackburn S, Zazulia AR, Videen T, et al. (2012) Relationship between angiographic vasospasm and regional hypoperfusion in aneurysmal subarachnoid hemorrhage. Stroke 43: 1788-1794.
- Mukaka MM (2012) Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J 24(3): 69-71.
- Crowley RW, Medel R, Dumont AS, Ilodigwe D, Kassell NF, et al. (2011) Angiographic vasospasm is strongly correlated with cerebral infarction after subarachnoid hemorrhage. Stroke 42(4): 919-923.
- Wellman GC, Koide M (2013) Impact of subarachnoid hemorrhage on parenchymal arteriolar function. Acta Neurochir Suppl 115: 173-177.
- Heit JJ, Ball RL, Telischak NA, Do HM, Dodd RL, et al. (2017) Patient outcomes and cerebral infarction after ruptured anterior communicating artery aneurysm treatment. Am J Neuroradiol 38(11): 2119-2125.
- Rabinstein AA, Weigand S, Atkinson JLD, Wijdicks EFM (2005) Patterns of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke 36(5): 992-997.
- Hwang DY, Silva GS, Furie KL, Greer DM (2012) Comparative sensitivity of computed tomography vs. magnetic resonance imaging for detecting acute posterior fossa infarct. J Emerg Med 42(5): 559-565.
- Abla AA, Wilson DA, Williamson RW, Nakaji P, McDougall CG, et al. (2014) The relationship between ruptured aneurysm location, subarachnoid hemorrhage clot thickness and incidence of radiographic or symptomatic vasospasm in patients enrolled in a prospective randomized controlled trial: Clinical article. J Neurosurg 120(2): 391-397.
- Kistler JP, Crowell RM, Davis KR, Heros R, Ojemann RG, et al. (1983) The relation of cerebral vasospasm to the extent and location of subarachnoid blood visualized by CT scan: A prospective study. Neurology 33(4): 424-436.
- Weidauer S, Lanfermann H, Raabe A, Zanella F, Seifert V, et al. (2007) Impairment of cerebral perfusion and infarct patterns attributable to vasospasm after aneurysmal subarachnoid hemorrhage: A prospective MRI and DSA study. Stroke 38(6): 1831-1836.
- Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, et al. (2010) Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke 41(10): 2391-2395.
- Velat GJ, Kimball MM, Mocco JD, Hoh BL (2011) Vasospasm after aneurysmal subarachnoid hemorrhage: Review of randomized controlled trials and meta-analyses in the literature. World Neurosurg 76(5): 446-454.
- Rabinstein AA, Lanzino G, Wijdicks EF (2010) Multidisciplinary management and emerging therapeutic strategies in aneurysmal subarachnoid haemorrhage. Lancet Neurol 9(5): 504-519.
- Malinova V, Dolatowski K, Schramm P, Moerer O, Rohde V, et al. (2016) Early whole-brain CT perfusion for detection of patients at risk for delayed cerebral ischemia after subarachnoid hemorrhage. J Neurosurg 125(1): 128-136.
- Dankbaar JW, de Rooij NK, Velthuis BK, Frijns CJM, Rinkel GJE, et al. (2009) Diagnosing delayed cerebral ischemia with different CT modalities in patients with subarachnoid hemorrhage with clinical deterioration. Stroke 40(11): 3493-3498.
- Geraghty JR, Testai FD (2017) Delayed cerebral ischemia after subarachnoid hemorrhage: Beyond vasospasm and towards a multifactorial pathophysiology. Curr Atheroscler Rep 19(12): 50.
- Rabinstein AA, Friedman JA, Weigand SD, McClelland RL, Fulgham JR, et al. (2004) Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke 35(8): 1862-1866.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Pathology and Toxicology Research
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)


