1018
Views & Citations18
Likes & Shares
Eisenmenger syndrome (ES) is one of the leading causes of perioperative death (up to 19%) in patients undergoing non-cardiac surgery. The decreased systemic vascular resistance associated with either general anesthesia or neuraxial blockade increases the degree of right to left shunting, thereby carrying substantial risk to the patient. Here, we present anesthetic management of two non-cardiac cases with Eisenmenger syndrome. Both the patients were successfully anesthetized with uneventful post-operative period and discharged with stable hemodynamic condition.
Keywords: Eisenmenger syndrome, Non- Cardiac Surgery, Anesthesia
INTRODUCTION
Congenital heart disease (CHD) accounts to 28% of all congenital anomalies [1]. Eight percent of patients with CHD and eleven percent of those with left to right shunts develop the Eisenmenger syndrome [2,3]. Eisenmenger syndrome refers to any untreated congenital cardiac defect with intra cardiac communication that leads to pulmonary hypertension, reversal of flow and cyanosis. Most Eisenmenger patients die from sudden cardiac death, Congestive heart failure, hemoptysis, cerebral abscesses, and thromboembolic events, from complications during pregnancy or due to non-cardiac surgery [4].
CASE PRESENTATION
Case 1
An 82-year-old female weighing 43kg with right sided inter trochanteric fracture femur was posted for Proximal Femur nailing (PFN). She was diagnosed to have Atrial Septal Defect (ASD) at the age of 60 years and was on following medications digoxin, carvedilol, nifedipine, diuretics and anti platelet at the time of surgery. Pre-operatively, apart from exertional dyspnea (grade II), she had no other symptoms. On examination, she had grade II clubbing, pulse rate of 64/min, blood pressure of 140/90 mmHg, oxygen saturation of 96% on room air with no rise in jugular venous pressure. Lungs clear on auscultation and ECG showed sinus rhythm. 2D ECHO and color doppler showed large ostium seconded ASD (26-28mm), dilated right atrium, right ventricle and pulmonary artery, bidirectional shunt with predominant left to right, severe tricuspid regurgitation with pulmonary artery systolic pressure (PASP) by TR jet 117 mmHg and good LV diastolic function. Blood investigations showed Hemoglobin 13.7gm%, TC 8700/cm, platelet 2.5 lakhs, PT/INR 14.52/1.07, RBS 202mg/dl, urea 25mg/dl, creatinine 0.9mg/dl, Na 132mEq/L, K 4.2mEq/L. Patient was seen by Cardiologist and given fitness under high risk for anesthesia (ASA grade III). Anti-platelet medications were stopped three days prior to the surgery and were started on low molecular weight Heparin.
On the day of surgery, informed consent was taken by patient and relatives for general anesthesia. Invasive lines included, 18G peripheral line, 20G left radial lines for arterial BP monitors and 7.5F 16cm catheter in right internal jugular vein (IJV) for central venous pressure (CVP) monitoring and drug administration. In view of ASD, special precautions were taken every time while injecting drugs to prevent air bubble entering the line. The other monitoring parameters included heart rate, rhythm, oxygen saturation, end tidal CO2 and urine output. Anti-aspiration and infective endocarditis prophylaxis were given before induction. Patient was induced with inj. midazolam 1mg, inj. Etomidate 10mg, inj. Fentanyl 100mcg and inj. Atracurium 25mg. Inj. xylocard was given prior to intubation to attenuate hemodynamic response while intubation. Anesthesia was maintained with oxygen 60%, air and sevoflurane. Volume control mode of ventilation was set to maintain end tidal CO2 30-35mmHG. Titrated dose of Inj. Nitroglycerine was administered intra operatively to maintain blood pressure (BP) and urine output (0.5ml/kg/h). The surgical duration was for 1h 30min. At the end of surgery, patient was extubated after reversing skeletal muscle relaxation with inj. neostigmine and glycopyrrolate. Post operatively patient was shifted to intensive care unit for monitoring. After 1h patient had hypotension and tachycardia with decrease trend in CVP which was suggestive of hypovolemia. Patient was stabilized with colloid infusion. Post-surgery Arterial Blood Gases (ABG) analysis showed pH 7.37, pCO2 33 mm of Hg, pO2 77 mm of Hg, HCO3 19.2mEq/L, BE 4.8. Patient was shifted to ward the next day and discharged after two days.
Case 2
A 29-year-old female weighing 55kg with CHD - large VSD and severe pulmonary arterial hypertension presented to Gynaec Out Patient Department with history of amenorrhea of 3 months with intra uterine fetal death for Dilatation & Curettage (D&E). Patient was on following medications Sildenafil, Torsemide, Ramipril and was instructed to avoid strenuous work. On examination, patient was asymptomatic with grade II dyspnea, grade II clubbing, heart rate 64/min, BP 117/65mmHg, SpO2 84% on room air. ECG showed normal sinus rhythm and Echocardiogram showed large VSD with bidirectional shunt, ejection fraction of 56%, severe pulmonary artery hypertension. Blood investigation showed Hemoglobin 12.3 gm%, WBC 11,310/cm, Platelet 2.86 lakhs. Ultrasonography showed intra uterine fetal death with Spalding’s sign positive. Cardiologist opinion was taken and planned total intravenous anesthesia (TIVA) for the procedure. 20 gauge peripheral IV line secured, aspiration and infective endocarditic prophylaxis given with utmost care not to inject any air bubble. Monitoring included ECG, heart rate, non-invasive blood pressure, and end tidal CO2 and oxygen saturation. Patient was put in lithotomy position and started with 100% oxygen through Bain’s circuit. She was given inj. ketamine 30mg followed by inj. Fentanyl 100mcg and inj. propofol 100mg in incremental doses. Procedure lasted for 15min and patient was hemodynamically stable with spontaneous ventilation maintaining saturation of 97%. Post procedure patient was monitored in post-operative unit for 3h and shifted to ward. She was discharged after 24h in stable condition.
DISCUSSION
Eisenmenger syndrome is a cyanotic congenital heart disease. The first clinical description was in 1897 by the Viennese Physician Victor Eisenmenger and in 1958 Paul Wood refined the path physiology of Eisenmenger syndrome as “Pulmonary hypertension due to a high pulmonary vascular resistance (PVR) with reversed or bidirectional shunt at aorta pulmonary, ventricular or atrial level” [5,6].
Hemodynamically, Eisenmenger syndrome is defined as an elevation of the pulmonary vascular resistance to 12 Woods units or to a Pulmonary-to-Systemic resistance ratio equal to or greater than 1 [7]. Eisenmenger may occur in congenital anomalies with a left-to-right shunt big enough to allow equalization of pressure between both ventricles and or pulmonary artery, and not in patients with small shunts or septal defects associated with significant pulmonary stenosis. Path physiology of the Eisenmenger syndrome has been shown in Figure 1.
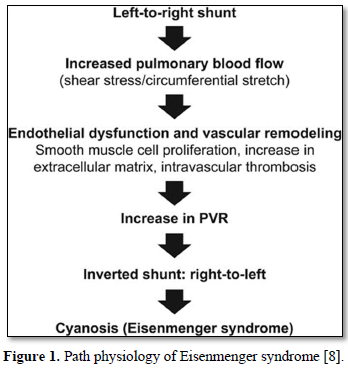
Eisenmenger syndrome is one of the leading causes of perioperative death (up to 19%) [4] in patients undergoing non-cardiac surgery (NCS). Therefore, perioperative risk and outcome depends on urgency, duration of surgery, anesthesia used and underlying pathology.
The goal of anesthetic management is to maintain the baseline PVR: SVR ratio in order to prevent an increase in right to left shunt. Either regional or general anesthesia may be used [9-11], each with its own risks and benefits. Regional anesthesia causes sympathetic blockade and decreases both preload and afterload which may be very hazardous in these patients.
During general anesthesia, intermittent positive pressure ventilation causes a decrease in venous return and cardiac output and an increase in pulmonary artery pressure, which together produce an increase in right to left shunt [9]. However, general anesthesia has been successfully used and is preferred in patients receiving antithrombotic drugs due to the increased risk of subdural hematoma following epidural or spinal anesthesia [12]. The two reasons to choose general anesthesia in case 1 patient for PFN were, first the patient was on anti platelet and anticoagulant, second to avoid sudden fall in the systemic vascular resistance in neuraxial blockade. Though the patient in case 2 was not on any anti platelet medication, the duration of surgery (D&E) prompted us to plan total intravenous anesthesia (TIVA).
The goal of monitoring in anesthesia is to detect sudden changes in the haemodynamics early so as to initiate appropriate treatment and prevent further complications. Invasive monitoring poses a specific risk in Eisenmenger patients as they are more prone for thrombus formation (due to polycythemia), have higher risk for infection and paradoxical air embolus [13]. In case 1, by considering risks and benefits, we obtained left radial line for continuous measurement of blood pressure and inserted a central venous catheter in right internal jugular vein to detect right heart failure as the right heart is ejecting against high pulmonary vascular resistance and to optimize the pre-load. In case 2, we opted for non-invasive monitoring as the patient was young and undergoing short duration procedure where the scope for wide fluctuation in heamodynamics was minimal.
Patients with Eisenmenger syndrome pose a challenge to anesthesiologists due to inability to adapt to sudden changes in haemodynamics because their pulmonary vascular bed is fixed [13]. Most of the agents used for induction and maintenance of general anesthesia depresses myocardial function and reduce systemic vascular resistance. Many authors also have recommended concomitant administration of vasopressors to avoid hypotension during induction. Combination of inj. Fentanyl and inj. Etomidate were chosen as induction agents in case 1 because of their more hemodynamic stability in Eisenmenger patients [14]. We observed no hypotension with this combination. Intubation was felicitated by Inj. Atracurium, a skeletal muscle relaxant with minimal effects on cardiovascular system. As arm-brain circulation time is shorter in Eisenmenger patients due to the right-to-left shunt, in case 2 the patient was put in lithotomy position before administering the drug in order to cutdown on anesthesia time. Inj. Ketamine [15] along with Inj. Propofol and Inj. Fentanyl was part of TIVA as it does not reduce systemic vascular resistance (SVR) in Eisenmenger patients. We avoided nitrous oxide in both the cases as it is a potent pulmonary vasoconstrictor [9], Benedikt et al. [16] have concluded Xenon as the choice of inhalational agent as it causes several physiological changes, which mediate protection of the brain and myocardium. It has lowest blood/gas partition co-efficient compared to all the inhalational agents. Also, sevoflurane shows suitable cardiovascular stability in one of the studies compared to others [17]. In case 2, as the surgical duration was less, we opted for TIVA to avoid even minimal depressant effect of inhalational agent.
As intermittent positive pressure ventilation can increase the right-to-left shunt if excessive transpulmonary pressures are used, [18] have recommended minute volumes of 5-8 l per minute with tidal volume of 5.5-6ml/kg body weight to maintain PaCO2 within normal limits in adults. Though the patient in case 2 was on spontaneous ventilation, there was no hypoxia or hypercarbia which are potent pulmonary vasoconstrictors.
CONCLUSION
Though the incidence of Eisenmenger syndrome has decreased over years due to early septal repairs, there is proportionate increase in load of these patients due to growing number of adult patients with CHD are surviving into adulthood and some into the sixth and seventh decade. Therefore, a good understanding of pathophysiology of this syndrome is essential for anesthetic management. The ultimate goal of any anesthesia technique is to maintain the baseline PVR: SVR ratio and to avoid factors that increase Pulmonary vascular resistance - like hypoxia, hypercarbia, metabolic acidosis, hypothermia, agitation, pain and tracheal suctioning.
- Hoffman JIE (1995) Incidence of congenital heart disease: 1. post-natal incidence. Pediatr Cardiol 16: 103-113.
- Young D, Mark H (1971) Fate of the patient with the Eisenmenger syndrome. Am J Cardiol 28(6): 658-669.
- Duffels MG, Engelfriet PM, Berger RM, Velde ET, Bresser P, et al. (2007) Pulmonary arterial hypertension in congenital heart disease: an epidemiologic perspective from a Dutch registry. Int J Cardiol 120(2): 198-204.
- Vongpatanasin W, Brickner ME, Hillis LD, Lange RA (1998) The Eisenmenger syndrome in adults. Ann Intern Med 128(9): 745-755.
- Eisenmenger V (1897) Dieangeborenen Defecte der Kammerscheide-wand des Herzens. Z Klin Med 32: 1-28.
- Partin C (2003) the evolution of Eisenmenger’ seponymic enshrinement. Am J Cardiol 92(10): 1187-1191.
- Wood P (1958) The Eisenmenger syndrome or pulmonary hypertension with reversed central shunt. I. Br Med J 2(5098): 701-709.
- Beghetti M, Galié N (2009) Eisenmenger syndrome a clinical perspective in a new therapeutic era of pulmonary arterial hypertension. J Am Coll Cardiol 53(9): 733-740.
- Kandasamy R, Koh KF, Tham SL, Reddy S (2000) Anesthesia for Caesarean section in a patient with Eisenmenger’s syndrome. Singapore Med J 41: 356-358.
- Pollack KL, Chestnut DH, Wenstrom KD (1990) Anesthetic management of a parturient anesthesia for Caesarean section in a patient with Eisenmenger syndrome. Anesth Analg 70: 212-215.
- Martin JT, Tautz TJ, Antognini JF (2002) Safety of regional anesthesia in Eisenmenger's syndrome. Reg Anesth Pain Med 27: 509-513.
- Gurumurthy T, Mohandas BS, Hegde Radhesh (2012) Anesthesia for a patient with Eisenmenger′s syndrome undergoing caesarean section. Indian J Anaesth 56: 291-294.
- Ammash NM, Connolly HM, Abel MD, Warnes CA (1999) Noncardiac surgery in Eisenmenger syndrome. J Am Coll Cardiol 33: 222-227.
- Bennett JM, Ehrenfeld JM, Markham L, Eagle SS (2014) Anesthetic management and outcomes for patients with pulmonary hypertension and intracardiac shunts and Eisenmenger syndrome: A review of institutional experience. J Clin Anesth 26(4): 286-293.
- Tweed W, Minuck M, Mymin D (1972) Circulatory responses to ketamine anesthesia. Anesthesiology 117: 1413-1414.
- Preckel B, Schlack W (2005) Inert gases as the future inhalational anesthetics? Best Pract Res Clin Anaesthesiol 19: 365-379.
- Thomas J (2011) Anesthesia for the child with congenital heart disease: Pointers and pitfalls. CME 29, No 11/12.
- Lumley J, Morgan M, Sykes M (1969) Changes in arterial oxygenation and physiological dead space under anesthesia. Br J Anaesth 41: 279-287.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Dermatology Clinics and Research (ISSN:2380-5609)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- International Journal of AIDS (ISSN: 2644-3023)
- Stem Cell Research and Therapeutics (ISSN:2474-4646)


