Review Article
The Chain is Broken: Limits of Global Blood Component Supply Become More Visible in Times of COVID-19
5640
Views & Citations4640
Likes & Shares
Annually, more than 100 million blood transfusions with donor blood take place worldwide. Blood transfusion supply is essential in order to facilitate a functional health-care system. However, most of these transfusions, 64%, are consumed in the high-income countries. Although blood and its components were recently added to the WHO Model List of Essential Medicines, insufficient blood hemovigilance systems in many low-income and middle-income countries still lead to preventable mortality and morbidity due to lack of blood components such as red blood cells (RBCs), platelets (Plt) and plasma. These countries are mostly located in Asia, Africa, Middle and South America, representing 82% of the global population. The shortage is calculated as the lack of more than 30 million donations. Also, complex processing whole blood into blood components carry considerable costs in order to maintain quality of these products. This makes it even more difficult to maintain blood hemovigilance systems in many low-income and middle-income countries. This weaknesses in the supply chain needs support by implementing patient blood management (PBM) and the expanded use of autologous blood in order to improve on allogeneic blood component supply. Simplification of production methods of these blood components is ongoing and will be highlighted in the current short review.
Keywords: Blood transfusions, Patient blood management, Supply chain needs
INTRODUCTION
The current pandemic crisis initiated by the COVID-19 viral outbreak, has increased the need for donor blood even more. In China, the number of whole blood donors dropped by 67% and the recruitment for donors dropped by 60%. The most important reason not to donate was the fear of acquiring COVID-19 during blood donation [1]. Also, same decreases in Zambia were reported not only caused by fear but also due to the partial lock-down that closed down all learning institutions. Since more than 90% is donated by mobile blood collection, the closing of these institutes caused donations to cease [2]. In numerous countries the crisis management ordered new rules in behavior such as issuing social distancing and escalated school and business closures. In response, elective surgical procedures and nonessential care were cancelled in many hospitals. These measures also caused cancellation of blood drives and reduced the number of blood donors who could be at a collection site, which severely affected the availability of blood products. Blood suppliers began notifying hospitals of these shortages, which led to preparations and actions to address the shortages while maintaining care delivery [3,4]. It is imperative to conclude that in times of a crisis, all health care systems are affected including blood donation programs. But in times of crisis, alternative solutions or programs are created or accelerated. Existing and new methods to alleviate the pressure on conventional donor blood supply are revisited for clinical practice. Even topics become political as is clearly the case with regard of using convalescent plasma (CP) as a passive immunization therapy in case of a COVID-19 infection [5]. Solutions that alleviate the pressure on blood hemovigilance systems are urgently needed, including well-supported strategies for implementation. When we will look back on the current health crisis, we probably will recognize that it has resulted in a number of innovations: new medication and medical devices, improved healthcare processes, manufacturing and supply chain breakthroughs and novel collaboration techniques. Most blood management innovations are existing but need a new evaluation and rethinking of their use into the blood supply chain. PBM systems, the use of autologous blood transfusions (ABT) and alternative or innovative methods of production of blood components support relieve pressure on the conventional blood management [6]. The conventional chain is broken by COVID-19 but repairing the supply chain is possible within reach by implementing these methods.
PATIENT BLOOD MANAGEMENT
PBM programs have existed for decades but are not embedded in most health care centers as a universal standard of care. PBM is defined as an evidence-based medical and surgical approach to minimize the need for and use of blood transfusion in patients as a means to improve their clinical outcomes [7].
It encompasses a comprehensive integration of a patient-centric, multidisciplinary standard of care centralized on blood health, which involves all functions of maintaining blood volume, anemia management, coagulation management, and surgical technique, not just blood transfusion therapy [8]. Most PBM programs are implemented in order to reduce allogeneic blood transfusions due to the current knowledge of its negative effects, both related to costs and healthcare. It is shown that when taking into consideration all costs related to providing RBC transfusions with an activity-based costing (ABC) model, the total costs were 3.2-to 4.8-fold higher than blood product acquisition costs [9]. The ABC model includes all major process steps, staff, and consumables to provide red blood cell (RBC) transfusions to surgical patients. From healthcare perspective, allogeneic blood transfusion is a potentially hazardous method similar to allogeneic organ transplantation. It may cause long-term effects on immunization, which would likely lead to micro-thrombosis, blood coagulation and hemolytic reactions [10]. Allogeneic blood transfusion has been associated with increased risk of tumor recurrence, postoperative infection, acute lung injury, perioperative myocardial infarction, postoperative low-output cardiac failure, and increased mortality [11].
Thanks to several strategies including donor screening and deferral, blood testing and pathogen inactivation, their risks have reached all-time low levels, particularly in high income countries. The underlying mechanism is likely to be related to immunosuppressive effects of allogeneic blood, storage lesions, iron content, and bacterial contamination. Judicious and evidence-based use of allogeneic blood components is needed to ensure that the potential benefits are worth the risks [12].
During a global pandemic, the main characteristics of PBM prove valuable in normal daily patient care. The practice and standards of PBM are universal across specialties as well as inpatient, outpatient, surgical, and medical disciplines. They focus on optimizing hemoglobin values, minimizing blood losses, implementing autologous blood transfusion, managing coagulopathy, and enhancing tolerance to anemia [13].
Practitioners of PBM consistently consider the hypothetical situation of caring for patients without the availability of blood products when describing its relevance and value.
AUTOLOGOUS BLOOD TRANSFUSION
With the improvement of medical technology and the growing discrepancy between blood supply and demand, Autologous blood transfusion (ABT) is a way to decrease the need of allogeneic transfusions and in this perspective different aspects are highlighted. The safety and effectiveness of ABT has gradually become a subject of interest. ABT can avoid the serious harm caused by allogeneic blood transfusion, alleviate blood shortage and save blood resources, while lightening the burden of patients. Therefore, ABT has gained more attention, and has become a common demand in clinical practice [14].
Intraoperative or postoperative auto transfusion refers to a method of transfusion in which blood in the body cavity of a patient, blood lost during surgery and postoperatively drained blood, can be recovered through a blood recovery device. Then, blood undergoes anticoagulation, filtration and washing, and is finally transfused back to the patient. The American Association of Blood Banks guidelines recommend that intraoperative or postoperative autotransfusion should be performed in surgeries where a large amount of bleeding (more than 20% total volume) is anticipated [15]. However, only 10% of peri-operative RBC need is provided by ABT due to lack of availability, staffing and costs although with the current technologies the benefit of a disease specific ABT program is widely proven [16].
In situations without access to blood banks and where the need is so desperate without any means of washing, the surgeons just collect by the with only filtering out debris and large clots before reinfusion. Surgeons are then often forced to resort to the ‘soup ladle technique’, scooping up blood spilled during a surgery and filtering it through gauze into a jar before returning it to the patient [17]. This method is both impractical and dangerous. In case of ABT after surgery this is sometimes also used with only filtering out large particles before reinfusion into the patient [18]. This is not recommended by surgical societies due to its adverse clinical effects it induces [19,20]. The mainstay technique up to now is all based on centrifugal devices, so called cell savers [21]. But as use of cell savers often is inaccessible, innovative blood processing technologies that not only effectively wash out contaminants but are also cost-effective are needed.
The future of allogeneic and autologous blood transfusion Innovation drives novel blood processing technology
There are several new developments that focus on different technologies in order to reach the same quality effect of conventional centrifugal cell savers but without its complexity. One proof of concept study was based on gravity driven sedimentation by using a 20 m long 1 mm in diameter tubing disposable device in order to wash stored red blood cells. The prototype of the gravity-driven washing operated at a flow rate of 0,5 ml/min produced a suspension of washed RBCs with low output hematocrit of 37% and washing waste with a hematocrit of 3.4%. In order to develop it further to the needed clinical capacity, a 40-fold increase of the device capacity would be needed. It was concluded that this a technical challenge to reach such a goal [22].
Another innovative device is HemoClear, using microfiltration technology that is specifically yields washed RBCs and platelets. In contrast to existing devices, as centrifugal cell savers merely recover RBCs, while blood concentrators such as the HemoSep device recover unwashed platelet-rich RBCs [23].
The HemoClear device is easy to operate (Figure 1). It uses crossflow filtration, driven by gravity, and is capable of capturing RBCs and separating them from other fluids and plasma at comparable quality that can be reached by cell savers [24]. The intended medical purpose of the HemoClear device is in recovering RBCs from patients in a setting where cell savers are not used due to lack of infrastructure, availability, or for financial reasons. The aim is to avoid wasting blood and provide a safe, easy and cost-effective way to re-infuse a patient’s own red blood cells. Specific types of surgery for which the technique seems especially useful include open heart and vascular surgery, orthopedic surgery such as total joint replacements and spinal surgery, liver transplantation, and ruptured ectopic pregnancy surgeries.
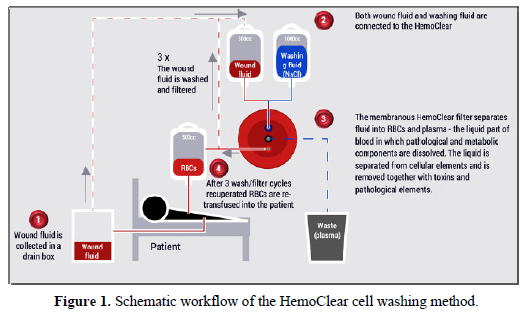
Novel processing technologies extend use of blood products for new indications
Passive immunization by convalescent plasma: In the absence of preventive measures for COVID-19, global effort focus on trails of various potentially effective treatments. One such treatment is convalescent plasma (CP) therapy, a classical adaptive immunotherapy. CP was used in previous epidemic infections, including MERS, SARS and Ebola [25-27]. The plasma of infected and recovered patients contains neutralizing antibodies against the virus, that slow down the viral replication. When CP is harvested from a former patient and administered to newly ill patients, passive immunization can be achieved. The evidence for efficacy of COVID-19 convalescent plasma treatment continues to grow but is also questioned due to the lack of clearly defined or under-powered small randomized trials [28-31]. Also, if CP is a possible therapeutic, there are still concerns about infectious side effects that cannot be excluded with this procedure.
Plasma collection by plasmapheresis represents another hurdle. The pandemic had increased the global demand for CP, whereas gold standard centrifugal plasmapheresis devices only are widely available in high-income countries. During the Ebola outbreak this hurdle was overcome by ad hoc training on use of donated plasmapheresis devices [32,33]. Nevertheless, capacity building for the current and future use of convalescent plasma therapy is an imperative. Sustainable expertise, infrastructure and medical technologies (such as the HemoClear device described above) should support preparedness for rapid CP acquisition in pandemics as a first line of defense if there is enough evidence available on its efficacy and safety as a therapy.
Peripartum use of autologuous cell saver techniques: Although originally a precautionary measure, support for the use of cell salvage in obstetric hemorrhage is provided by over 400 reported cases in which blood contaminated with amniotic fluid has been washed and re-administered without filtration, and various international associations have advocated the use of blood salvage in obstetrics [34,35]. Broad use of cell salvage in the obstetric field should greatly decrease maternal mortality, for which reported rates remain unacceptably high [36]. Post-partum hemorrhage accounts for nearly a third for annual maternal deaths, 99.7% of which occur in the emerging world [37]. Combined development of novel accessible salvage devices and increasing acceptance should greatly increase autologous blood availability in the obstetric field.
-
Wang Y, Han W, Pan L, Wang C, Liu Y, et al. (2020) Impact of COVID‐19 on blood centres in Zhejiang province China. Vox Sang 115: 502-506.
-
Kasanga M, Mudenda S, Gondwe T, Chileshe M, Solochi B, et al. (2020) Impact of COVID-19 on blood donation and transfusion services at Lusaka provincial blood transfusion centre, Zambia. Pan Afr Med J 35: 74.
-
Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, et al. (2020) Safety Update : COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients. Mayo Clin Proc 95: 1888-1897.
-
Shander A, Goobie SM, Warner MA, Aapro M, Bisbe E, et al. (2020) Essential Role of Patient Blood Management in a Pandemic: A Call for Action. Anesth Analg 131: 74-85.
-
President Donald J (2020) Trump Is Promoting Safe Plasma Donations To Protect Americans And Defeat COVID-19. Accesssed on: July 30, 2020. Available online at: https://www.whitehouse.gov/briefings-statements/president-donald-j-trump-promoting-safe-plasma-donations-protect-americans-defeat-covid-19/
-
Clark L (2020) Innovation in a Time of Crisis. Accessed on: March 26, 2020. Available online at: https://www.harvardbusiness.org/innovation-in-a-time-of-crisis/
-
Franchini M, Marano G, Veropalumbo E, Masiello F, Pati I, et al. (2019) Patient Blood Management: A revolutionary approach to transfusion medicine. Blood Transfus 17: 191-195.
-
Tolich D, Auron M, McCoy K, Dargis M, Quraishy N (2020) Blood management during the COVID-19 pandemic. Cleve Clin J Med pp: 1-5.
-
Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, et al. (2010) Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion 50: 753-765.
-
Hellings S, Blajchman MA (2009) Transfusion-related immunosuppression. Anaesth Intensive Care Med 10: 231-234.
-
Ashworth A, Klein AA (2010) Cell salvage as part of a blood conservation strategy in anaesthesia. Br J Anaesth 105: 401-416.
-
Shander A, Lobel GP, Javidroozi M (2016) Transfusion practices and infectious risks. Expert Rev Hematol 9: 597-605.
-
Sullivan HC, Roback JD (2019) The pillars of patient blood management: key to successful implementation (Article, p. 2840). Transfusion 59: 2763-2767.
-
Zhou J (2016) A review of the application of autologous blood transfusion. Braz J Med Biol Res 49(9): e5493.
-
Klein AA, Arnold P, Bingham RM, Brohi K, Clark R, et al. (2016) AAGBI guidelines: The use of blood components and their alternatives 2016. Anaesthesia 71: 829-842.
-
Shander A, Van Aken H, Colomina MJ, Gombotz H, Hofmann A, et al. (2012) Patient blood management in Europe. Br J Anaesth 109: 55-68.
-
Schantz-Dunn J, Nawal M (2011) The use of blood in obstetrics and gynecology in the developing world. Rev Obstet Gynecol 4: 86-91.
-
Sjöholm A, Älgå A, von Schreeb J (2020) A Last Resort When There is No Blood: Experiences and Perceptions of Intraoperative Autotransfusion Among Medical Doctors Deployed to Resource-Limited Settings. World J Surg 44: 4052-4059.
-
Ferraris VA, Force S of TSBCGT, Ferraris SP, Saha SP, Hessel EA, et al. (2007) Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg 83(5 Suppl): S27-86.
-
Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, et al. (2011) 2011 update to the society of thoracic surgeons and the society of cardiovascular anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg 91: 944-982.
-
Fourtounas M (2020) Cell saver physics - A review. South African J Anaesth Analg 26: S49-54.
-
Lu M, Lezzar DL, Vörös E, Shevkoplyas SS (2019) Traditional and emerging technologies for washing and volume reducing blood products. J Blood Med 10: 37-46.
-
McCollum C, Walunj A (2017) Hemosep for cell salvage. Natl Inst Heal Care Excell, pp: 1-11.
-
Hoetink A, Scherphof SF, Mooi FJ, Westers P, van Dijk J, et al. (2020) An In Vitro Pilot Study Comparing the Novel HemoClear Gravity-Driven Microfiltration Cell Salvage System with the Conventional Centrifugal XTRATM Autotransfusion Device. Anesthesiol Res Pract 2020: 1-10.
-
Arabi YM, Hajeer AH, Luke T, Raviprakash K, Balkhy H, et al. (2016) Feasibility of Using Convalescent Plasma Immunotherapy for MERS-CoV Infection, Saudi Arabia. Emerg Infect Dis 22: 1554-1561.
-
Arabi Y, Balkhy H, Hajeer AH, Bouchama A, Hayden FG, et al. (2015) Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: A study protocol. Springerplus 4: 709.
-
Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, et al. (2015) The Effectiveness of Convalescent Plasma and Hyperimmune Immunoglobulin for the Treatment of Severe Acute Respiratory Infections of Viral Etiology: A Systematic Review and Exploratory Meta-analysis. J Infect Dis 211: 80-90.
-
Joyner MJ, Klassen SA, Senefeld J, Johnson PW, Carter RE, et al. (2020) Evidence favouring the efficacy of convalescent plasma for COVID-19 therapy. medRxiv.
-
Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, et al. (2020) Significantly Decreased Mortality in a Large Cohort of Coronavirus Disease 2019 (COVID-19) Patients Transfused Early with Convalescent Plasma Containing High-Titer Anti-Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Protein IgG. Am J Pathol 191(1): 90-107.
-
Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, et al. (2020) Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ 371: m3939.
-
Perotti C, Baldanti F, Bruno R, Del Fante C, Seminari E, et al. (2020) Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica. 105(12): 2834-2840.
-
Epstein J, Burnouf T (2020) Points to consider in the preparation and transfusion of COVID-19 convalescent plasma. Vox Sang 115: 485-487.
-
Burnouf T, Emmanuel J, Mbanya D, El-Ekiaby M, Murphy W, et al. (2014) Ebola: A call for blood transfusion strategy in sub-Saharan Africa. Lancet 384: 1347-1348.
-
Esper SA, Waters JH (2011) Intra-operative cell salvage: A fresh look at the indications and contraindications. Blood Transfus 9: 139-147.
-
Waters JH, Biscotti C, Potter PS, Phillipson E (2000) Amniotic Fluid Removal during Cell Salvage in the Cesarean Section Patient. Anesthesiology 92: 1531-1536.
-
World Health Organization (WHO) Fact Sheet. Blood safety and availability. Accessed on: May 16, 2019. Available online at: https://www.who.int/en/news-room/fact-sheets/detail/blood-safety-and-availability
-
Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, et al. (2014) Global causes of maternal death: A WHO systematic analysis. Lancet Glob Health 2: e323-e333.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Oncology Clinics and Research (ISSN: 2643-055X)
- International Journal of AIDS (ISSN: 2644-3023)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Dermatology Clinics and Research (ISSN:2380-5609)



