Aims & Objectives: A single blinded randomized controlled study to evaluate the immediate and short-term effects of single per-cochleostomy dexamethasone injection on intracochlear electrode impedance and various behavioral parameters, thus analyzing its protective role on neural elements, both intra-operatively and on a regular basis up to 6 months.
Materials & Methods: It is a prospective, randomized, single blinded case-control study of 50 patients less than 5 years of age with congenital bilateral profound sensori-neural hearing loss, who were randomized into 2 groups, where one group received intra-cochlear dexamethasone and the other did not. Intra-cochlear electrode impedance and comfort and threshold levels for both the groups were evaluated at pre-defined follow-up periods.
Results: The steroid group showed better results in the form of reduced Impedance and better Comfort Levels along with a statistically significant increase in Dynamic Range (p value < 0.001) as compared to the control group.
Conclusion: Per-cochleostomy dexamethasone instillation can be a useful method to decrease the impedance of electrodes in postoperative period, helping in improving behavioral parameters of implantees with increased Comfort Levels and thus a wider Dynamic Range. However long-term follow-up study with larger sample size is required to validate the efficacy of such treatment.
Keywords: Cochlear implant, Residual hearing preservation, Dexamethasone, Impedance, Behavioral parameters
INTRODUCTION
Cochlear implant electrode insertion causes trauma to cochlear structures as well as molecular level changes resulting in loss of hair cells [1]. Leading to possible residual hearing loss, both immediate followed by progressive. Surgery also causes inner ear trauma (drilling and hydraulic pressure trauma, loss of perilymph and direct sudden injury to inner ear structures), causing deterioration in residual hearing [2]. It is now established that irrespective of the surgical techniques for cochlear implantation, various non-surgical factors contribute to improved rates of hearing preservation following surgery [3]. Residual hearing loss also occurs due to high electrical impedance at electrode-cochlear tissue interface due to surface electrochemical phenomenon and fibrotic tissue growth around electrode [4] and various metabolic factors like inflammation, oxidative stress, and apoptosis. Modulation of these pathways, through pharmacological treatment of inner ear, may be an effective adjunct to good electrode design and surgical technique for maintaining residual hearing. Dexamethasone receptors have been localized within cochlea [5,6]. The anti-inflammatory role of steroids is well known in preventing cellular injury and inhibiting cell death by blocking transcription of pro-inflammatory molecules, enhancing transcription of anti-inflammatory factors and inhibiting leukocyte migration [7,8]. Dexamethasone also enhances glutathione synthesis by spiral ganglion cell [9] which is an anti-oxidant. Present study evaluates the immediate and short-term protective effect of a single per-cochleostomy dexamethasone injection.
OBJECTIVES
The objective of the study was to assess the protective effect of per-cochleostomy dexamethasone injection in randomly selected patients undergoing cochlear implant surgery on intra-cochlear electrode impedance and neural response telemetry by analyzing the values of Threshold Levels and Comfort Levels (T & C level) and electrode impedances, both intraoperatively and on a regular basis up to 6 months in patients receiving steroids and in those who did not receive any.
MATERIALS AND METHODS
Fifty consecutive children (age less than 5 years) receiving cochlear implant at our Quaternary referral center were randomized into two groups A and B. Standard pre-operative evaluation by behavioral audiometry, brainstem evoked response audiometry, radiology (High resolution Computed Tomography of temporal bone, Magnetic Resolution Imaging of head and inner ear with 3D reconstruction) and neurological assessment was performed for all cases. Children with malformed cochlea and those with border-line intelligence (Intelligence Quotient less than 70) were excluded from the study. All patients were subjected to cochlear implant by a single surgeon (RCD) to eliminate surgeon bias. Surgery was performed by standard cortical mastoidectomy, posterior tympanotomy and cochleostomy approach in both the groups to ensure atraumatic scala tympanic insertion. Gentle, gradual drilling of cochleostomy, removal of endosteum and handling of electrode with gradual insertion was paramount in all subjects. Patients in Group A additionally received 0.5 ml (2 mg) of per-cochleostomy dexamethasone instillation in addition through an insulin syringe with steroid soaked gel-foam kept at the cochleostomy site, making sure no excess suctioning was done at the site. After wound closure immediate post-operative Neural Response Telemetry along with electrode impedance measurements in 4 modes (Common Ground, Mono Polar 1, Mono Polar 2, Mono Polar 1+2) was performed for all the patients using Custom Sound2 Software. Steroids were expected to interfere with intra-cochlear resistance/capacitance which was reflected by CG impedance. Device switch on was done 4 weeks after surgery. Impedance measurements for both the groups were repeated just after switch on. The readings were repeated 3 months and 6 months after implant. The Threshold level, Comfort level and Dynamic Range were also recorded during these visits (1st, 3rd and 6th months). Only those patients with a minimum follow up for six months were included in the study and the results were analyzed at the end of 2 years.
OBSERVATION AND RESULTS
Our study comprised of 33 males and 17 females. Group A had 18 males (72%) and 7 females (28%), whereas in Group B there were 15 males (60%) and 10 females (40%). The age ranged from 2-5 years in both the groups (mean of 3.56 years and 3.31 years in Group A and Group B respectively). Both the groups were matched for age and etiologies, with the commonest etiologies being idiopathic congenital sensorineural hearing loss, followed by ototoxicity, intra-uterine infections and prematurity.
Complications that were seen in our study included transient facial nerve weakness in 4 patients, button-holing of skin flap and CSF leak in 1 patient each, which were managed conservatively with no long-term sequelae, with no major complications.
On analysis, it was observed that the mean impedances in all the 4 modes (CG, MP1, MP2 and MP1+2 mode) decreased with time and stabilized after 6 months in both the groups, however, the steroid group showed a beneficial trend in the form of lower values of impedance than the non-steroid group at all subsequent follow-ups in all the 4 modes indicating some degree of protection incurred by the steroid instillation (Table 1), although the difference in the values in the two groups is not statistically significant (p value is > 0.05).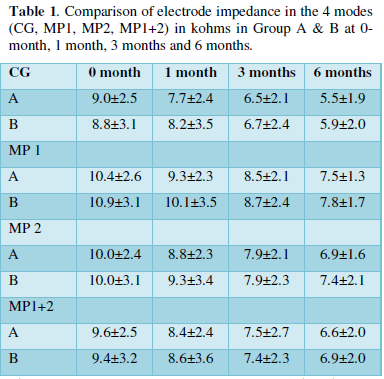
The minimum stimulus (average/mean) required to directly stimulate the cochlear nerve and get the best identifiable wave was lower in steroid group as compared to non-steroid group, implicating lesser current levels required to get the desired results. Though the values show a positive trend towards protective role of steroids, the results were not statistically significant.
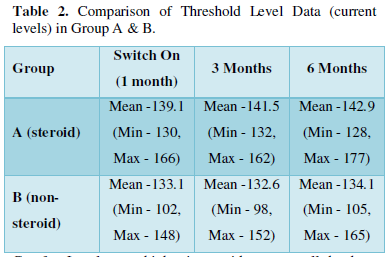
As seen in Table 2, although the minimum current level required to stimulate the electrodes and produce the sensation of sound was higher in steroid group at all the follow-up visits as compared to non-steroid group, the difference was not statistically significant (p value > 0.05).
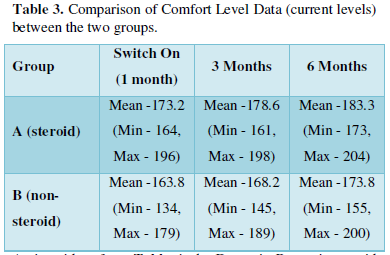
Comfort Levels were higher in steroid group on all the three occasions, depicting that the steroid group patients could tolerate higher levels of current levels as compared to the control group (Table 3).
As is evident from Table 4, the Dynamic Range in steroid group was higher as compared to non-steroid group on all the 3 occasions, but the difference is significant only in the 1st month, as the p value is 0.01.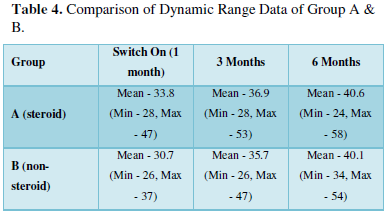
DISCUSSION
Vivero [10] proclaimed preservation of trauma-induced hearing loss using dexamethasone in his study. Adunka [11] ensured an atraumatic scala tympanic insertion of the electrode with antero-inferior cochleostomy. Hearing preservation may also be achieved by the use of a drug during and after implantation. The electrode array offers a potential vehicle for drug delivery (a thin, flexible electrode array with zigzag wires). Semi-chronic dexamethasone elution, acute drug delivery by intracochlear catheter, and longer-term delivery through diffusion from a reservoir were all shown to be feasible options.
Various proposals have been made to reduce the electrical impedance like Iridium oxide coating which decreases resistance between the electrode and the tissue [12,13], roughening the surface to increase the effective surface of the electrode [14]and application of steroids in the cochlea reducing the tissue growth around the electrode carrier [15].
Paasche [16] postulated the beneficial effects of steroid and iridium oxide coating of the electrode in decreasing impedance values. Our results are consistent with the other studies, showing the beneficial role of steroids as depicted by lower values of impedance in the test group, though not significant statistically, proving that steroids decrease the inflammatory reaction and connective tissue formation.
There is little literature available regarding the role of intra-cochlear steroid instillation on the Behavioral Parameters of the implantee. Studies on humans have showed a significant decrease in electrode impedance during the first few weeks after initial stimulation, after which levels stabilized for years [17,18]. Low Impedance levels are correlated with lower values of Threshold Level and higher values of Comfort Level and Dynamic Range.
In our study, values of Comfort Level and Threshold Level have increased with time initially and have stabilized 6 months post implant. Threshold Level values show an increasing trend initially for 3 months, which stabilize thereafter. Threshold values are normally seen to increase in the post-operative period after implant which remains static thereon. The Comfort Level, Threshold Level and Dynamic Range values are different in the two groups. The Comfort Levels and Dynamic Range are better in steroid group as compared to the control group, but the difference is not statistically significant. The Dynamic Range is more in the steroid group as compared to the non-steroid group at different periods of follow-up but the difference is statistically significant only in the 1st month of follow-up, proving the protective role of intra-cochlear steroids.
In our study to assess the role of per-cochleostomy steroid on intra-cochlear electrode impedance and its impact on the various Behavioral parameters like Threshold Level, Comfort Level and Dynamic Range, steroids have shown to decrease the Impedance values, which has manifested itself as a higher value of Comfort Level and subsequently a wider Dynamic Range. It shows a positive trend towards the protective role of per-cochleostomy dexamethasone instillation, though a larger series with a longer follow-up is required to study whether it actually translates to residual hearing preservation and is beneficial in subjects with substantial residual hearing.
CONCLUSION
Per-cochleostomy steroid instillation is a useful technique to preserve residual hearing by decreasing the Impedance values of the electrodes in the postoperative period which is reflected as improved values of the behavioral parameters of cochlear implantees in the form of increased Comfort Levels and a wider Dynamic Range. Beneficial effects of steroid were seen in the present study with single administration of intra-operative dexamethasone. This study has paved the path towards repeated use or prolonged use of steroid for preservation of residual hearing. The electrode can be a potential vehicle for drug delivery. Drug delivery by intracochlear micro-catheter, chronic dexamethasone elution and long-term delivery through diffusion from a reservoir are all new developments towards preserving residual hearing in cochlear implantees. However long term randomized controlled studies with larger sample size is required to validate the efficacy of such technique.






