Research Article
Dysregulation of the Autonomous Nervous System in Patients with Advanced Sick Building Syndrome.
5353
Views & Citations4353
Likes & Shares
Background: Exposure to dampness micro biota (DM) in moisture-damaged buildings can cause a spectrum of clinical manifestations with very frequent neurological sequelae. Sick Building Syndrome (SBS) is a reversible feature of the Dampness and Mold Hypersensitivity Syndrome that occurs after prolonged exposure.
Methods: Patients with advanced SBS (n=25) and age- and sex-matched controls (n=25) participated in this assessment of autoantibodies towards 13 different auto antigens.
Results: The average exposure to DM was 15 years. All patients reported profound fatigue, the prevalence of asthma was 19/24 (76%), multiple chemical sensitivity (MCS) was encountered in 21/22 (84%). A family history for autoimmune conditions was unexpectedly high, 68%. Autoantibodies to the α1-adrenergic receptor (α1AR) were significantly elevated compared to the healthy control group (p=0.022), whereas autoantibodies towards cholinergic muscarinic receptor m4ACh and IgM-class antibodies towards tri sulphated disaccharide component of heparin oligosaccharides (TS-HDS) were significantly reduced (p= 0.003 and p<0001, respectively).
Conclusions: This is the first report demonstrating an imbalance in autoantibodies in patients with advanced SBS. Although the findings cannot be applied for customized patient counseling, they are useful when investigating immune pathogenesis. It is evident that this illness is not anxiety-driven.
Keywords: Mycotoxins, Autonomous Nervous System, Dampness and Mold Hypersensitivity Syndrome (DMHS), Sick building Syndrome, Autoantibodies, Neurological Symptoms
INTRODUCTION
The term “sick building syndrome” (SBS) was linked in the early 1970s to a poor working environment [1-5]. Later, the syndrome was attributed to indoor air problems in the sufferers’ homes [6]. The name “SBS” is an umbrella term covering many different aspects. An occupant may feel unhealthy due to a variety of factors, such as an elevated concentration of volatile organic substances, or when air velocity, noise or the temperature are too high, or when ventilation is insufficient. The prominent feature of SBS is that the person experiences a series of transient symptoms in a building that makes him/her feel “sick”. When the individual leaves the building, his/her symptoms become relieved. SBS has also been related to “tight buildings” that “do not breathe” [7]. The problem of “tight buildings” arises when this kind of construction suffers from some form of water leakage. Notably, the symptoms experienced by individuals in buildings that have become infested with growing dampness micro biota that may expel bio toxins are also referred to as SBS, although the clinical course of this illness may differ from “feeling sick” e.g., from being in a too noisy environment. The impacts of microbial particles and mycotoxins on the initiation of SBS and on the subsequent health deterioration of many bodily functions were established more than a decade ago [8-11]. The symptoms may persist or worsen even if the consequences of the water damage have been corrected, the building has been remediated, and the person enters the building again [8,12]. In this article, we will use the term SBS only in conjunction with the harmful effects of dampness micro biota.
Despite a convincing body of scientific publications, the topic of the SBS related to the infestation of buildings with dampness micro biota remains controversial. Two major reasons are that there are still no biomarkers to prove the causality in an objective way, and because legal compensation issues are often involved. One medical reason for claiming that there is no connection between the mold infestation in a building and the symptoms experienced by users of that building is the observation that specific IgE-class antibodies are detected rarely. The assay of secondary mediators of inflammation has also been non-informative [13]. Nonetheless, the occupants of moldy environment experience a plethora of symptoms that are rarely related to allergic rhinitis or IgE-associated asthma. For example, objective parameters of systemic inflammation e.g., C-reactive protein (CRP) or Erythrocyte Sedimentation rate (ESR) may remain within reference ranges. Two to three decades ago, it was claimed that the nature of the mechanisms of action of long-term exposure to inhaled microbial particles and mycotoxins had not been adequately described and the SBS was summarized as a “what is it - if it is” issue [13]. In those days, questions were raised about whether there were truly any long-term health consequences to a mold environment; for example, it was claimed that there was no evidence in humans that the exposure to indoor air molds could evoke any form of non-mucosal pathology [14]. This point of view was justified from work conducted in the laboratory although most of the toxicological studies were done on animals with the toxins delivered primarily by ingestion. In one publication, the symptoms of SBS were likened to a form of mass hysteria [15], although the authors of publication did state that this interpretation was speculative and called for sufficiently funded and properly designed scientifically valid studies on SBS, chemical sensitivities and mycotoxicosis [15].
According to our experience and a survey of the literature, in the early phase of the so-called sick building syndrome, the occupants may experience reversible symptoms affecting the mucous membranes such as skin and eye irritation; headache, vocal problems, sore throat, non-productive cough, fatigue, and cognitive problems such as difficulties to concentrate, insomnia etc. Later, if there is prolonged or cumulative exposure to the products of dampness micro biota and decay components of the building constructions, the illness can become chronic and proceed to Dampness and Mold Hypersensitivity Syndrome (DMHS) [16]. The SBS is one of the five criteria for DMHS. In Valtonen’s definition, SBS is considered as a reversible state of reactivity from living in a moldy environment, whereas DMHS is a chronic condition that may not revert to normal even after the cessation of the exposure [16]. DMHS is an irreversible condition with a multiorgan involvement, with the development of Multiple Chemical Sensitivity (MCS) in almost every second patient. There is an increased rate of respiratory infections, reactivation of latent infections, profound fatigue, and a wide spectrum of yet under-recognized neurological symptoms such as “brain fog”, tremors, jerking movements, tic-like motions, cardiac palpitations, etc. [17-20]. In addition, potentially life-threatening hypersensitivity pneumonitis [21,22] or allergic alveolitis [23] can develop. Chronic fatigue syndrome (CFS), fibromyalgia, various types of hypersensitivities such as allergies, food intolerance, sensitivity to chemicals at concentrations tolerable by most of the population, sensitivity to electromagnetic fields, and even to daylight, may associate with DMHS [16]. Exposure to the secondary metabolite products of molds and mycotoxins in the indoor air may alter the functions of the endocrine system, especially the thyroid gland [24-28]. The spectrum of clinical conditions related to irreversible DMHS has been extensively reviewed [16,29-33]. In DMHS, there is a breakdown of oral, peripheral, and central tolerance that manifests itself clinically as multiple hypersensitivities including intolerances to several food products such as gluten, cow’s milk proteins, especially casein etc., (our clinical observation).
Respiratory problems have been recognized; these include an exacerbation and newly-onset asthma, allergic rhinitis and hypersensitivity pneumonitis also called exogenous allergic alveolitis caused by indoor air dampness micro biota [34-35]. Recently, we have described that the risks for neurological sequalae in the occupants of moisture-damaged buildings are as high as for respiratory symptoms, approximately three-fold when compared to the controls [17-19].
There is already some evidence that the exposure to dampness micro biota can cause the formation of autoantibodies against myelin basic protein (MBP), myelin-associated glycoprotein (MAG), ganglioside GM1, sulfa tide, myelin, oligodendrocyte glycoprotein, alpha-B-crystallin, chondroitin sulfate, tubulin and neurofilament [36], antinuclear (ANA), and autoantibodies against smooth muscle (ASM) [37]. The preliminary indication that neuronal autoantibodies can have a role in the pathogenesis of neurologic manifestations emerged from studies on patients with a postural orthostatic tachycardia syndrome (POTS), myalgic encephalomyelitis (ME/CFS), and chronic regional pain syndrome (CRPS) [38-44]; these are all conditions that may associate with advanced mold-related illness. We suspected that some patients might have manifestations of small fiber neuropathy (SFN). Therefore, we have determined the levels of some newly developed markers for this condition, such as fibroblast growth factor receptor 3 (FGF3) and IgM TS-HDS. The detection of these autoantibodies is a relatively new field of clinical research, and their medical significance is not yet fully established. However, the structure and functions of the receptors to which they bind have been described.
The angiotensin II receptors are G protein-coupled receptors that are ligands for angiotensin II [45]. The AT1 receptor subtype is found in the heart, blood vessels, kidney, adrenal cortex, lung as well as the circumventricular organs of brain, basal ganglia and brainstem. Endothelin type A receptors are also G protein-coupled receptors; these are widely distributed on blood vessels and their activation results in an elevation of the level of intracellular-free calcium, sodium retention and consequently to constriction of smooth muscles of the blood vessels [46]. The adrenoceptors are a class of G protein-coupled receptors; their endogenous ligands are norepinephrine (noradrenaline) and epinephrine (adrenaline). These receptors are located throughout the body; they mediate the activity of the sympathetic nervous system. The adrenoceptors are subdivided into the following subtypes: α1 and α2 and β1, β2 and β3. Muscarinic-type acetylcholine is also G-protein coupled receptors that are a part of the parasympathetic nervous system. There are 5 subtypes of muscarinic receptors. The FGFR3 belongs to the immunoglobulin superfamily that is structured as three immunoglobulin-like extracellular surface ligands, transmembrane domain, and an intracellular domain exhibiting tyrosine kinase activity. FGFRs bind fibroblast growth factors, a family comprising 22 members [47]. Patients with IgG antibodies towards FGFR3 often have neuropathies and immune-related disorders [48]. The acronym TS-HDS stands for trisulphated (TS) disaccharide (DS) component of heparin (H) oligosaccharides. TS-HDS has been identified as target carbohydrate antigen of some IgM proteins. These IgM immunoglobulins associate with a painful, predominantly sensory, polyneuropathy syndrome with axonal loss and deposition of C5b-9 complement molecules around veins [48]. The role of these IgM in the pathogenesis of the neuropathy remains to be determined. Clinically, some patients with advanced mold-related illness present with symptoms of polyneuropathy and vasculitis.
To clarify the mechanisms of neurological symptoms reported by many patients with advanced mold-related illness, we decided to estimate the levels of IgG autoantibodies to α1/2-adrenergic, and β1/2-adrenergic and muscarinic acetylcholine receptors (m1-m5ACh), endothelin-1 type A, angiotensin II, and FGFR3. In addition, we evaluated the levels of the IgM-class antibodies targeting the disaccharide component of glycosylation of heparin and heparin sulfate (TS-HDS). In other words, the aims were to obtain some proof for the concept that the chronic exposure to indoor air molds can be related inter alia to the development of an imbalance of the peripheral nervous system and the development of autoimmunity. One goal of this study was to narrow the gap between the so-called “unexplained medical condition” and evidence-based immunopathophysiology.
MATERIALS AND METHODS
Patients and matched controls
We collected sera from 42 exposed individuals and 38 age and sex matched controls. All 42 individuals replied to our first screening questionnaire. The presence of autoantibodies was analyzed in 25 patients who were selected according to the severity of their symptoms, and in 25 sex and age-matched controls. Thereafter, to confirm the correlation between the symptoms and laboratory findings, we devised an extended questionnaire through Google Forms (Appendix A) that inquired about the demographic data, social status and a history of their exposure to dampness microbiota. In addition, the patients were asked about their co-morbidities, conditions that might have a confounding effect on the results such as silicone breast implants, tattoos, smoking, their own personal and family history of autoimmune diseases and current medication, etc. (Table 1). From our clinical experience, we were aware that advanced SBS is associated with the development of MCS, and sensitivity to electromagnetic fields (EMF), and therefore we collected information on the prevalence of these symptoms and the duration of these conditions in our study cohort. Twenty-three out of 25 patients filled in our questionnaire.
The patients were enrolled from the whole of Finland via social media. Some of the patients were known to one of the authors (SH) and some of them had participated in our earlier studies [17,18]. The inclusion criteria for the patients were: 1). Prolonged symptoms during an exposure to moldy environment in their workplace or at home. 2). Neurologic and cardiac symptoms that were associated with the exposure. 3). No known other cause of neurologic or cardiac symptoms such as fatigue, paresthesia, or palpitation. Each patient had an age and sex matched healthy control who had known exposure to dampness microbiota either at home or at his/her workplace. The exclusion criteria for the controls were: 1). No known prolonged symptomatic history of the exposure to mouldy environments. 2). No known chronic neurologic or cardiac diseases or symptoms. Neither migraine nor smoking were on the exclusion list. Although our patients had a history of long-lasting exposure to indoor air mold and manifestations of chronic mold-related illness, we will refer to them as SBS patients because they were not interviewed according to Prof. Valtonen’s questionnaire [16] and therefore DMHS could not be confirmed.


Venepuncture, sample shipment and storage
The patients attended their local clinical laboratories or contacted our clinicians for venous blood which was withdrawn into three 9 mL vacutainer tubes without gel. The blood was centrifuged after 30 min at 3000 g and the serum fraction was divided into 3 aliquots and sent overnight at room temperature to the Pathology Department of Lapland Central Hospitalwhere the aliquots were stored at -80°C until retrieved and sent on dry ice for autoantibody analyses to CellTrend.
Determination of autoantibodies with ELISA
Altogether we detected 13 autoantibodies: the IgG-class anti-adrenergic receptors (α1, α2, β1, β2), cholinergic anti-muscarinic receptors (m1-m5), fibroblast growth factor receptor 3 (FGFR3) and IgM-class antibodies to the glycosylated moieties of heparan and heparan sulphate (TS-HDS) autoantibodies. These were detected with the CE marked for Research Use Only (RUO) commercial ELISA kits whereas anti-endothelin receptor type A (ETAR) and anti-angiotensin II type 1 receptor (AT1R) autoantibodies were detected with the CE marked For in vitro diagnostics ELISA kits (CellTrend GmbH Luckenwalde, Germany). Each sample was analyzed in duplicate and the respective optical densities were read against the calibration curve constructed from the defined sample in five dilutions. The level of autoantibodies for each sample was expressed as arbitrary units/ml. The ELISA methods were validated according to the FDA’s “Guidance for industry: Bioanalytical method validation”.
Statistics
Initially, scale variables were plotted and tested for normality. Thereafter, all variables were assessed by descriptive statistics with either number (percentage), mean (SD) or median (minimum, maximum) and stratified by study group. Comparisons were made following Wilcoxon Signed Rank Test according to the variable’s nature and distribution. Point biserial correlations were used for the correlation to fit for the variable’s nature. All tests were 2 tailed with P<0.05 marking statistical significance, analyses and plots were made with R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
RESULTS
Demographics, family history and exposure data
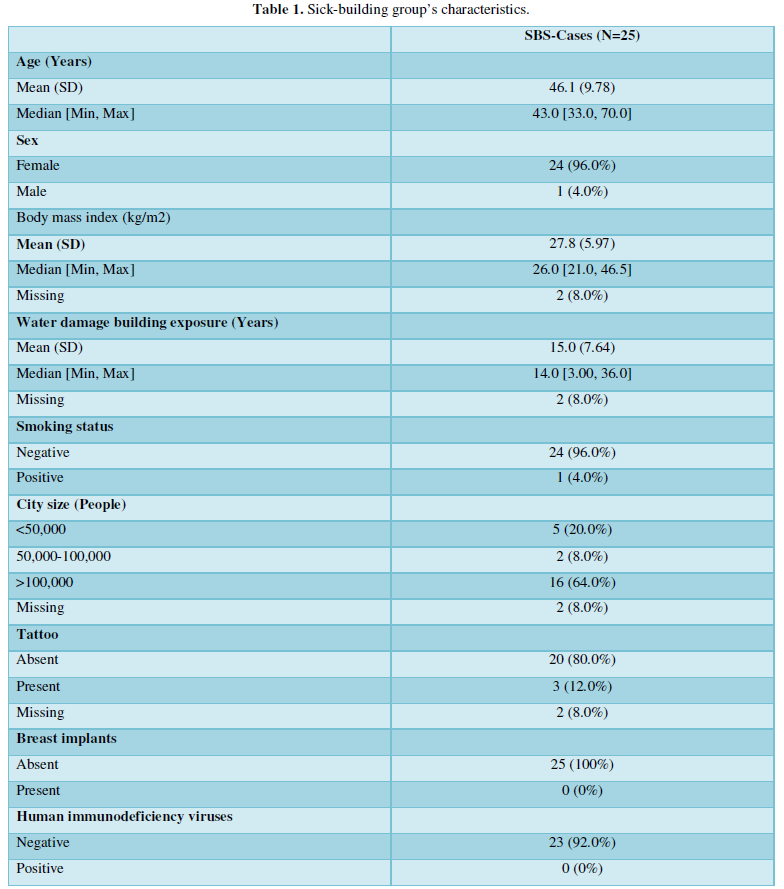
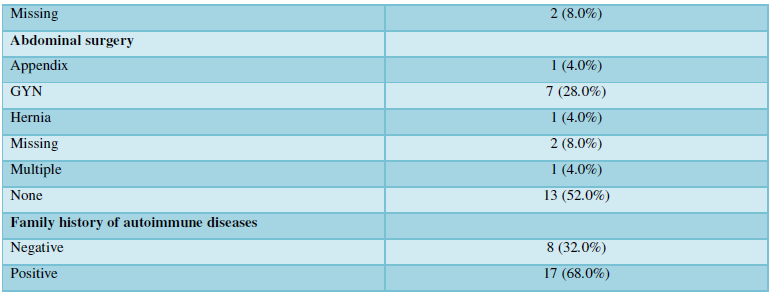
The mean age of the 25 patients was 46 years (min 33 - max 70), all except one were females. The average age of the corresponding controls was 45 years old (min 30 - max 62), three were males and 22 females. There were no obese patients, all except one were non-smokers, three reported that they had tattoos, none of them had silicone breast implants, 23/25 were HIV negative and two did not reply to this question. In the patients, the average exposure time to moisture dampness was 15 years (min 3 - max 36). Interestingly, a family history for autoimmune disease was reported by 17 patients (68%). These data were not available from the controls. A summary of the patients’ characteristics is presented in the (Table 1).
The symptoms and co-morbidities reported by the patients
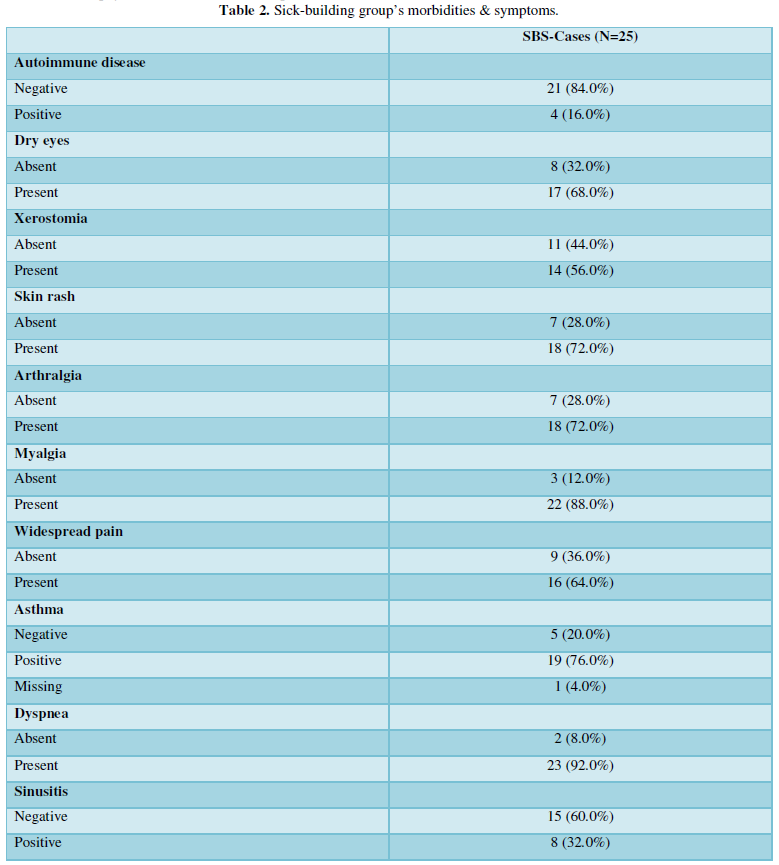
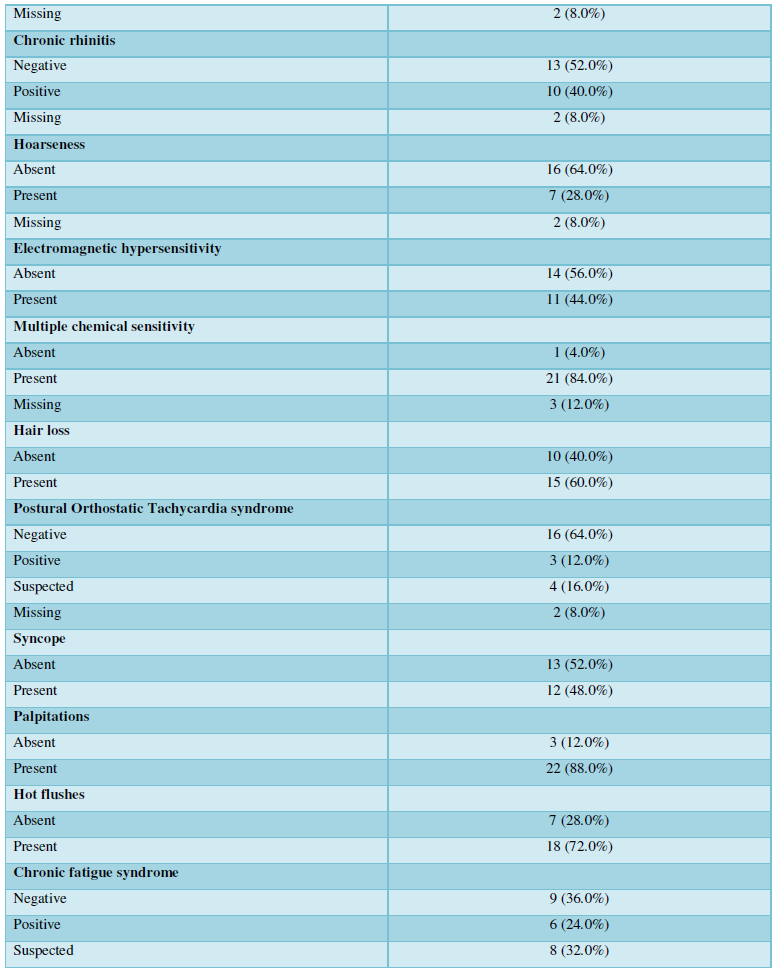
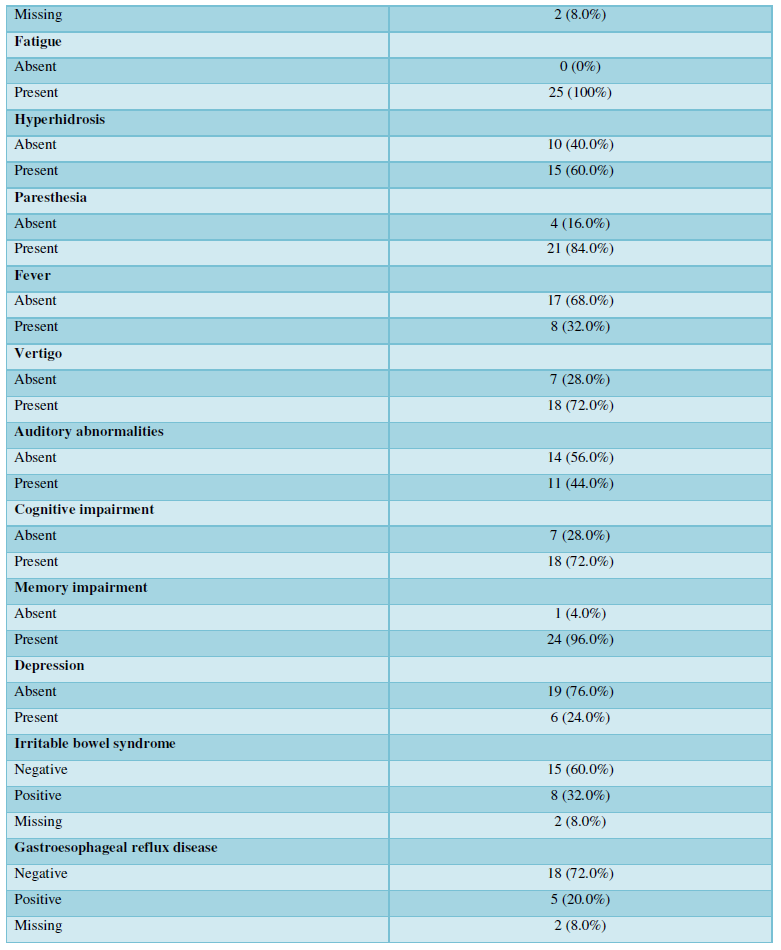
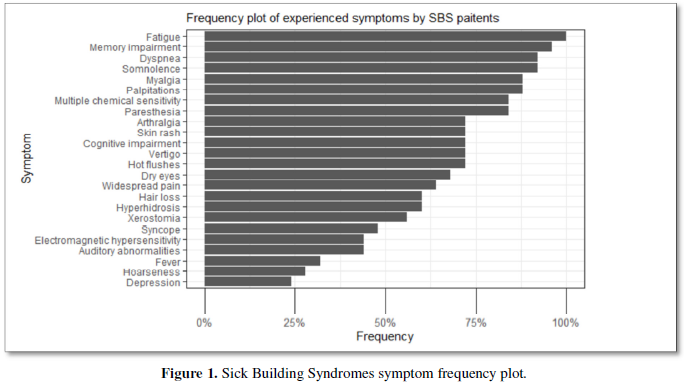
As expected, asthma was very prevalent among the diagnosed diseases 19/24 (76%). An inflammation of the upper respiratory tract was also prevalent: chronic sinusitis and chronic rhinitis were reported by 38/23 (32%) and 10/23 (40%) of the participants, respectively. Importantly, 6/25 (24%) of patients had chronic fatigue syndrome, and in a further 8/23 (32%), this was suspected; in fact, all the patients reported profound fatigue. The features of the advanced mold-related disease such as the development of MCS was reported by 21/22 (84%). Cognitive, memory problems, depression and somnipathy as the signs of a central nervous system dysfunction were prevalent; myalgias, arthralgias and widespread pain were also reported by most of the patients. The patients for this study were enrolled on the basis of the presence of dysautonomia that was either physician-diagnosed or suspected (POTS, palpitations, paraesthesia, hyperhidrosis, thermoregulation problems, etc.). These symptoms were very prevalent. The diseases and symptoms reported by patients and grouped by the anatomical locations are presented in the Table 2, and the frequency plot of the symptoms is illustrated in Figure 1.




Autoantibodies
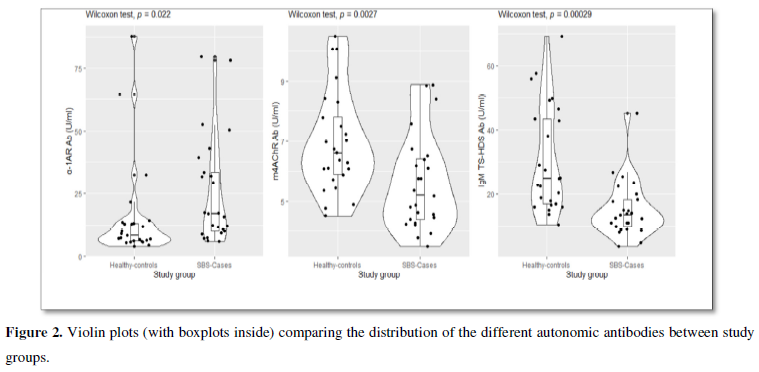
There was no statistically significant difference between the levels of the following autoantibodies AGTR1, ETAR1, α2AR, β1AR, β2AR, m1AChR, m2AChR, m3AChR, m5AChR and FGFR3 when comparing the patients’ levels with the corresponding values in the controls. The levels of α1AR antibodies were significantly elevated in patients compared to the controls, p= 0.022. In contrast, the amounts of IgG m4AChR and IgM TS-HDS antibodies were significantly decreased in the study cohort, p= 0.008 and p<0.001, respectively (Figure 2).
There was no correlation between the abnormal levels of autoantibodies (Spearman’s correlation test). Negative correlations were found between the presence of m1AChR with respect to memory impairment and somnolence, r = -0.98 and -0.66 respectively and m5AChR for the same symptoms, r = -0.61 and -0.43, but the significance of these findings remains unclear.

DISCUSSION
Here we present laboratory-based evidence of the dysregulation of the autonomous nervous system in patients with advanced SBS. We found a statistically significant elevation of the levels of IgG α1AR autoantibodies and a reduction of IgG levels towards m4ACh autoantibodies and IgM antibodies against TS-HDS. Although at this stage of knowledge, the detection of these autoantibodies cannot be used for individualized patient counselling, they may be useful when trying to clarify the mechanisms behind the many “unexplained symptoms” experienced by the occupants of moisture-damaged buildings. By having simultaneously evaluated age and sex-matched controls, we have shown at a group level that there is an imbalance in the relative quantities of these autoantibodies.
Patients with advanced mold-related illness often complain about fatigue, cognitive impairment, arthralgia, myalgia, pyrexia, dry eyes, cardiac palpitation, self-reported POTS, paraesthesia, thermoregulation problems etc. Many of these complaints point to an imbalance of the autonomic nervous system as well as an involvement of the central nervous system. Indeed, the prevalence of profound fatigue was reported by every individual in our study group. Furthermore, every second patient experienced other symptoms such as memory impairment, somnolence and cognitive impairment suggesting some degree of impairment of the central nervous system. By definition, this patient group had a high prevalence of symptoms indicative for autonomous dysregulation, such as palpitations, paraesthesia, vertigo, syncope. The prevalence of asthma and symptoms of the chronic inflammation of the upper respiratory tract were high which agrees with the published data [34,35]. In contrast, auditory abnormalities have not yet been linked to the chronic form of SBS. In agreement with our earlier findings, chronic mold-related illness was associated with the development of environmental hypersensitivities described by many patients, e.g., 21/25 (84%) suffered from MCS. These hypersensitivities are usually not inquired by treating physicians, and therefore they will remain undiagnosed. One unexpected finding was high prevalence of autoimmune disorders in the families, 68%. This prevalence is higher than generally in a non-selected Finnish population. Unfortunately, we do not have respective data for the controls. From their responses of the control group to our questionnaire, they did not appear to exhibit elevated risks for autonomous dysregulation attributable to other external confounding factors, such as tattoos or silicone breast implants. Therefore, it seems plausible that prolonged exposure (15 years on the average) to dampness microbiota may well have been the causative factor behind the reported symptoms in the patients.
Indoor air dampness microbiota release biotoxins, microbial nanoparticles, microbial volatile organic compounds, and decay products from the building materials and a prolonged exposure to these compounds may evoke a low-grade inflammation and neuroinflammation. It is possible that they can stimulate some parts of the immune system while simultaneously blocking the function of other parts. Many indoor air mycotoxins are both neurotoxic and cytotoxic [32]. At the cellular level, they may increase the production of reactive oxygen species (ROS), inhibit ATP synthesis, alter mitochondrial membrane potential (ΔΨ) and facilitate the release of mitochondrial proteins into the cytosol [49]. Exposure to mycotoxins activates the inflammasome machinery in the cell that triggers the production of the pro-inflammatory cytokine, IL-1β, a marker of the activation of innate immunity [50]. When IL-1β cytokine binds to its cognate receptor, IL-1R1, and the consequent intracellular signal transduction will lead to a vicious cycle of inflammation [51]. The breakdown of the blood brain barrier (BBB) causes neuroinflammation [25-24] that may manifest in several forms, e.g., epilepsy [55], seizures [56] and thermoregulation problems [57], the conditions reported by DMHS patients.
Some of the symptoms experienced by patients with advanced mold-related illness (such as fatigue, memory and cognitive impairment, and somnolence) are like those reported by the silicon breast implant (SBI) patients. The prolonged exposure (>10 years) for both SBS (dampness microbiota) and SBI (silicone material) conditions to the foreign non-self-material might be a potential causative factor for the reported subjective and “unexplained” symptoms experienced by the patients. Implanted silicone is currently considered as an adjuvant that may 'bleed' and migrate from the implant into the body and subsequently act as a chronic stimulus for the immune system. Silicones are distributed throughout the body and can be detected in tissues and the central nervous system. Likewise, the chronic inhalation of microbial components may also act as a continual stimulus for the immune system. Mycotoxins may disrupt the blood brain barrier (BBB) and penetrate into the brain via the circulation or directly through nervus olfactorius [58]. Chronic stimulation of the immune system e.g., by silicone or microbial components, has been reported to trigger the development of an autoimmunity and inflammatory condition currently named ASIA (autoimmune/inflammatory syndrome caused by adjuvants) [59]. Although the topic of parenteral adjuvants is not relevant for SBS, like many other conditions, this is a clinical syndrome where adjuvants play a decisive role. Mucosal fungi colonization, dysbiosis in the gut and the overgrowth of a commensal yeast Candida (often diagnosed in chronic SBS patients) may act as an intrinsic adjuvant and stimulate immune cells to produce broad-spectrum antibodies, some protective [60] but some also harmful. The production of autoantibodies in this scenario cannot be excluded. There are other similarities between SBS and SBI; for example, SBI patients may also experience an amelioration of the symptoms after the removal of the silicone implant and early SBS may revert to healthy state by avoidance of the mouldy environment as long as the precautions are taken early enough. A higher rate of autoimmune conditions, immune deficiencies and even malignancies have been documented in both clinical entities [61-36]. Nonetheless, there are also some clinical dissimilarities between the SBI and advanced SBS. Patients with SBS often complain about the disappearance of the voice, cough, dyspnoea, other pulmonary manifestations and there was a high prevalence of these symptoms in our study group (Figure 1). Physician-diagnosed diseases of the respiratory tract, such as atypical or IgE-driven asthma, are not uncommon. Patients exposed to indoor air molds are prone to recurrent infections and reactivation of latent infections, such as herpes simplex virus and develop different types of hypersensitivities, with MCS being very common. One could argue that the MCS may represent a hyperactivation of the sensory compartment of the autonomous nervous system as a consequence of chronic stimulation of sensory receptors and the insidious presence of low-grade inflammation. Consequently, using the same ELISA technology (CellTrend) an immune imbalance was demonstrated in women with SBIs as compared with their aged matched healthy controls: instead of the expected elevation of autoantibodies, a significant decline of the following autoantibodies was detected: anti-α1AR (p<0.001), anti-AT1R (p<0.001) and anti-ETAR (p=0.001) [64].
In some other studies, elevated levels of autoantibodies have been reported. Interestingly, the levels of AT1R and ETAR autoantibodies were higher levels in patients with systemic sclerosis than in healthy or disease controls; the higher the levels, the more severe was the disease. These autoantibodies are not silent bystanders but are recognized as exerting biological effects on those endothelial cells that expressed more transforming growth factor beta (TNF-β) [65]. Likewise, in biopsy-proven SFN, autoantibodies to FRFR3 and TS-HDS were more frequent than in the controls [48]. In our study, on the contrary, the level of TS-HDS antibodies was lower in comparison to the age and sex-matched healthy controls. One plausible reason for this discrepancy is that the ELISA methods available in clinical immunology are poorly standardized. Indeed, the purification and preservation protocols of the conformational epitopes or probable post-translational modification of recombinant antigens are not reported by the manufacturers. There might also be biological reasons for this inconsistency; the autoantibodies measured from the circulation might be bound to the respective tissue antigens or alternatively the dysregulation might be caused by the natural anti-idiotypic antibody network that traps the autoantibodies, forming immune complexes. These speculations will need to be examined in the future with more sophisticated assay techniques. Although it is important to compare the levels of antibodies in disease subjects to those of matched controls, it would be even more interesting to clarify the functions of these specific antibodies e.g., using recombinant cell lines that overexpress human receptors.
The two limitations of our study are that we analyzed a relatively small cohort of patients with advanced SBS and that we were unable to collect more information on the controls to examine how well these individuals match the patients in terms of possible confounding factors.
The strength of this study is that this is the first time that an imbalance of the autonomic nervous system has been linked with the symptoms experienced by individuals with SBS. This imbalance was evident in the responses to the questionnaire and in the reported symptoms. The clinical findings were supported by laboratory data. All the samples were analyzed in a blinded manner in terms of group assignment. Another strength is that we used novel ELISA techniques to detect autoantibodies to 13 different autoantigens.
CONCLUSION
An imbalance in the amounts of autoantibodies has been demonstrated. While not yet allowing customized patient counselling, this finding provides additional evidence that chronic mold-related illness is not an anxiety-driven condition but has a pathophysiological foundation. We encourage clinicians to take this condition seriously and call for WHO to provide an ICD-11 code for chronic mold-related illness to improve the patient’s medical management and their social security.
ETHICAL CONSIDERATION
We obtained an Ethical recommendation from the Ethical Committee from the Northern Ostrobothnia, EETTMK 10/ 2020 to study biomarkers in patients exposed to dampness microbiota.
AUTHOR CONTRIBUTIONS
Conceptualization, J.L., T.T. and A.W..; methodology, S.H., I.K., K.V., J.L.; software, I.K.; validation, G.H. and H.H.; formal analysis, G.H., A.W., H.A.; investigation, S.H.; resources, H.H., Y.S.; data curation, G.H.; writing - original draft preparation, T.T.; writing - review and editing, I.K.; G.H., K.V., visualization, I.K.; supervision, Y.S.; project administration, Y.S.; funding acquisition, Y.S, H.H.; All authors have read and agreed to the published version of the manuscript.
FUNDING
This research received no external funding.
ACKNOWLEDGMENTS
We thank all the patients and the controls who volunteered to participate in our study without any monetary or any other form of compensation or benefit. Cell Trend is acknowledged for analyzing all the samples.
DEDICATION
This work is dedicated to all the sufferers from an indoor air moisture-damaged environment who have not yet obtained sufficient medical or social help.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Appendix A. The questionnaire for the patients.
Demographic, social status and basic information on the exposure
Hight
Weigh
Unemployment yes / no
Has the incidence of moisture damage affected the income yes / no
Caused unemployment yes / no
Caused disability yes / no
Caused partial incapacity for work yes / no
Residence size:
less than 10,000
10-50,000
50-100,000
more than 100,000:
Duration of moisture damage exposure in years (e.g., 1996-2004) kindergarten, school, study, workplace, home.
Confounding conditions
Smoking yes / no
Tattoo yes / no, how many?
Silicone breast implants yes / no
Audit score: Risks of alcohol use AUDIT-laskuri / testi | Laskurini.fi
HIV infection yes / no
Gastric surgery: Weight loss surgery, gastric hernia surgery, bile surgery, year:
Family history for autoimmune diseases yes / no
Any of: Diabetes type1 / coeliac disease / Inflammatory bowel disease / hypothyroidism / arthritis
rheumatoid / SLE / autoimmune hepatitis / MS / platelet disease / pernicious anemia / other
autoimmune disease / no autoimmune diseases
Autoimmune diseases before SBS yes /no
Have you had before the present contact?
Elevated CRP/ ESR/ ANA-Ab/ RF/ CCPAb/ ANCA-Ab/ ENA-Ab?
Common symptoms reported by ASIA patients:
Normal vision (with glasses) yes / no
Dry eyes yes / no
Dry mouth yes / no
Hearing abnormalities yes / no
Sleep disturbances yes/ no
Memory impairment yes / no
Depression yes / no
Cognitive impairment yes / no
Palpitations yes / no
Hair loss yes / no
Headache yes / no
Vertigo yes / no
Heat sensation yes / no
Arthralgia yes / no
Myalgia yes / no
Widespread pain yes / no
Diarrhea yes / no
Constipation yes / no
Dyspnea yes / no
Paresthesia yes / no
Excessive sweating yes / no
Syncope yes / no
Skin rash yes / no
Tachycardia yes / no
Weight loss yes / no
Arthritis yes/ no
Myositis yes / no
Fever (over 37.5 °C) yes / no
Allergic reactions yes / no
Fatigue yes / no
Lymphadenopathy yes / no
Symptoms often reported by DMHS patients:
Migraine yes / no
Biological treatment of migraine yes / no
Asthma yes / no
COPD yes / no
Reflux disease yes / no
Celiac disease yes / no
Food intolerances (to what allergens?) yes / no
Dietary restrictions yes / no
Inflammatory bowel disease yes / no
Irritable bowel syndrome yes / no
Recurrent or chronic sinusitis yes / no
Sinus surgery, punctures yes / no
Ear infections yes / no
Chronic rhinitis yes / no
Laryngeal lesions, yes / no
Allergic alveolitis, yes / no
Head or neck injuries with residual disability yes / no, year
Pituitary hormone abnormality, yes / no
What is the anomaly, if known:
Multiple chemical sensitivity (MCS) yes / no
Duration of MCS (how many years)
Sensitivity to electromagnetic fields (how many years) yes / no
- Sundell J (2017) Reflections on the history of indoor air science, focusing on the last 50 years. Indoor Air 27(4): 708-724.
- Skov P (1992) The sick building syndrome. Ann N Y Acad Sci 641: 17-20.
- Riesenberg DE, Arehart-Treichel J (1986) “Sick building” syndrome plagues workers, dwellers. JAMA 255(22): 3063.
- Robertson A (1989) Sick building syndrome. Practitioner 233(1475): 1250-1252.
- Lyles WB, Greve KW, Bauer RM, Ware MR, Schramke CJ, et al. (1991) Sick building syndrome. South Med J 84(1): 65-71, 76.
- Ishibashi M, Tonori H, Miki T, Miyajima E, Kudo Y, et al. (2007) Classification of patients complaining of sick house syndrome and/or multiple chemical sensitivity. Tohoku J Exp Med 211(3): 223-233.
- Hicks JB (1984) Tight building syndrome: when work makes you sick. Occup Health Saf pp: 51-56.
- Straus DC (2009) Molds, mycotoxins, and sick building syndrome. Toxicol Ind Health 25(9-10): 617-635.
- Straus DC (2011) The possible role of fungal contamination in sick building syndrome. Front Biosci (Elite Ed) 3: 562-580.
- Pieckova E (2012) Adverse health effects of indoor moulds. Arh Hig Rada Toksikol 63(4): 545-549.
- Newman A (1995) Do microbes contribute to sick building syndrome? Environ Sci Technol 29(10): 450A.
- Sauni R, Verbeek JH, Uitti J, Jauhiainen M, Kreiss K, et al. (2015) Remediating buildings damaged by dampness and mould for preventing or reducing respiratory tract symptoms, infections and asthma. Cochrane Database Syst Rev 2: CD007897.
- Chang CC, Ruhl RA, Halpern GM, Gershwin ME (1993) The sick building syndrome. I. Definition and epidemiological considerations. J Asthma 30(4): 285-295.
- Assoulin-Daya Y, Leong A, Shoenfeld Y, Gershwin ME (2002) Studies of sick building syndrome. IV. Mycotoxicosis. J Asthma 39(3): 191-201.
- Chang C, Gershwin ME (2004) Indoor air quality and human health: Truth vs mass hysteria. Clin Rev Allergy Immunol 27(3): 219-239.
- Valtonen V (2017) Clinical Diagnosis of the Dampness and Mold Hypersensitivity Syndrome: Review of the Literature and Suggested Diagnostic Criteria. Front Immunol 8: 951.
- Hyvonen S, Lohi J, Tuuminen T (2020) Moist and Mold Exposure is Associated With High Prevalence of Neurological Symptoms and MCS in a Finnish Hospital Workers Cohort. Saf Health Work 11(2): 173-177.
- Hyvonen S, Poussa T, Lohi J, Tuuminen T (2020) High prevalence of neurological sequelae and multiple chemical sensitivity among occupants of a Finnish police station damaged by dampness microbiota. Arch Environ Occup Health pp: 1-7.
- Hyvonen SM, Lohi JJ, Rasanen LA, Heinonen T, Mannerstrom M, et al. (2020) Association of toxic indoor air with multi-organ symptoms in pupils attending a moisture-damaged school in Finland. Am J Clin Exp Immunol 9(5): 101-113.
- Empting LD (2009) Neurologic and neuropsychiatric syndrome features of mold and mycotoxin exposure. Toxicol Ind Health 25(9-10): 577-581.
- Welch LS (1991) Severity of health effects associated with building-related illness. Environ Health Perspect 95: 67-69.
- Welch LS, Sokas R (1992) Development of multiple chemical sensitivity after an outbreak of sick-building syndrome. Toxicol Ind Health 8(4): 47-50.
- Thorn A, Lewne M, Belin L (1996) Allergic alveolitis in a school environment. Scand J Work Environ Health 22(4): 311-314.
- Kumar SN, Telang AG, Singh KP, Jain AK, Afroz M, et al. (2011) Experimentally Induced Toxicity of Ochratoxin A and Endosulfan in Male Wistar Rats: A Hormonal Disorder. J Anim Vet Adv 10: 1750-1755.
- Hassan AA, Rashid MA, Koratum KM (2010) Effect of Aflatoxin B1, Zearalenone and Ochratoxin A on Some Hormones Related to Fertility in Male Rats. Life Sci J 7: 64-72.
- Reddy KE, Jeong JY, Lee Y, Lee HJ, Kim MS, et al. (2018) Deoxynivalenol- and zearalenone-contaminated feeds alter gene expression profiles in the livers of piglets. Asian-Australas J Anim Sci 31(4): 595-606.
- Somppi TL (2017) Non-Thyroidal Illness Syndrome in Patients Exposed to Indoor Air Dampness Microbiota Treated Successfully with Triiodothyronine. Front Immunol 8: 919.
- Dennis D, Robertson D, Curtis L, Black J (2009) Fungal exposure endocrinopathy in sinusitis with growth hormone deficiency: Dennis-Robertson syndrome. Toxicol Ind Health 25(9-10): 669-680.
- Tuuminen T, Lohi J (2018) Immunological and toxicological effects of bad indoor air to cause Dampness and Mold Hypersensitivity Syndrome. AIMS Allergy Immunol pp: 14.
- Tuuminen T, Lohi J (2018) Revising the criteria for occupational mold-related disease: Arguments, misconceptions and facts. EMJ Allergy Immunol 3: 128-135.
- Tuuminen T, Lohi J (2018) Dampness and Mold Hypersensitivity Syndrome is a biotoxicosis that should be diagnosed promptly. In Adv Clin Toxicol [Online] pp: 1-4.
- Tuuminen T, Vaali K, Valtonen V 2019) Dampness and Mold Hypersensitivity Syndrome as an Umbrella for Many Chronic Diseases - The Clinician’s Point of View. Elsevier: Amsterdam, The Netherlands, Vol: 2.
- Tuuminen T (2020) The Roles of Autoimmunity and Biotoxicosis in Sick Building Syndrome as a “Starting Point” for Irreversible Dampness and Mold Hypersensitivity Syndrome. Antibodies (Basel) 9(2).
- US, I. o. M (2004) In Damp Indoor Spaces and Health, Washington (DC).
- WHO (2009) Damp and Mould: Health Risks, Prevent and Remedial Actions. Organization, W. H., Ed. Copenhagen.
- Campbell AW, Thrasher JD, Madison RA, Vojdani A, Gray MR, et al. (2003) Neural autoantibodies and neurophysiologic abnormalities in patients exposed to molds in water-damaged buildings. Arch Environ Health 58(8): 464-474.
- Gray MR, Thrasher JD, Crago R, Madison RA, Arnold L, et al. (2003) Mixed mold mycotoxicosis: immunological changes in humans following exposure in water-damaged buildings. Arch Environ Health 58(7): 410-420.
- Ryabkova VA, Churilov LP, Shoenfeld Y (2019) What Role for Autoimmunity, Neuroinflammation, and Small Fiber Neuropathy in Fibromyalgia, Chronic Fatigue Syndrome, and Adverse Events after Human Papillomavirus Vaccination? Int J Mol Sci pp: 20.
- Ruzieh M, Batizy L, Dasa O, Oostra C, Grubb B (2017) The role of autoantibodies in the syndromes of orthostatic intolerance: A systematic review. Scand Cardiovasc J 51(5): 243-247.
- Gunning WT, Kvale H, Kramer PM, Karabin BL, Grubb BP (2019) Postural Orthostatic Tachycardia Syndrome Is Associated with Elevated G-Protein Coupled Receptor Autoantibodies. J Am Heart Assoc 8(18): e013602.
- Wirth K, Scheibenbogen C (2020) A Unifying Hypothesis of the Pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Recognitions from the finding of autoantibodies against ss2-adrenergic receptors. Autoimmun Rev 19(6): 102527.
- Shoenfeld Y, Agmon-Levin N (2011) ‘ASIA’ - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun 36(1): 4-8.
- Fedorowski A, Li H, Yu X, Koelsch KA, Harris VM, et al. (2017) Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace 19(7): 1211-1219.
- Tuuminen T, Jaaskelainen T, Vaali K, Polo O (2019) Dampness and mold hypersensitivity syndrome and vaccination as risk factors for chronic fatigue syndrome. Autoimmun Rev 18(1): 107-108.
- de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T (2000) International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52(3): 415-472.
- Boron WF, Boulpaep EL (2009) Medical physiology a cellular and molecular approach 2nd; Elsevier: Philadelphia, PA.
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, et al. (1996) Receptor specificity of the fibroblast growth factor family. J Biol Chem 271(25): 15292-15297.
- Levine TD, Kafaie J, Zeidman LA, Saperstein DS, Massaquoi R, et al. (2020) Cryptogenic small-fiber neuropathies: Serum autoantibody binding to trisulfated heparan disaccharide and fibroblast growth factor receptor-3. Muscle Nerve 61(4): 512-515.
- Tonshin AA, Teplova VV, Andersson MA, Salkinoja-Salonen MS (2010) The Fusarium mycotoxins enniatins and beauvericin cause mitochondrial dysfunction by affecting the mitochondrial volume regulation, oxidative phosphorylation and ion homeostasis. Toxicology 276(1): 49-57.
- Kankkunen P, Valimaki E, Rintahaka J, Palomaki J, Nyman T, et al. (2014) Trichothecene mycotoxins activate NLRP3 inflammasome through a P2X7 receptor and Src tyrosine kinase dependent pathway. Hum Immunol 75(2): 134-140.
- Said-Sadier N, Ojcius DM (2012) Alarmins, inflammasomes and immunity. Biomed J 35(6): 437-449.
- Meggs WJ (1993) Neurogenic inflammation and sensitivity to environmental chemicals. Environ Health Perspect 101(3): 234-238.
- Meggs WJ (1995) Neurogenic switching: A hypothesis for a mechanism for shifting the site of inflammation in allergy and chemical sensitivity. Environ Health Perspect 103(1): 54-56.
- Meggs WJ (1997) Hypothesis for induction and propagation of chemical sensitivity based on biopsy studies. Environ Health Perspect, 105(Suppl 2): 473-478.
- Vezzani A, Baram TZ (2007) New roles for interleukin-1 Beta in the mechanisms of epilepsy. Epilepsy Curr 7(2): 45-50.
- Balosso S, Maroso M, Sanchez-Alavez M, Ravizza T, Frasca A, et al. (2008) A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1beta. Brain 131(Pt 12): 3256-3265.
- Sanchez-Alavez M, Tabarean IV, Behrens MM, Bartfai T (2006) Ceramide mediates the rapid phase of febrile response to IL-1beta. Proc Natl Acad Sci U S A 103(8): 2904-2908.
- Islam Z, Amuzie CJ, Harkema JR, Pestka JJ (2007) Neurotoxicity and inflammation in the nasal airways of mice exposed to the macrocyclic trichothecene mycotoxin roridina: Kinetics and potentiation by bacterial lipopolysaccharide coexposure. Toxicol Sci 98(2): 526-541.
- Soriano A, Butnaru D (2014) Shoenfeld Y Long-term inflammatory conditions following silicone exposure: the expanding spectrum of the autoimmune/inflammatory syndrome induced by adjuvants (ASIA). Clin Exp Rheumatol 32(2): 151-154.
- Doron I, Leonardi I, Li XV, Fiers WD, Semon A, et al. (2021) Human gut mycobiota tune immunity via CARD9-dependent induction of anti-fungal IgG antibodies. Cell 184(4): 1017-1031 e14.
- Tuuminen T, Rinne KS (2017) Severe Sequelae to Mold-Related Illness as Demonstrated in Two Finnish Cohorts. Front Immunol 8: 382.
- Watad A, Rosenberg V, Tiosano S, Tervaert CJW, Yavne Y, et al. (2018) Silicone breast implants and the risk of autoimmune/rheumatic disorders: A real-world analysis. Int J Epidemiol 47(6): 1846-1854.
- Watad A, Bragazzi NL, Amital H, Shoenfeld Y (2019) Hyperstimulation of Adaptive Immunity as the Common Pathway for Silicone Breast Implants, Autoimmunity, and Lymphoma of the Breast. Isr Med Assoc J 21(8): 517-519.
- Halpert G, Watad A, Tsur AM, Dotan A, Quiros-Lim HE, et al. (2021) Autoimmune dysautonomia in women with silicone breast implants. J Autoimmun 120: 102631.
- Riemekasten G, Philippe A, Nather M, Slowinski T, Muller DN, et al. (2011) Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis 70(3): 530-536.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Advance Research on Alzheimers and Parkinsons Disease


