Research Article
Retrospective Evaluation of Hepatitis B Seroprevalence in Children Followed in a Family Health Center
680
Views & Citations10
Likes & Shares
Background: The aims of this study are to determine hepatitis B seroprevalence and vaccination rates in children aged 4-10 years who were followed in a primary health center and to compare hepatitis B seroprevalence and vaccination rates in children before and after the introduction of family medicine model (FMM) in primary health care in Turkey.
Methods: A total of 2,500 children aged between 4-10 years registered in five units of the XXXXXX Family Health Center constituted the universe of the study. Patient records from December 2014 to May 2017 were retrospectively scanned. Hepatitis B markers of children were studied from the health records and those who met the criteria were invited to the outpatient clinic through a phone call, and verbal and written consents were obtained from their parents. Participants were randomly selected (N=415). The sociodemographic characteristics, general health status, anti-HBs and HBsAg results of the participants were obtained from the health records.
Results: Of all the participants who participated in this survey, %73,6 had an anti-HBs level of 10 mIU/mL and higher, while 26,5% had an anti-HBs level lower than 10. The anti-HBs levels were 10 mIU/mL and above in 78.8% of those born before and 10 mIU/mL and above in 70.2% of those born after FMM (p=0.033).
Conclusion: The lower anti-HBs levels in pre-FMM patients can be explained by the increased attention to the cold chain, the active use of vaccine tracking systems, and the efforts of family practitioners.
Keywords: Hepatitis B, Seroprevalence, Prevention, Vaccination, Family medicine
INTRODUCTION
Prevention of hepatitis B infection, especially in children, through vaccination is one of the most important responsibilities of primary health care services. The risk of developing chronic hepatitis after contact with the hepatitis B virus (HBV) is 5% in adults, but 30-50% in children aged 1-5 years, and 90% in children aged one year and below [1]. The primary aim of this study is to determine HBV seroprevalence and vaccination rates in children aged 4-10 years who were followed up by primary care. The secondary aim is to evaluate the difference between hepatitis B seroprevalence and vaccination rates in children before and after the family medicine system in Turkey, and the tertiary aim is to evaluate the elevation of anti-HBs values by administering a single dose vaccine to those with anti-HBs levels of
The research hypotheses are as follows:
- H0: There is no difference between children born before and after the implementation of FMM in terms of hepatitis B seroprevalence and vaccination rates.
- H1: There is a difference between children born before and after the implementation of FMM in terms of hepatitis B seroprevalence and vaccination rates.
A total of 2,500 children aged between 4-10 years registered in five units of the XXX XXX Family Medicine Center (FMC) constituted the universe of the study. There was a total of 2,500 children aged between 4-10 years and five units in the aforementioned FMC. The formula for calculating the known sample size of the universe is (n=Nt2pq/d2(N-1)+t2pq), and N=2500. The required sample size was determined to be 335 for 95% statistical power. We interviewed 426 patients that were randomly selected from the five units. A random numbers table was used for randomization. Eleven participants were excluded from the study due to incomplete data, and the study was completed with 415 participants. This study is significant in that it compares the hepatitis B serology of children born before and after the introduction of the Family Medicine Model (FMM) in XXX, Turkey. FMM was started in XXX in December 13th, 2010.
Study inclusion criteria:
- Being registered in XXX XXX Family Medicine Center
- Being aged between four and ten
- Having hepatitis markers tested between December 2014 and May 2017 for any reason
- Written and verbal parental consent for participation in the study.
Study exclusion criteria: Although they met the inclusion criteria, those whose parents did not give verbal and written consent were excluded from the study. The study was started after obtaining ethical approval from the University of Health Sciences, XXX Training and Research Hospital with the date of 22/06/2017 and decision number of 11/03. The study is an observational and retrospective study. Patient records of the XXX FMC were scanned (between December 2014 and May 2017). Hepatitis B markers were tested between the specified dates, children in the 4-10 years age group were determined and patients who met the criteria according to the records were invited to the outpatient clinic through a phone call, and verbal and written consents were obtained from their parents. The sociodemographic characteristics, general health status, anti-HBs and HBsAg results of the participants were obtained from the health records. Number, percentage, mean and standard deviation were used for descriptive statistics in the study. Normality of data distribution was determined by the Kolmogorov-Smirnov test. Numerical data were evaluated using one of the appropriate tests from the Mann-Whitney U-test, Student's t-test, the one-way ANOVA test, and the Kruskal-Wallis test. Categorical data were evaluated using the Fisher's chi-square test. Data were analyzed using the SPSS version 18.0 at a confidence interval of 95% and a statistical significance level of p <0.05.
The research algorithm showing each phase of the study, participants and those who were excluded from the study is given in Figure 1.
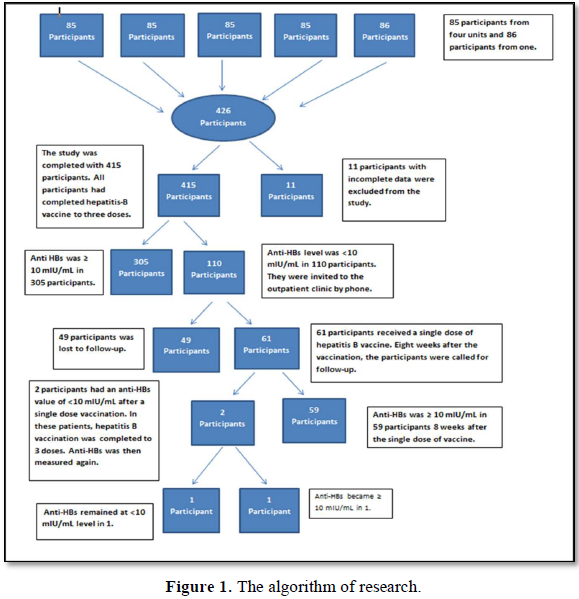
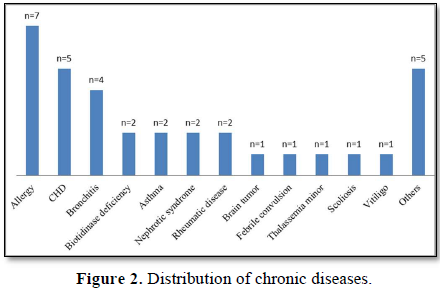
The study included 415 participants between the ages of 4-10. Eleven participants were excluded from the study due to incomplete data. The mean age of the participants was 7.07±1.74 years. The distribution of the participants' chronic diseases is presented in Figure 2.
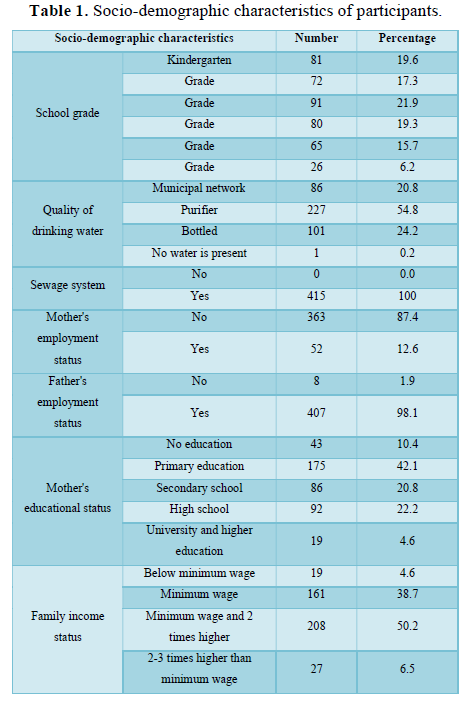
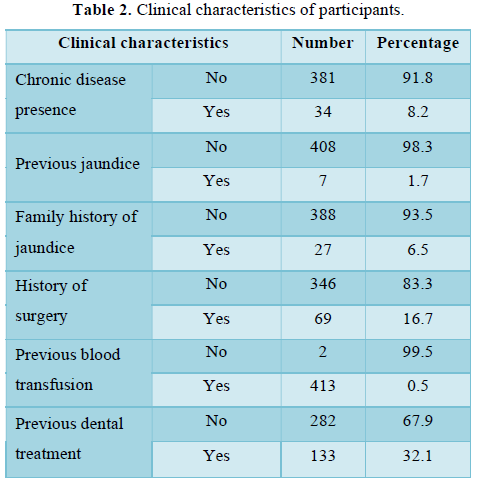
All participants had received all vaccines required by the National Vaccination Program. There were no participants who developed an adverse reaction to vaccination and/or had a contraindication to vaccine. Some socio-demographic and clinical descriptive characteristics of the participants are presented in Table 1 and Table 2.
Of the participants, 26.5% (n=110) had insufficient level of anti-HBs (
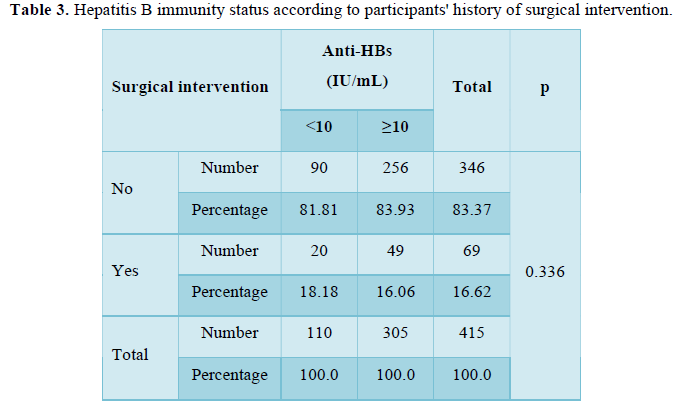


Of the participants, 26.5% (n=110) had insufficient level of anti-HBs (

The participants were divided into two groups: those who were born before and after December 13th, 2010, which is the date of starting FMM (Figure 3).

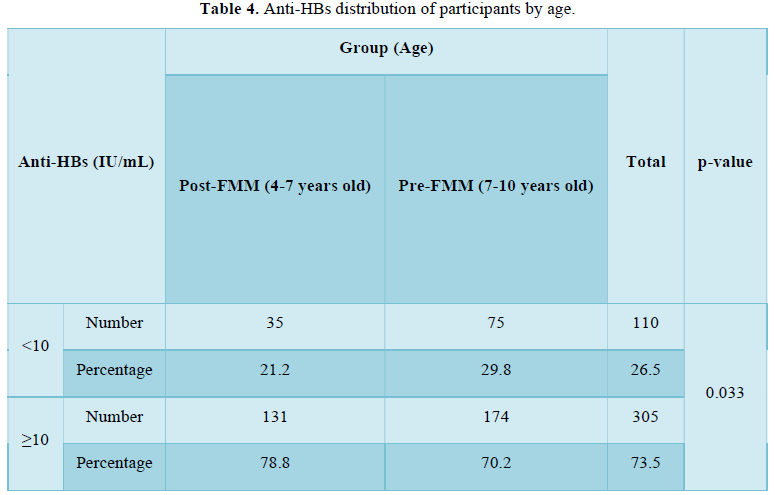
Considering an anti-HBs level of ≥10 mIU/mL as positive and an anti-HBs level of Table 4).

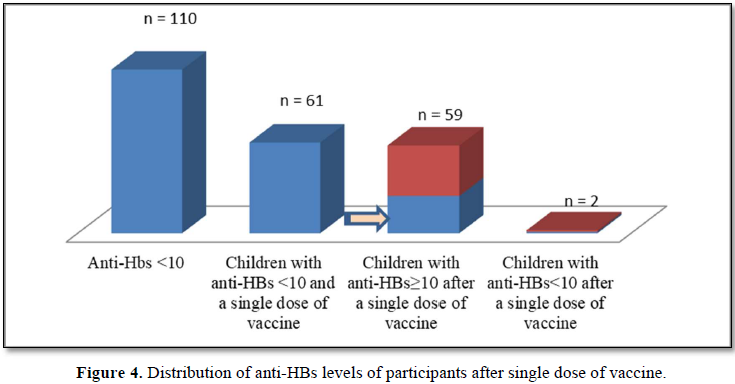
One hundred and ten patients with an anti-HBs level of 10 mIU/mL, while the anti-Hbs levels of two children (3.28%) did not (p=0.000) (Figure 4).
We found that 26.5% of the patients were seronegative and 73.5% were seropositive. A similar study by Iscan et al. was conducted on 180 children aged 1-12 who were admitted to the hospital preoperatively and completed their vaccine scheme. It was found that of the patients, 21.6% had an anti-HBs level below 10 mIU/mL and 78.4% had an anti-HBs level above 10 mIU/ml [2]. The study by Nalbantoglu et al. investigated hepatitis B seroprevelance and vaccination status of 302 children aged 9 months to 8 years presented to the hospital with any complaint other than hepatitis found that of the children, 13.9% were not vaccinated for hepatitis B, 2% had a single dose, 1.7% had two doses and 82.5% had three doses of hepatitis B vaccine. As a result, it was also found that 83.1% of the patients were anti-HBs positive [3]. Kader et al. investigated the seroprevalence of hepatitis B among students of the university. They found that 89.4% were anti-HBs positive, and that 120 of the anti-HBs positive students (94.5%) were immunized by vaccination; 9.2% that tested negative for HBsAg, anti-HBc IgG and anti-HBs were included in the hepatitis B vaccination program. When asked about their hepatitis B vaccination status, 57.0% mentioned that they were vaccinated, 23.2% did not know whether they were vaccinated, and 19.7% stated that they had not been vaccinated. Among the 28 students that stated they had not been vaccinated, 25 were found to be anti-HBc IgG-positive [4]. Inci et al. studied a total of 292 employees of the Kayseri Obstetrics and Pediatrics Hospital, 71.6% of which were female. They found that three (1.1%) participants were HBsAg-positive and 62.7% were anti-HBs-positive. Anti-HBs positivity was due to vaccination in 44.2% and natural causes in 18.5% [5]. Korkmaz et al. retrospectively studied the health records of 586 healthcare workers. They found that 86% were anti-HBs positive and 0.9% were HBsAg-positive [6]. The limitations of our study are as follows: the small geographic region which limits accuracy in reflecting the entire Turkish population, and the retrospective design. Further studies are needed for more comprehensive and generally applicable results. Our results are still valuable. In that we found that 26.5% of all vaccinated children were not successfully immunized against hepatitis B. The single dose increased the immunization rate to 96.72% when these children received single dose of vaccine again. However, 26.5% of the children would be vulnerable against hepatitis B infections if they were not evaluated for anti-HBs levels. Hepatitis B immunity can be determined 4 to 8 weeks after the final dose [7]. Therefore, patients should be called in for an anti-HBs test at the eighth month, i.e., eight weeks after the third dose of the vaccine.
The financial impact of anti-HBs evaluation can be estimated as follows:
The cost of testing anti-HBs of all eight-month-olds, the cost of the booster dose of the non-immunized children, and the repeat anti-HBs measurement is 35,714,007 TRY [(number of eight-month-olds X cost of anti-HBs) + (number of anti-HBs-negative babies X (Anti-HBs cost + booster dose cost)] = [(1,301,049 X 7.6) + 343,477 (7.6 + 67.59)] = 35,714,007 TRY [8,9]. Although the prevalence of hepatitis B in Turkey varies by region, it is around 2-8%,9 with a median value of 5%. Assuming that of babies who have not developed immunity to hepatitis B and who have not been vaccinated again, 5% are infected with HBV, 90% of the infants infected with HBV would develop chronic hepatitis B due to the age group. The 10-year hepatitis B treatment cost of a two-year-old is 18,451 TRY without accounting for HCC, cirrhosis or liver failure (Lamivudine 100 mg/day and interferon 18 mIU/week for 24 weeks: 95,04 X 12 X 10 + 293.63 X 24 = 18,451 TRY) [10,11]. Therefore, the ten-year treatment cost of patients that will likely develop chronic hepatitis B would cost 2,851,897,266 TRY. In short, preventive measures that will cost 35,714,007 TRY (5,912,915 USD as of 2019) can prevent spending 285,344,715 TRY (47,216,841 USD as of 2019) in treatment costs. After the completion of hepatitis B vaccination; the children can be evaluated for anti-HBs prior to starting pre-school, where they will be participant to more human contact, in order to make up for potentially missed vaccination opportunities.
- Akarca US (2010) Hepatitis B. In: Tinci C, Dabak R, editors. Gastroenterology, hepatology, diagnosis and treatment. Istanbul: Offset Preparation. pp: 255-272.
- Iscan G, Tasar MA (2018) The Evaluation of Pre-Op Anti-Hbs Levels at the Children Who Were Completed the Vaccine Schedule. Konuralp Tıp Dergisi 10(2): 244-247.
- Nalbantoglu B, Nalbantoglu A, Kulcu NU, Say A (2008) Seroprevalence of Hepatitis B and Immunization Status of Children Aged Between 9 Months - 8 Years Old. J Child 10(3): 116-121.
- Kader C, Yolcu S, Erbay A, Akca KN, Yuzer S, et al. (2012) Investigation of Hepatitis-B and C Seroprevalences in Bozok University School of Health Students. Viral Hepatit Dergisi 19(2): 49-53.
- Inci M, Aksebzeci AT, Yagmur G, Kartal B, Emiroglu M, et al. (2009) Investigation of HBV, HCV and HIV Seropositivity in Healthcare Workers. Turk Hijyen ve Deneysel Biyoloji Dergisi 66(2): 59-66.
- Korkmaz P, Çevik-Çağlan F, Aykın N, Alpay Y, Güldüren HM, et al. (2013) Seroprevalences of HBV, HAV, HCV and HIV Infection among Health Personnel in a State Hospital. Klimik Dergisi 26(2): 64-67.
- Tözün N, Yurdadin C, Özdogan CA, İdilman R, Kaymakoglu S, et al. (2019) Turkey Hepatitis B Roadmap. Available online at: https://www.vhsd.org/tr/files/download/p1be3700991q4hlip1fjbrgqj64.pdf
- SBU Antalya Training and Research Hospital Central Laboratory Examinations (2019) Available online at: http://10.7.16.20/sarusplus/his/HISExplorer.htm
- Hepatit B Vaccine Price (2019) Available online at: https://www.ilacrehberi.com/v/engerix-b-eriskin-20-mcg-10-ml-im-enjeksiyon-ce15/kt/nasil-kullanilir/#fiyatlar
- Lamivudin Price (2019) Available online at: http://www.ilacabak.com/zeffix-100-mg-28-tablet-3130
- Interferon Price (2019) Available online at: http://www.ilacabak.com/intron-a-pen-18-miu-1-flakon-5670
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Astronomy and Space Research
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)





