Research Article
Formulation and Evaluation of Ibuprofen (100 mg) Chewable Tablet by Direct Compression
5740
Views & Citations4740
Likes & Shares
A simple formula was developed for the preparation of chewable ibuprofen tablets by direct compression. All the ingredients except the lubricant were weighed accurately and passed through a 20 µm sieve, and were taken in a plastic bag to be mixed thoroughly for 15 min. Subsequently the lubricant was weighed and added to the mixture. Then mixed for additional 3 min before the particle size distribution. The chewable tablets were evaluated in terms of friability, hardness, and dissolution profile and drug content percent.
The blended powder showed a good flowability characteristic. The chewable tablet had weight variation in the range of 495-505 mg. The weight loss percentage was less than 1%. Hardness values were 3,551 kg/cm2 with relative standard deviation 0.96. The dissolution test proved that almost 90% of the drug released after 45 min. The formulation of ibuprofen chewable tablet was found to be palatable with and uniform drug content thus it is applicable for production on large scale.
Keywords: Ibuprofen, Non-steroidal anti-inflammatory drug, Evaluation, Direct compression
INTRODUCTION
In the present times, we are living in an urban modern or Ibuprofen is one of the propionic class of the non-steroidal anti-inflammatory drug (NSDAID). It is mostly administered orally and topically to relieve acute pain and fever. This drug is poorly soluble in aqueous media and thus the rate of dissolution from the currently available solid dosage forms is limited [1,2]. This leads to poor bioavailability at high doses after oral administration, thereby increasing the risk of unwanted adverse effects. Generally, to improve bioavailability of poorly soluble drugs, tablets are formulated as chews for oral administration. Chewable tablets which are intended to be chewed within the buccal cavity and thus are disintegrated in the mouth. Ideally, chewing tablets must be easy to chew, palatable, of appropriate size and shape and able to disintegrate readily to facilitate dissolution to deliver the desired therapeutic effect [3-6]. Ibuprofen exhibits poor flow, compaction (tableting) and dissolution behavior due to its hydrophobic structure, high cohesive, adhesive and viscoelastic properties therefore consistent research efforts have been directed to establish the desired improvement in the flowability, compression and dissolution properties of ibuprofen [7-11]. In the present study ibuprofen chewable (100 mg) tablet is formulated by direct compression.
MATERIALS & METHODS
Materials
Ibuprofen was received as gift from Blue Nile pharmaceutical industry, Khartoum, Sudan. Mannitol, orange flavor and magnesium stearate were received as gift from G.M.C industry Khartoum, Sudan. Acacia gum was purchased from pharmaceutical market. All chemicals and reagents used were of analytical grade.
METHOD
Preparation of the chewable tablet
All the ingredients except the lubricant were weighed accurately and passed through a 20 sieve, then were taken in a plastic bag to be mixed thoroughly for 15 min. Subsequently the lubricant was weighed and added to the mixture. Then mixed for additional 3 min before the blend had undergone direct compression (Table 1).
Characterization of the prepared formulation
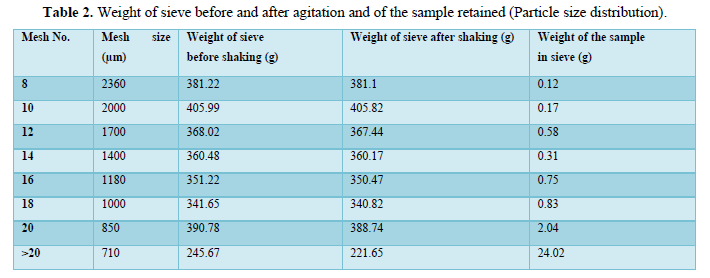
Particle size distribution: 30 g of the powder mix were weighed and placed on the top of the sieve set. Then it was carefully closed and placed in the sieve shaker for 10 minutes. Each sieve was weighed before and after the agitation then the difference was calculated to show the weight of the powder retained in each sieve according to their particle size. Finally, the particle size distribution graph was plotted (Table 2).
Angle of repose: A funnel was fixed in the stand. The powder drug was weighed and transferred to the funnel while the orifice of the funnel was closed by the thumb. Then the thumb was removed letting the powder to flow. A height (h) was measured and the circle was drawn at the edges to estimate the diameter. The following equation was used to calculate the angle:
Angle of repose (θ) = tan-1 h/r, r is the radius
Bulk density (BD): The powder sample was weighed then slowly poured into 50 ml graduated cylinder. The volume was recorded to calculate BD by dividing the sample weight by volume.
Tapped density (TD): It was measured by tapping cylinder which contains the sample 100 times. TD was calculated by dividing the sample weight by its final volume.
Carr’s index (CI): The percentage compressibility was calculated by the following equation:
(Tapped density – Bulk density x 100)/ Tapped density
Hausner’s ratio (HR): It was determined by the following formula:
HR = Tapped density/Bulk density.
1.png)
1.png)

Evaluation of ibuprofen chewable tablet
Friability: Twenty tablets were weighed all together and transferred to the chamber of the friability tester that revolves at twenty-five rpm. After four minutes the tablets were removed and re-weighed collectively. The percent friability was determined using the following equation:
% Friability = Initial weight–Final weight/Initial weight.
Hardness: The tablet was placed in the tester to measure its strength. The average of twenty tablets was calculated. The force was measured in kilograms per centimeter square.
Weight variation test: Twenty tablets were weighed individually. Average weight was calculated from the total weight of all tablets. The individual weights were compared with the average weight. The percent deviation was calculated using the following formula:
Percentage deviation = [(standard deviation /Average weight] ×100
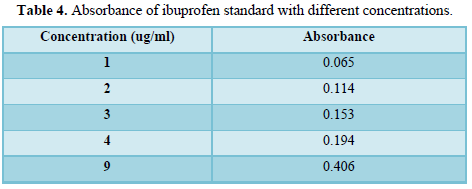
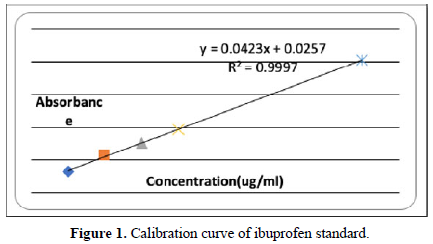
Calibration curve: 25 g of Ibuprofen was weighed and placed in volumetric flask. Phosphate Buffer (pH 6.8) was added and the volume is completed to 100 ml. This solution is then diluted to give different concentration. Finally, the absorbance of each solution was determined at a wavelength 221nm and the calibration curve was plotted.
Dissolution test: The dissolution profile of ibuprofen from chewable tablets were studied using the linear regression equation. The chewable tablets were placed in a rotating basket (100 rpm) filled with 900 ml of the phosphate buffer solution of pH 6.8, held at 37.0 °C ± 0.5°C. At time intervals of 10 min for 45 minutes, the samples (1 ml) were withdrawn and placed immediately in fresh dissolution medium. The amount of ibuprofen in the samples was determined spectrophotometrically at 221 nm.
Drug content: Twenty tablets were placed in a mortar and crushed using the pestle. A quantity equivalent to 0.2g was taken in 100 ml volumetric flask. Then 100 ml phosphate buffer solution (PH 6.8) was added. One ml was taken and diluted with 100 ml of buffer solution. The absorbance was measured against the blank which is the PBS (pH 6.8) at 221 nm using UV visible spectrophotometer.
Taste evaluation: Physiologically, taste is a sensory response resulting from a chemical stimulation of the taste buds on the tongue. There are four basic type of taste; salty, sour, sweet and bitter.
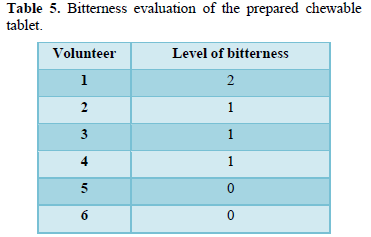
Six volunteers were randomly selected. The control was the pure drug. Prepared formulae were chewed in the mouth by each volunteer and the bitterness level was recorded against pure drug using a numerical scale (classifying bitter taste into five levels: level 0: tasteless, level 1: acceptable bitterness, level 2: slight bitterness, level 3: moderately bitterness and level 4: strong bitterness).
RESULTS
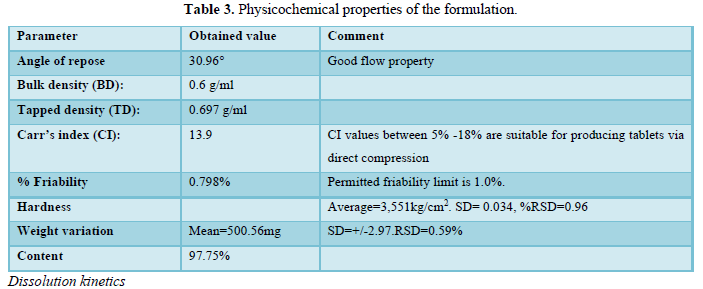
The results of the physical tests are shown in Table 3.
A series of 1,2,34, and 9 ug/ml of ibuprofen standard were prepared and their absorbances were obtained as shown in Table 4 and the constructed calibration curve is shown in Table 4 and the dissolution profile is shown in Figure 1.
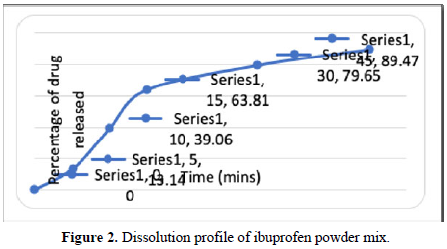
The obtained drug release profile is shown in Figure 2.





DISCUSSION
The chewable ibuprofen tablet was formulated by direct compression method. This technique was chosen for tablet production since it decreases processing steps which include wetting and drying process thus reducing the cost of manufacturing. The composition of the tablet is shown in Table 1.
Particle size distribution of the powder was found to be homogenous and of small size. The homogeneity and particle size distribution of the powder affect two major processes; the first one is the flow of the powder through the table ting machine and into the dye, and secondly it affects the dissolution in that the smaller particle size the higher the surface area and hence the higher dissolution (Table 2).
Physical properties and characterization of ibuprofen chewable tablet mixture prepared by direct compression technique was evaluated for angle of repose (30.9°), bulk density, tapped density, Carr’s index (13.9) and Hausner ratio (1.16). These results are in line to those obtained by A. Helmy [12] which indicate that the powder mix has good flow-ability characteristics and it’s suitable for manufacturing via direct compression (Table 3).
All tablets passed the weight variation tests since no tablet deviate from the average weight by 5%. The average of hardness values was found to be 3.551kg/cm2 with relative standard deviation 0.96%. These results are agreeing with the findings by researcher [13] (Table 3).
Good mechanical stability was confirmed by the percentage weight loss which was found to be 0.798%. This result is similar to what’s found by researcher [14]. The estimation of drug content using the linear regression equation of the calibration curve was performed. The result was found to be within the USP (2007) range (91-101%) which is equal to 98.3% (Table 3).
The dissolution test was made in accordance with USP conditions where the amount of drug release was measured at the intervals of 5, 10, 15, 30, and 45 min using calibration curve. In which 89.47% of the drug was released after 45 minutes. This finding was similar to that of Helmy et al study [12]. (Figures 1 and 2 and Table 4).
Most volunteers reported the lowest bitterness level against pure drug on the proposed numerical scale which indicates that the chewable tablets are acceptable in their taste (Table 5).
CONCLUSION
The preparation of chewable tablets was carried out by direct compression and gave satisfactory results. The tablet showed immediate drug release. From the above research work, it was concluded that ibuprofen tablets (100 mg) were formulated in a cost-effective manner and this is advantageous from an economical point of view.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the authors.
- Chaturvedi K, Gajera BY, Xu T, Shah H, Dave RH (2018) Influence of processing methods on physico-mechanical properties of Ibuprofen/HPC-SSL formulation. Pharm Dev Technol 23(10): 1108-1116.
- Ehlers H, Räikkönen H, Antikainen O, Heinämäki J, Yliruusi J (2009) Improving flow properties of ibuprofen by fluidized bed particle thin coating. Int J Pharm 368(1-2): 165-170.
- Michele TM, Knorr B, Vadas EB, Reiss TF (2002) Safety of chewable tablets for children. J Asthma 39(5): 391-403.
- Vanprapar N, Cressey TR, Chokephaibulkit K, Muresan P, Plipat N, et al. (2010) A chewable pediatric fixed-dose combination tablet of stavudine, lamivudine, and nevirapine: Pharmacokinetics and safety compared with the individual liquid formulations in human immunodeficiency virus-infected children in Thailand. Pediatr Infect Dis J 29(10): 940-944.
- Nirun V, Tim RC, Kulkanya C, Petronella M, Nottasorn P, et al. (2010) A chewable pediatric fixed-dose combination tablet of stavudine, lamivudine, and nevirapine: Pharmacokinetics and safety compared with the individual liquid formulations in human immunodeficiency virus-infected children in Thailand, Mahidol University, pubmed.gov. Pediatr Infect Dis J 29(10): 940-944.
- Pharmapproach (2020) Formulation, Manufacture and Evaluation of Chewable tablets [Internet]. Pharmapproach.com. 2019. Available online at: https://www.pharmapproach.com/formulation-manufacture-and-evaluation-of-chewable-tablets-2/
- Apeji YE, Oyi AR, Isah AB, Allagh TS, Modi SR, et al. (2018) Development and optimization of a starch-based co-processed excipient for direct compression using mixture design. AAPS Pharm SciTech 19(2): 866-880.
- Pawar A, Paradkar A, Kadam S, Mahadik K (2004) Agglomeration of Ibuprofen with talc by novel crystallo-co-agglomeration technique. AAPS Pharm SciTech 5(4): e55.
- The study of physicochemical properties of solid dispersions of ibuprofen in the presence of chitosan. Research Gate. Available online at: https://www.researchgate.net/publication/282124363_The_study_of_physicochemical_properties_of_solid_dispersions_of_ibuprofen_in_the_presence_of_chitosan
- Choi Y, Min KA, Kim CK (2019) Development and evaluation of dexibuprofen formulation with fast onset and prolonged effect. Drug Dev Ind Pharm 45(6): 895-904.
- Kothapally NK, Devareddy S (2013) Formulating taste masked orally disintegrating tablets of a bitter drug ibuprofen. Int Res J Pharm 4(11): 71-78.
- Helmy A, Elkady S, Khames A, Abdelbary A (2011) Preparation, characterization, and in-vitro/vivo evaluation of indion-based chewable tablets of paracetamol and ibuprofen for pediatric use. J American Sci 7(12): 831-844
- Kumar VA, Deepthi KL, Klyani R, Padmasri B, Prasanth D (2016) Formulation and evaluation of almotriptan chewable tablets. Int J Pharm Anal Res 5(3): 389-399.
- Poluri K, Babu P, Gary S, Muniyandy S (2015) Formulation development and evaluation of taste masked ibuprofen chewable tablets. Inventi J 9(4): 316-318.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)




