Short Communication
A Review of “The Will to Bud”: A Three Rivers Evolution Lecture Presented September 22, 2018
3587
Views & Citations2587
Likes & Shares
Hydra virid is cultured under different temperatures and feeding schedules bud continuously with clockwork precision. At different temperatures, buds are initiate at different rates, and hydras support different numbers of developing buds for different periods, but freshly detached buds have the same six to seven tentacles (statistically identical). Similarly, hydras fed on different schedules support different number of buds having different numbers of digestive cells but, within statistical limits, the same number of tentacles (six-seven).
Since buds develop from parental gastric region and peduncle cells converging on the budding region, modules containing a minimum number of parental cells would seem to initiate bud development, determine tentacle number, break with parental symmetry, and grow out ward. Additional cells provided to buds by the movement of parental cells and intrinsic growth do not alter tentacle number.
Keywords: Hydra virid, Statistical limits, Alter tentacle number
INTRODUCTION
Budding in hydras
Under laboratory conditions, hydras achieve a steady state, neither elongating nor shrinking, while, at the same time their cell populations grow, buds sprout, elongate, develop a head of tentacles and hypostome, a body column of gastric region and peduncle, and a terminal adhesive pad before detaching [1-9].
Hydra’s two epithelial tissues, an outer epidermis (“Ectodermal epithelial cells,” epitheliomuscular cells, ectoderm) and an inner gastrodermis (“Endodermal gland cells”) lie on either side of the mesoglea (matrix or extracellular material [ECM]). In addition, interstitial (basal cells, neoblast) is concentrated in intercellular spaces between hydra's epidermal cells.
Hydra's epithelial and interstitial cells are self-sustaining cell populations that differentiate locally into non-dividing cells of the head (hypostome and tentacles) and foot. Interstitial cells have stem-cell properties, dividing and giving rise to cells that become nerve, gland cells, and cnidoblasts that subsequently differentiate into various cnidocytes [10]. Cnidocytes migrate from the body column to tentacles where they large among epithelial battery cell and function in predation and defense. Other interstitial cells become sperm, egg and probably, adhesive gland cells of the foot [11-13].
Bud dynamics
Buds form in the budding region at the juncture of the gastric region and peduncle. The reorientation of circular gastrodermal musculature may be disturbed at this juncture, hence, encircling a cellular module and thrusting it outward upon contraction [14].
New mesogleal components are added as buds develop while moving downward (Figure 1). The budding region is the site of local production of new mesogleal components [15-17]. The " ECM is continuous at the sites of bud formation and what occurs is simply an increase in the expression of [mesogleal components] as evagination of the bud progresses” [18].
Beyond epithelia, interstitial cells play an essential role in budding. Indeed, they are required for the eruption of a developing bud, since hydras deprived of their interstitial cells (known as “epithelial hydras”: [7,19] do not sustain budding. Epithelial hydras may enlarge and form supernumerary tentacles when force-fed, but epithelial cells alone do not restore interstitial cells [20] or the products of interstitial cell differentiation.
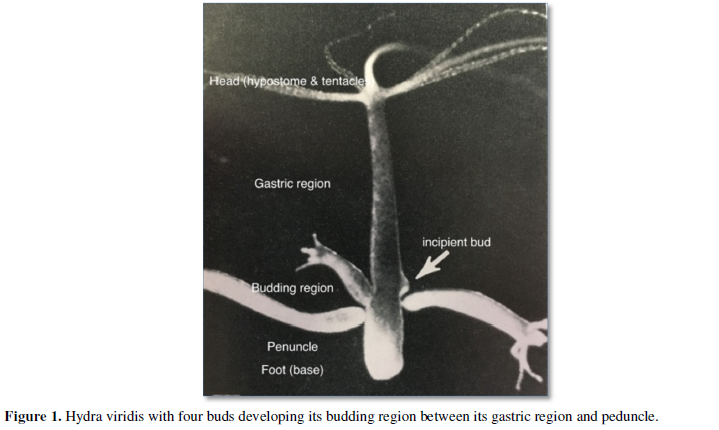

Bud dynamics
Estimates of the size of the initial bud module are made from estimates of the number of digestive cells. Digestive cells were counted (Figure 2) because they can be distinguished unambiguously from the other cells comprising hydras.
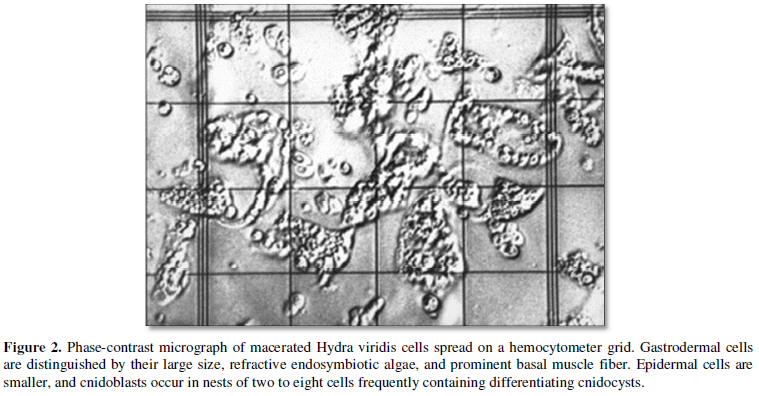
Hydras fed three days a week were cultured at different temperatures (Table 1), 18º, 21º, and 28º C or fed one to four days a week while cultured at 21º C.
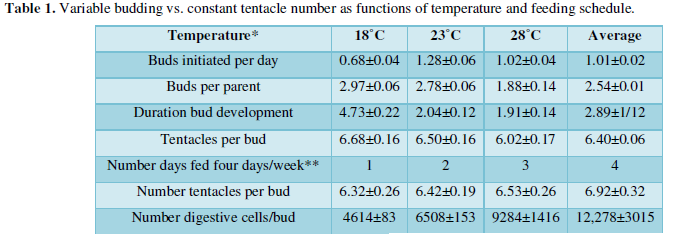

Moreover, the budding rate for my hydra, Chlorohydraviridisima, was optimal in the vicinity of 23º (actually closer to 21º) centigrade, trailing off both above and below this optimum. As one might imagine, the number of buds initiated per day and the duration of bud development varied as functions of temperature, but the number of tentacles on each bud did not change significantly. Thus, hydras maintained on different feeding schedules and incubated at different temperatures, produced buds at different rates and with different numbers of cells, but with the same number of tentacles. The determination of tentacle numbers was, therefore, under different controls than the rate of budding and size of buds.
Since then, it was suggested that hydra’s budding region occurs at the point where downward moving cells from the gastric region encounter upward moving cells from the peduncle. When a minimum number of these cells accumulate, they constitute a bud module and initiate a bud’s development while determining the number of a bud’s tentacles that will ultimately be present on the bud. The module proceeds to break with the “parent” hydra’s symmetry redirecting growth outward. Ultimately, the rate at which buds develop and detach is a function of feeding rate and temperature as parental cells continues to feed buds that also grow intrinsically. Thus, the size of a bud module determines the number of tentacles produced by the bud while the number and size of buds produced per unit time is governing by the rate of cell division on the “parent” and bud.
Under both regimes, the number of tentacles per buds ranged from [5-7]. Larger animals fed more often tended to produce buds with slightly more tentacles (i.e., the regression of tentacles per feeding days differed significantly [21-23]. But, the number of tentacles per bud did not differ statistically as either a function of temperature or feeding schedule. The number of buds initiated per day, the number buds developing on a parent at any time, and the duration of bud development did differ significantly as a function of temperature; and the number of digestive cells per bud differed significantly as a function of feeding schedule.
How many parental cells comprise a bud module?
One may answer this question with data in (Table 1) and assumptions about the average rate of cell division in hydras fed on different schedules. Extrapolating back, the 12,278±3015 (rounded to 12,000) digestive cells present in freshly detached hydras cultured at 21º C and fed 4 days a week would have been produced in 2.04±0.12 (two days) by an initial mass of 2000 to 3000 digestive cells dividing twice a day. Likewise, the 6500 digestive cells present in freshly detached buds of parents fed 2 days a week would have been produced by 2000-3000 digestive cells dividing once every day, and the 4000-5000 digestive cells present in buds from parents fed one day a week would have been produced by about 2000-3000 digestive cells dividing once in two days.
Thus, the bud module would contain 2000-3000 digestive cells at the initiation of bud development in parents fed one to four days a week (and maintained at 21ºC). If the number of digestive cells is less than half the number of epidermal cells, and the entire epithelium is half the size of the interstitial cell population [24], then an initial bud module would consist of about 15,000 cells.
Intriguingly, an estimate of 200-600 digestive cells found in tentacle rudiments during regeneration [22] is consistent with the estimate of 2000-3000 digestive cells present in bud modules were the size of the initial module to determine the tentacle number on buds.
- Burnett A L (1961) The growth process in J Exp Zool 146: 21-84.
- Campbell RD (1965) Growth and tissue renewal patterns in Hydra Littoralis. Thesis the Rockefeller Institute.
- Campbell R D (1967a) Tissue dynamics of steady state growth in Hydra Littoralis. I Patterns of cell division. Dev Biol 15: 487-502.
- Campbell R D (1967b) Tissue dynamics of steady state growth in Hydr Littoralis. II Patterns of tissue movement J Morphol 121: 19-28.
- Campbell R D (1967c) Tissue dynamics of steady state growth in Hydra Littoralis. III Behavior of specific cell types during tissue movement J Exp Zool 164: 379-392.
- Campbell R D (1974) Cell movement in Hydra. Am Zool 14: 523-535.
- Campbell RD, Subtelny S (1979) Development of Hydra lacking interstitial and nerve cells (Epithelial Hydra) Determinants of Spatial Organization. 37th Symposium of the Society for Developmental Biology Alan R Liss New York 267-293.
- Shostak S, Patel NG, Burnett AL (1965) The role of mesoglea in mass cell movement in Hydra. Dev Biol 112: 434-450.
- Shostak S, Bisbee JW, Ashkin C, Tammariello RV (1968) Budding in Hydra viridis. J Exp Zool 169: 423-430.
- Shostak SV, Kolluri (1995) Symbio genetic origins of cnidarian cnidocysts. Symbiosis 19: 11-29.
- Lentz TL (1966) The Cell Biology of Hydra. New York NY John Wiley 158: 1036.
- Littlefield CL (1985) Isolation of a subpopulation of interstitial cells that is developmentally restricted to sperm production. Dev Biol 112: 185-193.
- Littlefield CL (1991) Cell lineages in hydra: Isolation and characterization of an interstitial stem cell restricted to egg production in Hydra oligactis. Dev Biol 143: 378-388.
- Aufschnaiter R, Wedlich-Söldner R, Zhang X, Hobmayer B (2017) Apical and basal epitheliomuscular F-actin dynamics during Hydra bud evagination. Biol Open 6: 1137-1148.
- Shostak S, Kankel DR (1967) Morphogenetic movements during budding in Hydra. Dev Biol 15: 451-463.
- Burnett AL, Hausman RE (1969) Mesoglea of Hydra II Possible role in morphogenesis. J Exp Zool 171: 15-24.
- Aufschnaiter R, Zamir EA, Little CD, Özbek S, Münder S, et al. (2011) In vivo imaging of basement membrane movement: ECM patterning shapes Hydra J Cell Sci 124: 427-438.
- Shimizu H, Zhang X, Zhang J, Leontovich A, Fei K, et al. (2002) Epithelial morphogenesis in hydra requires de novo expression of extracellular matrix components and matrix metalloproteinases. Develop 129: 1521-1532.
- Marcum BA, Lenhoff HM (1982) Culturing epithelial hydra. Hydra: Research Methods New York: Plenum Press 287-290.
- Marcum BA, Campbell RD, Lenhoff M (1982) “Eliminating all nonepithelial cells using colchicine” in Howard (ed) Hydra: Research Methods. New York NY: Plenum 281-290.
- Shostak S (1967) Bud movement in Hydra, Science. 155: 1567-1568.
- Shostak S (1982) Structural cell number and regeneration in Chlorohydra viridissima. J Exp Zool 222: 69-75.
- Shostak S, Globus M (1966) Migration of epithelia-muscular cells in Nature 210: 218.
- Bode HS, Berking CN, Gierer DA, Schaller H, Trenkner E, et al. (1973) Quantitative analysis of cell types during growth and morphogenesis in hydra. Wilhelm Roux Arch Entwickl Mech Org 171: 269-285.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Astronomy and Space Research
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)




