Review Article
Functional and Structural Effects of Vitamin D on Diabetic Kidney
4305
Views & Citations3305
Likes & Shares
Vitamin D is vitamin and hormone with huge range of calcium-dependent and calcium-non-dependent functions. Chronic kidney disease and diabetic nephropathy patients have a high incidence of cardiovascular and infectious morbidities. Increasing evidence indicates a relationship between vitamin D deficiency and cardiovascular and infectious mortality risks. Kidney is a target organ for Vitamin D3. This article is to review and summarize the pleiotropic effects of Vitamin D in patients with Diabetes Mellitus and Diabetic Nephropathy.
Keywords: Vitamin D, Kidney, Damage, T1D, Diabetic nephropathy
INTRODUCTION
Vitamin D is a lipid-soluble vitamin and the only vitamin that can be synthesized by humans. Evolutionally, vitamin D has been synthesized by a photochemical process in land vertebrates to satisfy the requirement for a calcified skeleton for more than 350 million years [1]. Vitamin D is metabolized by 25-hydroxylase and 1α-hydroxylase in the liver and kidneys, respectively, and converted to the active form, 1.25-dihydroxyvitamin D [1.25(OH)2D] [2]. Vitamin D acts through activation of the vitamin D receptor (VDR), which involves several pleiotropic effects.
Level of Vitamin D synthesis and its bioavailability depends on kidney cells condition. Vitamin D deficiency is a prominent feature of kidney disease including Chronic Kidney Disease (CKD) and diabetic nephropathy (DN). Vitamin D deficiency is related to albuminuria, CKD progression, and subsequent cardiovascular diseases [3]. VDR is highly expressed in the kidney; therefore, the kidney can be considered a classic Vitamin D target organ [4].
Diabetes mellitus (DM) is a major public health problem worldwide, with ever-increasing incidence and prevalence in recent years. The Institute for Alternative Futures (IAF) expects that the total number of people with type 1 and type 2 DM in the United States will increase by 54%, from 19,629,000 to 54,913,000 people, between 2015 and 2030 [5].
Diabetic Nephropathy (DN) affects about one-third of patients with DM and currently ranks as the first cause of end-stage kidney disease in the Western world. The major clinical manifestations of DN are proteinuria, hypertension and progressive reduction of renal function: United States Renal Data System registered that approximately 35-50% of end-stage renal disease (ESRD) cases are secondary to DN complications [6]. Thus, studies of molecular mechanisms preserving kidneys which Vitamin D involved in are important in terms of prevention of proteinuric kidney diseases, i.e., Diabetic Nephropathy.
FUNCTIONAL EFFECTS OF VITAMIN D ON DIABETIC KIDNEY
The complexity of interactions of Vitamin D is directly related with progressive long-term changes implicated in the worsening of renal function. These changes result in a dysregulation of the Vitamin D-dependent pathways. Various studies demonstrated a pivotal role of Vitamin D supplementation in regression of albuminuria and glomerulosclerosis, contrasting the increase of glomerular basement membrane thickening and podocyte effacement. These, in turn, improves renal and cardiovascular outcomes. The homeostasis and regulation of the nephron’s function are absolutely dependent from the cross-talk between endothelium and podocytes.
A pivotal role for the management of DM could be found in the pleiotropic actions of vitamin D. Studies conducted by Afzal [7], Schöttker [8], Tsur [9], have shown a link between low serum levels of vitamin D and the increasing risk of developing DM.
An association of a proper status of vitamin D with a better modulation of glucose homeostasis, due to a regulation of insulin secretion and tyrosine phosphorylation of the insulin receptor sown [10]. Data of the Third National Health and Nutrition Examination Survey (NHANES III) found an inverse association between the level of vitamin D and the prevalence of albuminuria.
In our previous studies conducted in Department of Pediatrics №4 of Bogomolets National Medical University we found decreased level of Vitamin D in iabetic patients. In group with T1D Vitamin D level was in insufficiency rage, in patients with DN Vitamin D deficiency found [11,12].
The study of Karnchanasorn et al. [13] showed modulatory effect of vitamin D. Authors demonstrated a correlation between appropriate vitamin D status and both β-cell function and insulin sensitivity.
A protective role of vitamin D in type 1 DM has been demonstrated in a recent where serum 25-(OH) D concentrations sustained with cholecalciferol supplementation 3000 IU/day for one year improved metabolic control and slowed the decline of residual β-cell function in children with T1DM [14]. The meta-analysis by Gregoriou [15] also showed that vitamin D supplementation in the form of 1α(OH)vitamin and cholecalciferol appears to be beneficial in the treatment of T1DM patients by attenuating the natural history of the disease.
Activation of the VDR is essential in reducing proteinuria. Traditionally, administration of RAAS blockers can reduce albuminuria [16]. 1.25(OH)2D3 is known as a RAS inhibitor by its negative regulatory effect on renin production to provide additional renoprotection [17].
STRUCTURAL EFFECTS OF VITAMIN D ON DIABETIC KIDNEY
The renoprotective effects of vitamin D can improve proteinuria, glomerulosclerosis, and interstitial infiltration and reduce renal oxidative stress [18]. Combined treatment with paricalcitol and losartan suppressed the induction of fibronectin, transforming growth factor β (TGF-β) and monocyte chemoattractant protein-1 (MCP-1), and reversed the decline of the slit diaphragm proteins nephrin, Neph-1, ZO-1, and alpha-actinin-4 [19].
Another emerging issue linked with DN development and lower levels of Vitamin D is urinary megalin excretion in T2DM patients. [19]. Megalin, as a member of the low-density lipoprotein receptor family [20]. Megalin plays an important role in the reabsorption of Vitamin D binding protein from glomerular filtrates. It was reported that megalin-mediated (auto)lysosomal dysfunction in primary tubular epithelial cells is decisive for the development of kidney disease in a High-Fat-Diet-induced diabetes model. Exocytosis-mediated urinary megalin excretion increases along with the progression of DN, giving further contributions in the understanding of the pathogenesis of Vitamin D loss in these subjects, their findings also suggest a potential role of megalin urinary excretion as indicator of progression of DN [21-23]. Megalin has shown to have an A1 adenosine receptor (A1AR)-mediated effect. It was shown that A1AR played a protective role in proximal tubular megalin loss associated albuminuria by inhibiting the pyroptosis-related caspase-1/IL-18 signaling in DN [24].
It was found a higher level of urinary megalin in chronic microvascular complications of diabetes with associated metabolic derangements. Vitamin D supplements have positive effect on urinary megalin levels in diabetic nephropathy patients with vitamin D hypovitaminosis [25].
Serum Vitamin D levels are also decreased in CKD. It was found the role of C-megalin excretion in Vitamin D metabolism in 153 pre-dialysis CKD patients. Urinary C-megalin exhibited negative correlations with serum 25(OH)D, 1,25(OH)2D and 24,25(OH)2D. Multiple regression analysis showed that urinary C-megalin had a significantly negative association with 25(OH)D. Serum 1,25(OH)2D and 24,25(OH)2D, as well as 1,25(OH)2D/25(OH)D and 24,25(OH)2D/25(OH)D ratios, showed positive correlations with eGFR. Thus, urinary C-megalinemerged as an independent factor positively associated with 1,25(OH)2D/25(OH)D and 1,25(OH)2D/24,25(OH)2D [26].
CKD and DN always associated with high rate of cardiovascular complications. There are data showing that paricalcitol and calcitriol are both able to modulate the thrombomodulin expression in human aortic smooth muscle cells. Moreover, Maestroni [27] and D’Arrigo [28] demonstrated a raise of soluble thrombomodulin levels in patients with CKD by the administration of paricalcitol.
VDR knockout in diabetic mice was associated with severe albuminuria and glomerulosclerosis. Alternatively, vitamin D might slow the progression of diabetic nephropathy by improving insulin secretion, delaying destruction of β islet cells, affecting osteocalcin, and consequently assisting in glucose metabolism. TGF-β, MCP-1, hepatocyte growth factor, thrombospondin-1, and plasminogen activator inhibitor are other possible molecular targets of vitamin D action [29].
Increasing evidence from experimental and clinical studies has unveiled a pathological role of macrophages in the development of glomerulosclerosis by the production of inflammatory chemokines, cytokines and fibrogenic factors, release of proteolytic enzymes and production of reactive oxygen species. A central role in these mechanisms is played by the monocyte chemoattractant protein (MCP)-1, a chemokine produced by mesangial cells (MCs) and renal tubular cells that has the responsibility of recruiting macrophages into the kidney [27]. It was demonstrated that calcitriol can inhibit the synthesis and activity of MCP-1 and contrast the glomerular injury in diabetic mice. Their data suggest that vitamin D may protect against renal injury in DN by preventing or reducing macrophage infiltration [30,31].
A proper vitamin D regulation also has an important role against inflammation secondary to DN. Indeed, diabetes leads to an increase in the expression of inflammatory factors and inappropriate immune activity. Inflammatory response likely contributes to DM occurrence by worsening insulin resistance and it is in turn intensified in the presence of hyperglycemia to exacerbate long-term complications of diabetes [32].
Vitamin D exerts protective effects against inflammatory agents by inhibiting the expression of interleukin (IL)-6, IL-8, RANTES (regulated on activation, normal T cell expressed and secreted), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), platelet-endothelial cell adhesion molecule-1 (PECAM-1), receptor of advanced glycation end products (RAGE) and E-selectin through a nuclear factor-κB (NF-κB)–mediated mechanism, partly by disrupting DNA binding of NF-κB.
Moreover, vitamin D represses the expression of cyclooxygenase (COX)-2 and upregulates the expression of 15-hydroxyprostaglandin dehydrogenase (15PGDH), the enzyme initiating prostaglandin catabolism, in this way reducing prostaglandin levels and suppressing the production of several proinflammatory cytokines. The immunomodulatory action of vitamin D has been well demonstrated also in the study of Lucisano [33], where an acute paricalcitol supplementation induced a significant reduction of IL-17, IL-6, IL-1β, TNF-α and IFN-γ in a cohort of CKD patients. Chronic inflammation leads to sclerotic processes in kidney. Reduction of the inflammatory rate can prevent irreversible morphological changes in kidneys.
In the study conducted on human incubated monocytes taken from patients with DM and DN with uremia, it was found that Vitamin D may exert an anti-inflammatory effect by regulating the signal transduction pathways that control VDR and signal transducer and activator of transcription 5 (STAT5) expression.
These findings have been further investigated. It was found more anti-inflammatory effects of Vitamin D and VDR on phosphorylated STAT5 (p-STAT5) in serum-incubated monocytes from patients with DM and uremia caused by DN: lipopolysaccharide associated with IL-15 upregulated the expression of p-STAT5, whereas pre-treatment with 1,25-(OH)2D3 significantly inhibited this effect [34].
In recent years, CKD has become a global public health problem, and the incidence of ESRD is increasing. DN is the main cause of ESRD so it is extraordinarily significant to study the new effective treatment of DN.
Recently, the renoprotective effects mediated by Vitamin D and Vitamin D receptor (VDR) have been evidenced. VDR is a transcription factor located at chromosome 12 containing 9 exons, is one of the nonsteroid nuclear hormone receptor superfamily, which participates in transcriptional regulation of genes in tissue- and cell-specific ways. It is now well recognized that Vitamin D/VDR plays an important role not only in regulating blood calcium and phosphorus levels. It was demonstrated that Vitamin D/VDR signaling pathway possesses a variety of kidney-protective structural effects in DN patients, such as antiproteinuria, antifibrosis, anti-inflammatory, and preventing podocyte damage [35].
Experimental as well as observational studies and clinical trials conducted in the past years suggest the effective role of Vitamin D and the synergic action with RAAS inhibitors to counteract the worsening of DN and to preserve the glomeruli and the integrity of glomerular filtration barrier. Moreover, Vitamin D seems to exert many extra-renal functions essential for the body homeostasis. The results of these studies emphasize the need for better awareness among researchers and clinicians about the consequences of insufficient Vitamin D levels and the importance of monitoring its status in high-risk populations. Summarized scheme of Vitamin D effects in Diabetes Mellitus and Diabetic nephropathy given in Figure 1.
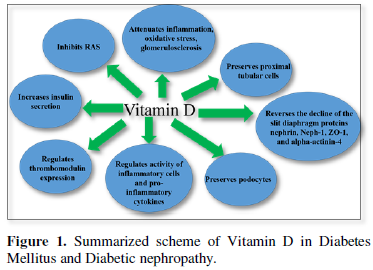

Even if growing evidence proves that vitamin D may have antiproteinuric, anti-inflammatory, renoprotective and cardioprotective effects in patients with DN, it is still required randomized controlled trials in larger patient groups studying molecular background of the nephroprotective effects of Vitamin D.
- DeLuca HF (2004) Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80: 1689-1696.
- Henry HL (2011) Regulation of vitamin D metabolism. Best Pract Res Clin Endo Crinol Metab 25: 531-541.
- Franca Gois PH, Wolley M, Ranganathan D, Seguro AC (2018) Vitamin D Deficiency in Chronic Kidney Disease: Recent Evidence and Controversies. Int J Environ Res Public Health 15: 1773.
- Bikle D, Feingold KR, Anawalt B, Boyce A (2000) Vitamin D: Production, Metabolism, and Mechanisms of Action. [Updated 2017 Aug 11] editors Endotext [Internet] South Dartmouth (MA): MDText.com, Inc. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK278935/
- Gembillo G, Cernaro V, Salvo A (2019) Role of Vitamin D Status in Diabetic Patients with Renal Disease. Medicina (Kaunas) 55: 273.
- Alicic RZ, Rooney MT, Tuttle KR (2017) Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol 12: 2032-2045.
- Afzal S, Bojesen SE, Nordestgaard BG (2013) Low 25-hydroxyvitamin D and risk of type 2 diabetes: A prospective cohort study and meta-analysis. Clin Chem 59: 381-391.
- Schöttker B, Herder C, Rothenbacher D, Perna L, Müller H, et al. (2013) BrennerSerum 25-hydroxyvitamin D levels and incident diabetes mellitus type 2: A competing risk analysis in a large population-based cohort of older adults. Eur J Epidemiol 28: 267-275.
- Tsur BS, Feldman I, Feldhammer MB, Hoshen G, Leibowitz RD (2013) Balicer Decreased serum concentrations of 25-hydroxycholecalciferol are associated with increased risk of progression to impaired fasting glucose and diabetes. Diabetes Care 36: 1361-1367.
- Flores M (2005) A role of vitamin D in low-intensity chronic inflammation and insulin resistance in type 2 diabetes mellitus? Nutr Res Rev 18: 175-182.
- BurlakaIe (2016) Beyond Proteinuria: Apoptosis and Vitamin D3 State in Children with Diabetic Nephropathy. J Clin Exp Pathol 6: 266.
- Maidannyk V, BurlakaIe (2016) Association of Vitamin D deficiency, cellular hypoxia, and caspase-3 with renal disease in pediatric diabetic nephropathy. J Translational Sci 2: 130-133.
- Karnchanasorn R, Ou HY, Chiu KC (2012) Plasma 25-hydroxyvitamin D levels are favorably associated with β-cell function. Pancreas 41: 863-868.
- Panjiyar RP, Dayal D, Attri SV, Sachdeva N, Sharma R, et al. (2018) Sustained serum 25-hydroxyvitamin D concentrations for one year with cholecalciferol supplementation improves glycemic control and slows the decline of residual β cell function in children with type 1 diabetes. Pediatr Endocrinol Diabetes Metab 2018(3): 111-117.
- Gregoriou E, Mamais I, Tzanetakou I, Lavranos G, Chrysostomou S, et al. (2017) The Effects of Vitamin D Supplementation in Newly Diagnosed Type 1 Diabetes Patients: Systematic Review of Randomized Controlled Trials. Rev Diabet Stud 14: 260-268.
- Anavekar NS, Gans D J, Berl T, Rohde RD, Cooper W, et al. (2004) Redictors of cardiovascular events in patients with type 2 diabetic nephropathy and hypertension: A case for albuminuria. Kidney Int Suppl 92: S50-S55.
- Pilz S, van Den Hurk K, Nijpels G, Stehouwer CDA, Van’t Riet E, et al. (2012) Vitamin D status, incident diabetes and prospective changes in glucose metabolism in older subjects: The Hoorn study. Nutr Metab Cardiovasc Dis 22: 883-889.
- Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, et al. (2001) Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861-869.
- De Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, et al. (2004) Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309-2320.
- Ogasawara S, Hosojima M, Kaseda R, Kabasawa H, Yamamoto-Kabasawa K, et al. (2012) Significance of urinary full-length and ectodomain forms of megalin in patients with type 2 diabetes. Diabetes Care 35: 1112-1118.
- Saito A, Pietromonaco S, Loo AK, Farquhar MG (1994) Complete cloning and sequencing of rat gp330/‘megalin’ a distinctive member of the low density lipoprotein receptor gene family. Proc Natl Acad Sci. USA 91: 9725-9729.
- De S, Kuwahara S, Hosojima M, Ishikawa T, Kaseda R, et al. (2017) Exocytosis-Mediated Urinary Full-Length Megalin Excretion Is Linked with the Pathogenesis of Diabetic Diabetes 66: 1391-1404.
- Diez-Sampedro A, Lenz O, Fornoni A (2011) Podocytopathy in diabetes: A metabolic andendocrine disorder. Am J Kidney Dis 58: 637-646.
- Tian D, Shi X, Zhao Y (2019) The effect of A1 adenosine receptor in diabetic megalin loss with caspase-1/IL18 signaling. Diabetes Metab Syndr Obes 12: 1583-1596.
- Kasabri V, Akour A, Bulatova N (2020) A Pre-Post Study of Vitamin D Supplement Effects on Urinary Megalin: The Emerging Predictive Role of Megalin in Diabetic Nephropathy Progression. Endocr Metab Immune Disord Drug Targets 20: 1552-1557.
- Toi N, Inaba M, Ishimura E (2019) Significance of urinary C-megalin excretion in vitamin D metabolism in pre-dialysis CKD patients. Sci Rep 9: 2207.
- Maestroni S, Zerbini G (2018) Glomerular endothelial cells versus podocytes as the cellular target in diabetic nephropathy. Acta Diabetol55(11): 1105-1111.
- D’arrigo G, Pizzini P, Cutrupi S, Tripepi R, Tripepi G, et al. (2018) Vitamin D receptor activation raises soluble thrombomodulin levels in chronic kidney disease patients: A double blind, randomized trial. Nephrol Dial Transplant 34(5): 819-824.
- Zhang Z, Sun L, Wang Y, Ning G, Minto AW, et al. (2008) Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int 73: 163-171.
- Hammer Y, Soudry A, Levi A, Talmor-Barkan Y, Leshem-Lev D, et al. (2017) Effect of vitamin D on endothelial progenitor cells function. PLoS One 12(5): e0178057.
- Zhang Z, Yuan W, Sun L, Szeto FL, Wong KE, et al. (2007) 1,25-Dihydroxyvitamin D3 targeting of NF-κB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int 72: 193-201.
- Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP (2013) Diabetes mellitus and inflammation. Curr Diab Rep 13: 435-444.
- Lucisano S, Arena A, Stassi G, Iannello D, Montalto G, et al. (2015) Role of Paricalcitol in Modulating the Immune Response in Patients with Renal Disease. Int J Endocrinol 2015: 765364.
- Yang M, Yang B O, Gan H, Li X, Xu J, et al. (2015) Anti-inflammatory effect of 1,25-dihydroxyvitamin D3 is associated with crosstalk between signal transducer and activator of transcription 5 and the vitamin D receptor in human monocytes. Exp Ther Med 9: 1739-1744.
- Lei M, Liu Z, Guo J (2020) The Emerging Role of Vitamin D and Vitamin D Receptor in Diabetic Nephropathy. Biomed Res Int 2020: 4137268.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Astronomy and Space Research
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)



