Research Article
Digital Analysis of Gtb Karyotype-A Cost Effective New Approach in Diagnostic Cytogenetics Laboratories
3331
Views & Citations2331
Likes & Shares
In Diagnostic Cytogenetic Laboratories World over used routine GTB banding analysis and its well accepted as a Gold standard method. The same approach could help to detect most of the chromosomal syndromes except micro-deletion (sub-microscopic deletion
If it works, it would make a viable option (cost effective ways) to third World poor countries an option for Cytogenetic Laboratories and possible to detect all type of chromosomal disorders in the same specimen for many unfortunate patients.
Keywords: Chromosome Microarray Analysis, Chromosomal profiling, Cytogenetic Laboratories, Chromosomal disorders
INTRODUCTION
Chromosome studies or karyotyping is a test that evaluates the number and structure of a person’s chromosomes in order to detect abnormalities. Chromosomes are thread-like structures within each cell nucleus and contain the body's genetic blueprint. Each chromosome contains thousands of genes in specific locations. These genes are responsible for a person’s inherited physical characteristics and they have a profound impact on growth, development, and function.
Humans have 46 chromosomes, present as 23 pairs. Twenty-two pairs are found in both genders (autosomes), and one pair (sex chromosomes) is present as either XY (in males) or XX (in females). Normally, all cells in the body that have a nucleus will contain a complete set of the same 46 chromosomes, except for the reproductive cells (eggs and sperm), which contain a half set of 23. This half set is the genetic contribution that will be passed on to a child. At conception, half sets from each parent combine to form a new set of 46 chromosomes in the developing fetus.
Chromosomal abnormalities include both numerical and structural changes. For numerical changes, anything other than a complete set of 46 chromosomes represents a change in the amount of genetic material present and can cause health and development problems. For structural changes, the significance of the problems and their severity depends upon the chromosome that is altered. The type and degree of the problem may vary from person to person, even when the same chromosome abnormality is present.
CONVENTIONAL METHODS TO DO KARYOTYPE
Taking a sample of a person’s cells and culturing them in nutrient-enriched media to promote cell division in vitro. This is done in order to select a specific time during the cells’ growth phase when the chromosomes are easiest to distinguish. Isolating the chromosomes from the nucleus of the cells, placing them on a slide, and treating them with a special stain. Taking microphotographs of the chromosomes.
In jigsaw puzzle fashion, rearranging the pictures of the chromosomes to match up pairs and arrange them by size and structure, from numbers 1 to 22, followed by the sex chromosomes as the 23rd pair.
The pictures also allow the chromosomes to be vertically oriented. Each chromosome looks like a striped straw. It has two arms that differ in length (a short arm (p) and a long arm (q), a pinched-in area between the arms called a centromere and a series of light and dark horizontal bands. The length of the arms and the location of the bands help determine top from bottom.
Once the chromosome photo arrangement is completed, a laboratory specialist evaluates the chromosome pairs and identifies any abnormalities that may be present.
MATERIALS & METHODS
All the specimens were collected from Neonatology Ward of Dr B.C. Roy Post Graduate Institute of Pediatric Sciences, Kolkata along with patient data sheet contain all basic clinical information’s and investigation reports assigned by the attending consultant pediatricians as per our guidelines. The project proposal was approved by the Institute Ethical committee [1-7].
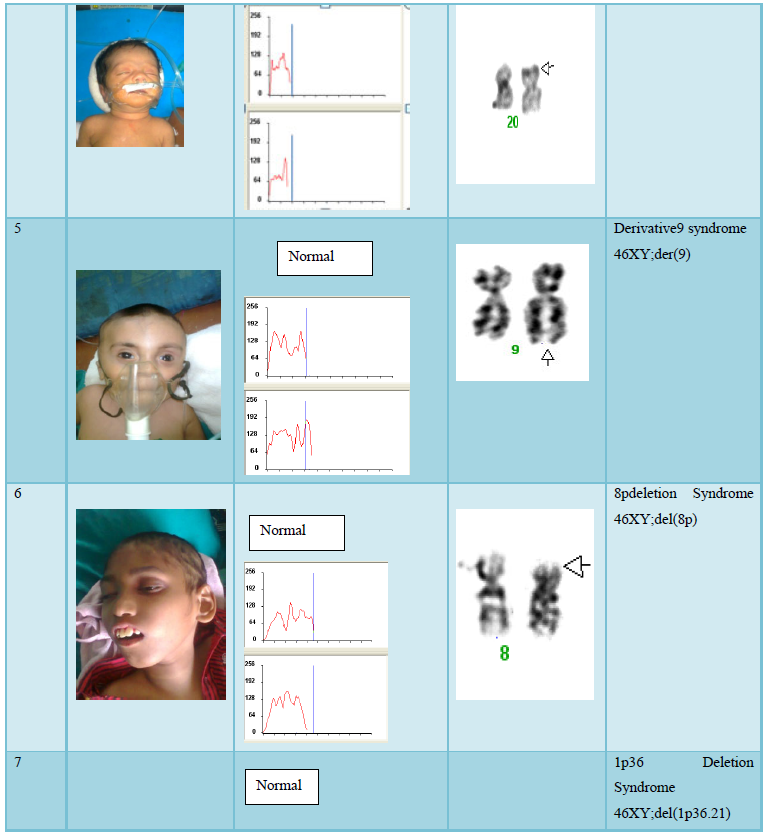
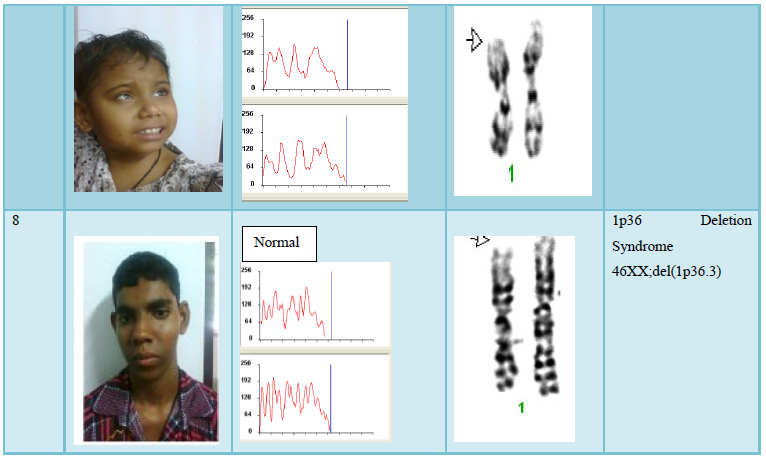
The Cells (Peripheral Blood) were cultured in culture media. The culture was then processed using standard protocol of lecukocyte culture. After processing of the culture, metaphases plate were prepared and subjected to G-T-G Banding technique for analysis of the chromosome abnormalities, 20 metaphases plates were then analyzed by using automated karyotype system-cytovision verson 3.6 Applied Imaging (Figure 1).


RESULT
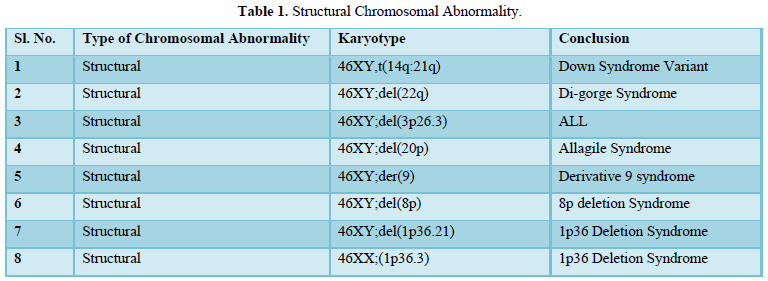
The result shows that among 120 indicated cases (patient with dysmorphic features associated with congenital defects) 22 cases have chromosomal abnormality (14 cases have numerical defects and 8 cases with structural chromosomal instabilities). Among the 8 cases of structural chromosomal abnormality, (out of 120 cases) one have the karyotype 46XY,t(14q:21q)- it is a case of Down’s Syndrome, one have the karyotype 46XY;del (22q)-it is a case of Di-gorge Syndrome, one have the karyotype 46XY;del(3p26.3), one have the karyotype with 46XY;del (20p) -it is a case of Allagile Syndrome and one have the karyotype with 46XY;der(9) and a single case of 8p deletion syndrome with karyotype 46XY; del(8p). We also found 1 male and 1 female of 1p36deletion syndrome with the karyotype of 46XY; del (1p36.21) and 46XX; (1p36.3) respectively.
FISH analysis results shows single red signal of 22q locus specific probes instead of two due to deletion in specific locus and two green signals in centromere position in both the interphasic and metaphasic condition. The blue signals for counter stain DAPI. This result confirmed the small deletion in 22q location.
DISCUSSION
Routine cytogenetic diagnostic laboratory microscopic chromosome variation usually carried out based on an International System for Human Cytogenetic Nomenclature (ISCN-2005) and are not actually easy to identify. These structural abnormalities usually detected with optical microscopes are aneuploidies, marker chromosome, gross rearrangements and variation in chromosome size. The frequency of these abnormalities in human population is thought to be as 1 every 375 live births by putative information [8,9]. Some genetic diseases are suspected to be caused by these structural variations, but the relationships are not very certain. It is not plausible to divide these variants into two classes as “normal” or “disease”, because the actual output of the same variant will also vary. Structural variations also have its function in population genetics. In order to understand chromosomal structural variation in our region (West Bengal) this present collaborative project was initiated, in which we have screened chromosome abnormalities among the neonates and children with dysmorphic features associated with congenital defects admitted each day in the hospital. All the patients were clinically examined by attending clinician, registered and selected for present studies. Most of the patients were admitted with severe critical condition and follow up for repeat specimens were failed due to sad demise or referring to other Hospitals for further managements. In 8 out of 22 patients in this study (Table 1) the aberration occurred de novo; two of them had complex rearrangements with both duplication and a deletion (Table 2). As shown by testing the parental samples, none of the abnormalities detected in this study was derived from a parental balanced translocation. Lacking familial cases is not in line with the observations in large studies [10,11] who assessed that 50% of pathogenic imbalances, respectively, originated from a parent with a balanced translocation. However, paternity was not checked in the present study.

CONCLUSION
Human chromosomes harbor hundreds of structural differences including deletions, insertions, duplications, inversions, and translocations. Collectively, these differences are known as “structural variation” (or, “SV”) at present Genetic Science. Any two humans differ by thousands of structural variants which vary greatly in size and phenotypic consequence. The present study (screening) result shows presence of structural variation (SV) along with recognized syndromes like Down Syndromes [4] Edwards Syndrome [2] Patau Syndrome [2] Turner Syndrome [2]. Sex Chromosomes Abnormality [1] others [4] and chromosome rearrangements [8]. Among them two cases have shown typical clinical Phenotype (Beta-Thalassaemia associated with microcephaly, congenital Cataract and rare casese of microdletion 1p36) reported probably first time from India. Though any correlation between the thalassaemia and 1p36 deletion could not be established in this present scope, we hope this kind of novel information will be extremely valuable for further in detail study and enrich the growing literature [9-11]. Our present observation in understanding the role of SV to congenital developmental defects and present preliminary survey data on frequency of chromosome anomalies are also well correlated with other international studies [12,13]. Present survey for chromosomal aberration among of sick neonates and children with dysmorphic features (Hospital record shows 75 unselected cases per month admitted in neonatal and pediatric intensive care unit or NICU & PICU) seems to be a significant information or report from India and will be useful in Birth Defects Databases.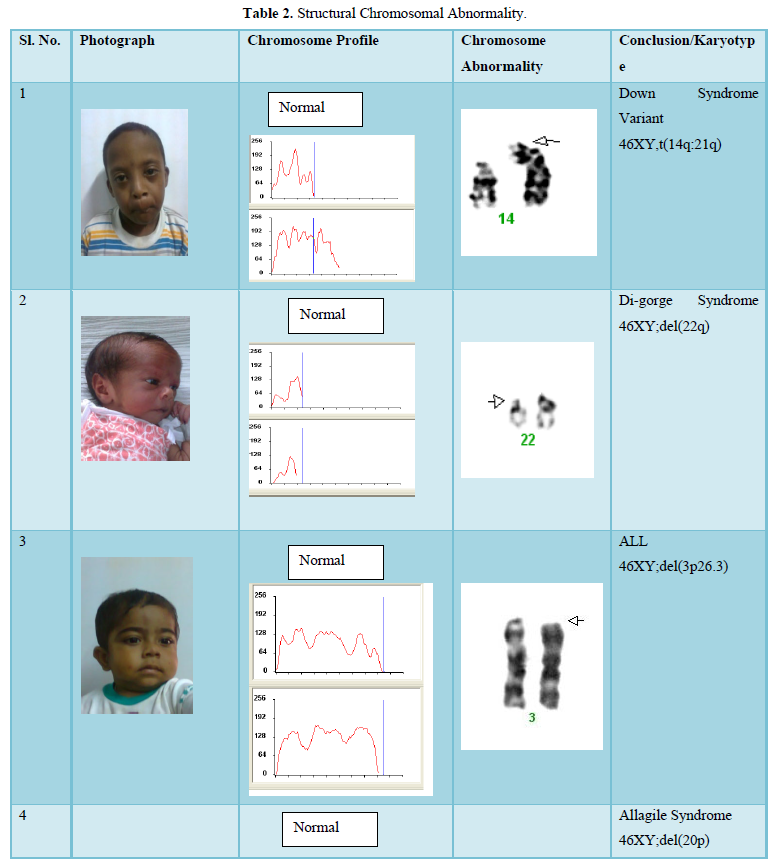



- Le Caiqnec C (2005) Detection of genomic imbalances by array based comparative genomic hybridization in fetuses with multiple malformations. J Med Genet 42: 121-128.
- Boyd PA, Loane M, Garne E, Khoshnood B, Dolk H, et al. (2011) Sex chromosome trisomies in Europe: Prevalence, prenatal detection and outcome of pregnancy. Eur J Hum Genet 19: 231-234.
- Wellesley D, Dolk H, Boyd PA, Greenlees R, Haeusler M, et al. (2012) Rare chromosome abnormalities, prevalence and prenatal diagnosis rates from population-based congenital anomaly registers in Europe. Eur J Hum Genet 20: 521-526.
- Connor O (2008) Chromosomal Abnormalities: Aneuploids. Nature Edition 1: 172.
- Verna RS, Babu A (1989) Human chromosomes: Manual of basic techniques. Pergamon Press, New York 71-72.
- Seabright M (1971) A rapid banding technique for human chromosomes. Lancet 2: 971-972.
- Driscoll DA, Salvin J, Sellinger B (1993) Prevalence of 22q11 micro deletions in DiGorge and velo-cardio-facial syndromes: Implications for genetic counseling and prenatal diagnosis. J Med Genet 30: 813-817.
- Old JM (1991) Methods in molecular biology, Protocols in human molecular genetics, Detection of mutations by the amplification refractory mutation system (ARMS). 9: 77-84.
- Wyandt HE, Tonk VS (2004) Atlas of human chromosome heteromorphisms. Netherlands: Kluwer Academic.Ravnan JB, Tepperberg JH, Papenhausen P, Lamb AN, Hedrick J, et al. (2006) Subtelomere FISH analysis of 11 688 cases: An evaluation of the frequency and pattern of subtelomere rearrangements in individuals with developmental disabilities. J Med Genet Jun 43: 478-489.
- Knight SJ, Regan R, Nicod A, Horsley SW, Kearney L, et al. (1999) Subtle chromosomal rearrangements in children with unexplained mental retardation. Lancet 354: 1676-1681.
- Shaffer LG, Coppinger J, Beth SA, Torchia A, Theisen A, et al. (2008) Comparison of microarray-based detection rates for cytogenetic abnormalities in prenatal and neonatal specimens prenatal diagnosis. 28: 789-795.
- Baker E, Hinton L, Callen DF, Altree M, Dobbie A, et al. (2002) Study of 250 children with idiopathic mental retardation reveals nine cryptic and diverse subtelomericsub telomeric chromosome anomalies. Am J Med Genetics 107: 285-293.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)



