214
Views & Citations10
Likes & Shares
Conclusions: Drug therapy of cancer is costly. Ivon prolonged PFS was worthy of the proposed 9-month $188,000 cost. Durva costs were justified by activities in non-small and small lung cancer. Neoadjuvant Durva activity was demonstrated at bargain costs. More neoadjuvant therapy is needed.
Abbreviations: Durvalumab (Durva), Ivonescimab (Ivon), Pembrolizumab (Pembro), Progression-free-survival (PFS), Programmed cell death ligand 1 (PD-L1), United States (US).
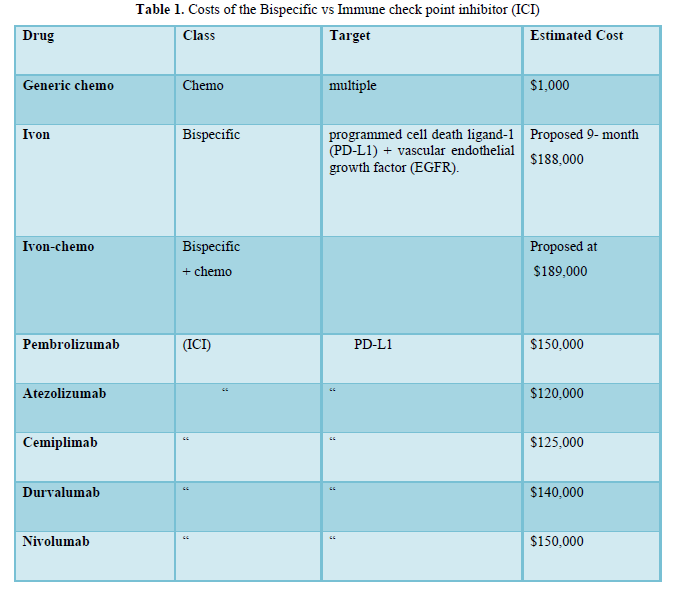
Pembrolizumab (Pembro), the 1st immune check point inhibitor (ICI), was introduced in 2015 for advanced/metastatic a/m NSCLC treatment (1). It blocks the programmed cell death ligand 1 (PD-L1) and has since received universal application in > 15 cancer types. Soon after Pembro discovery, other ICI were developed including Nivolumab, Atezolizumab, Durvalumab (Durva) and Cemiplimab. Durva pharma was wise, avoiding competion with Pembro, used Durva as 2nd -line (2).
The bispecific class Ivonescimab (Ivon) was a fresh glimpse of hope against a/m NSCLC treatment targeting both the PD-L1 and vascular endothelial growth factor (EGFR), essentially working as a double hitter (3,4). Ivon progression-free survival (PFS) was 11.4 months as compared with Pembrolizumab 5.8 at 0.54 hazard ratio (HR) in a/m-NSCLC with negative anaplastic lymphoma Kinase rearrangement (ALK) and epidermal growth factor receptor (EGFR) (HARMONi-2).
The drug has not yet been approved in the US and Europe but is in the process of approved. In view of Ivon reported outcome, originality and sophisticated multistep synthesis, we previously proposed 9-month cost at $188,000 (5).
Ivon- chemo improved PFS at 0.52 HR (95% CI: 0.41-0.66; p< 0.00001). Toxicity was manageable with acceptable safety profile. There was a positive overall survival (OS) trend in the primary analysis without a statistically significant benefit.
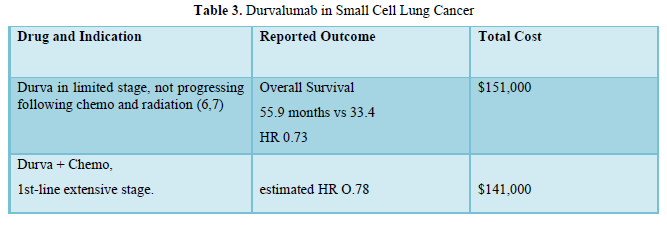
Durvalumab (Durva) is a 2nd-generation ICI, human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody that blocks PD-L1. It was approved in 2017- 2018 in NSCLC and as 1st-line treatment of small cell lung cancer.
(6-8). We planned to compare the 2025 Ivon data and costs with Durva in non-small and small cell lung cancer.
RESULTS
The 5- median annual 2023 cost of 5 monoclonal antibodies: namely Pembro, Durva, Nivolumab, Atezolizumab and Cemiplimab was $163,640. Direct negotiation between the US government and pharma, have significantly decreased drug prices. ICI costs dropped to $120,000-$150,000.
In Table 1, costs of Ivon vs ICI were shown. Ivon as a single agent cost was previously proposed at 9-month at $188,000 (5). Ivon-chemo similarly demonstrated similarly impressive PFS prolongation with acceptable safety data.
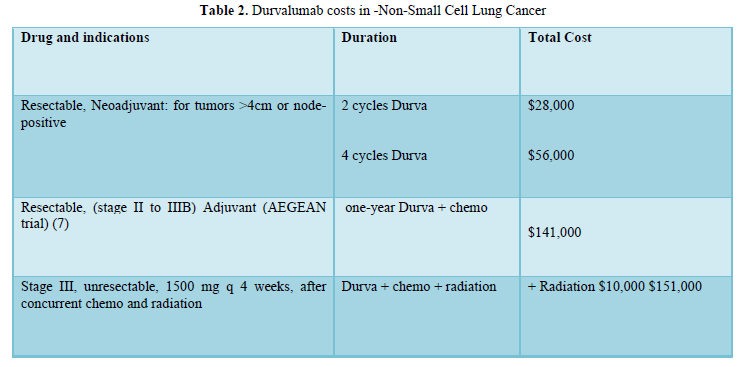
All Durva data were obtained either from the parent company published data or the US Federal Drug Administration (FDA) approval decisions. The AEGEAN trial (7) described Durva use in resectable stage 3 NSCLC following chemo-radiation (Table 2).
Durva role and costs in non-small and small cell in lung cancer are shown in Table 2,3.
DISCUSSION
The attention of drug authorities has been focused on value and cost effectiveness {9,10). Cost matters and counts in limited-budget countries affecting more the no- to low-income patients (11). Important factors in determining cost include the number of eligible consumers (5), originality and the ease vs sophistication of the synthesis process.
Cancer drug costs across the globe are generally considered excessive and unaffordable. In general, drug costs are highly negotiable depending on buyers and amount purchased. The financial toxicity of cancer drugs has been previously portrayed (12-13). The US government has already intervened with direct negotiation with pharma. Cost control is indeed warranted and necessary, provided it keeps the pharmaceutical industry not only afloat but also successful.
The main goal should always remain focused on effective and safe drug development at affordable costs. Any future overall drug cost policy should primarily consider drug superiority and originality. We previously proposed for 9-month Ivon $188,000 cost.
Ivonescimab combined with chemotherapy in patients with EGFR-mutant non-squamous non-small cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor treatment was the basis of (HARMONi-A). The Ivon-chemo cost would be $189,000.
Durva has many indications in various cancers including biliary tract, hepatocellular, mismatch repair deficient endometrial and muscle invasive bladder. Durva activity in non-small as well as small lung cancer was demonstrated at reasonable costs. Durva, 1st-reported on 2017-2018, will lose its drug patency around 2027-2028 or shortly after and prices would probably fall to approximately $100,000.
Of interest is the bargain cost of neoadjuvant therapy. It is essentially a small fraction if compared with the whole treatment cost. It is also necessary to emphasize the overall presumed lesser toxicity of short application of neoadjuvant therapy.
Summary: Ivon and Ivon-chemotherapy significant PFS prolongation in a/m NSCLC deserves wide international approval. Outcome was considered worthy at the proposed but negotiable Ivon $188,000 costs. Durva costs were justified by activities in non-small and small lung cancer. Neoadjuvant Durva activity was demonstrated at bargain costs. More neoadjuvant therapeutic development is warranted. A drug destined for approval and success should be effective, safe and affordable. If unaffordable, value would lose its worth.
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small cell lung cancer. N Engl J Med. 372:2018–28, 2015.
- Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable non–small-cell lung cancer. N Engl J Med.2023;389(18):1672-1684 (Including Supplement and Protocol).
- Zhou C, Chen J Wu L, et al: Phase 3 study of ivonescimab (AK112) vs pembrolizumab as first-line treatment for PD-L1-positive advanced NSCLC: Primary analysis of HARMONi-2. 2024 World Conference on Lung Cancer. Abstract PL02.04. Presented September 8, 2024.
- Wang L, Luo Y, Ren S, et al: A phase 1b study of ivonescimab, a programmed cell death protein-1 and vascular endothelial growth factor bispecific antibody, as first- or second-line therapy for advanced or metastatic immune-therapy-naive NSCLC. J Thorac Oncol 19:465-475, 2024.
- Guirgis, HM. Cost Comparison of The Bispecific Ivonescimab, Immune Check Point Inhibitors and Target Therapy in advanced/metastatic Lung Cancer. J Cancer Sci Treatment, 7(1): 228-231.
- IMFINZI®(durvalumab) [Prescribing Information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2025.
- Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable NSCLC: updated outcomes from the phase 3 AEGEAN trial. Presented at: IASLC 2024 World Conference on Lung Cancer; September 7-10, 2024; San Diego, CA. Abstract OA1
- Cheng Y. et al. Durvalumab after Chemoradiotherapy in Limited-Stage Small-Cell Lung Cancer. N Engl J Med 2024; 391:1313-1327.
- Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology statement: A framework to assess the value of cancer treatment options. J Clin Oncol. June 22, 2015.
- Cherny NI, Sullivan R, Dafni U, et al. A standardized, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies. The European Society for Medical Oncology: magnitude of clinical benefit scale (ESM-MCBS): Oxford University Press; 2015.
- Guirgis, H. Cost matters. journal of Cancer & Oncology (OAJCO) Commentary Volume 7 Issue 1, April 19, 2023. DOI: 10.23880/oajco-16000184.
- Li M, Liao K, Pan I-Wen et al. Growing Financial burden from high cost targeted oral anticancer medicines among Medicare beneficiaries with cancer. JCO oncology practice ,18,11,759, 2002.
- Peppercorn, J. The impact of financial toxicity on cancer care. Clin adv, Hem & Onc. 21, October 2023. specialty pharmacy. Ascopubs.org/journal/op, 20 February 2024.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Rheumatology Research (ISSN:2641-6999)
- BioMed Research Journal (ISSN:2578-8892)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)


