Objective: Some women develop long-term changes in bowel function following radiotherapy, severely impacting on quality of life. Studies in patients treated with pelvic radiotherapy have shown elevated levels of fecal calprotectin in those with chronic gastrointestinal toxicity suggesting that fecal calprotectin, a biomarker of acute GI inflammation, is a viable indicator of long-term GI toxicity. This study aimed to ascertain whether there was an association between GI symptom burden and fecal calprotectin up to two years after radiotherapy.
Design: A longitudinal observational study including women treated with pelvic radiotherapy for gynecological cancer measuring fecal calprotectin from baseline up to 2 years after completion of treatment compared with GI symptom burden using the modified Inflammatory Bowel Disease Questionnaire- Bowel subset (IBDQ-B). A general linear mixed model (GLMM) was used to test the association between IBDQ-B and fecal calprotectin.
Results: A high IBDQ-B score indicates good bowel function; low fecal calprotectin levels indicate no intestinal inflammation. Median baseline fecal calprotectin of 23 women was 35 mcg/g and median IBDQ-B score was 68 (range: 51-70; maximum score 70). Two years after radiotherapy median fecal calprotectin was 14mcg/g and the median IBDQ-B score was 63.5 (range: 31-70). In the GLMM, with fecal calprotectin as the independent variable, the fixed beta coefficient between IBDQ-B scores and fecal calprotectin was -0.20 overall (p=0.030). However, when adding the time interaction, this effect was not statistically significant across the time points (p=0.64). A correlation was found between fecal calprotectin levels at the end of treatment and modified IBDQ-B scores one-year post RT, r= -0.17 (p=0.46) and two years post RT, r= -0.45 (p=0.04). However, when applying a bootstrap analysis of 10.000 samples, the statistical significance was lost two years post RT (p= 0.36).
Conclusion: The results suggest that fecal calprotectin has a weak correlation with IBDQ-B scores and is a poor indicator of long-term radiotherapy-induced GI toxicity.
Keywords: Gynecological Cancer, Fecal Calprotectin, IBDQ-B, Chemoradiation, Gastrointestinal Consequences
Objective: Some women develop long-term changes in bowel function following radiotherapy, severely impacting on quality of life. Studies in patients treated with pelvic radiotherapy have shown elevated levels of fecal calprotectin in those with chronic gastrointestinal toxicity suggesting that fecal calprotectin, a biomarker of acute GI inflammation, is a viable indicator of long-term GI toxicity. This study aimed to ascertain whether there was an association between GI symptom burden and fecal calprotectin up to two years after radiotherapy.
Design: A longitudinal observational study including women treated with pelvic radiotherapy for gynecological cancer measuring fecal calprotectin from baseline up to 2 years after completion of treatment compared with GI symptom burden using the modified Inflammatory Bowel Disease Questionnaire- Bowel subset (IBDQ-B). A general linear mixed model (GLMM) was used to test the association between IBDQ-B and fecal calprotectin.
Results: A high IBDQ-B score indicates good bowel function; low fecal calprotectin levels indicate no intestinal inflammation. Median baseline fecal calprotectin of 23 women was 35 mcg/g and median IBDQ-B score was 68 (range: 51-70; maximum score 70). Two years after radiotherapy median fecal calprotectin was 14mcg/g and the median IBDQ-B score was 63.5 (range: 31-70). In the GLMM, with fecal calprotectin as the independent variable, the fixed beta coefficient between IBDQ-B scores and fecal calprotectin was -0.20 overall (p=0.030). However, when adding the time interaction, this effect was not statistically significant across the time points (p=0.64). A correlation was found between fecal calprotectin levels at the end of treatment and modified IBDQ-B scores one-year post RT, r= -0.17 (p=0.46) and two years post RT, r= -0.45 (p=0.04). However, when applying a bootstrap analysis of 10.000 samples, the statistical significance was lost two years post RT (p=0.36).
Conclusion: The results suggest that fecal calprotectin has a weak correlation with IBDQ-B scores and is a poor indicator of long-term radiotherapy-induced GI toxicity.
Keywords: Gynecological Cancer, Fecal Calprotectin, IBDQ-B, Chemoradiation, Gastrointestinal Consequences
SUMMARY BOX
- What is already known about this subject? Fecal calprotectin is a biomarker for acute gastrointestinal inflammation routinely used in inflammatory bowel disease. Papers have suggested fecal calprotectin could also be a potential marker for chronic radiotherapy induced fibrosis.
2. What are the new findings? This study showed a very weak longitudinal correlation between fecal calprotectin levels and modified Inflammatory Bowel Disease Questionnaire- Bowel subset (IBDQ-B) scores which was not statistically significant across time points in the acute or
SUMMARY BOX
- What is already known about this subject? Fecal calprotectin is a biomarker for acute gastrointestinal inflammation routinely used in inflammatory bowel disease. Papers have suggested fecal calprotectin could also be a potential marker for chronic radiotherapy induced fibrosis.
2. What are the new findings? This study showed a very weak longitudinal correlation between fecal calprotectin levels and modified Inflammatory Bowel Disease Questionnaire- Bowel subset (IBDQ-B) scores which was not statistically significant across time points in the acute or
SUMMARY BOX
1. What is already known about this subject? Fecal calprotectin is a biomarker for acute gastrointestinal inflammation routinely used in inflammatory bowel disease. Papers have suggested fecal calprotectin could also be a potential marker for chronic radiotherapy induced fibrosis.
2. What are the new findings? This study showed a very weak longitudinal correlation between fecal calprotectin levels and modified Inflammatory Bowel Disease Questionnaire- Bowel subset (IBDQ-B) scores which was not statistically significant across time points in the acute or chronic setting
3. How might it impact on clinical practice in the foreseeable future? This study is only the second study including long-term GI toxicity data and suggests that fecal calprotectin testing in patients with symptoms of long-term radiotherapy-induced GI toxicity has limited value if no acute inflammatory pathology is suspected as it does not predict long-term GI toxicity.
BACKGROUND AND AIM
In the UK, 21,000 women are diagnosed yearly with a gynecological cancer. Some women develop long-term changes in bowel function following treatment that severely impact on quality of life but there is no single biomarker to identify long-term GI toxicity.
Fecal calprotectin is a biomarker of inflammation in the GI tract. Calprotectin is a protein produced by neutrophils within the intestinal epithelium when there is epithelial damage through infection and can be found in stools [1-4].
Fecal calprotectin is commonly used in clinical practice to distinguish inflammatory (inflammatory bowel disease, IBD) from non-inflammatory (IBS) causes of diarrhea, and also in known IBD to determine whether a patient has an acute flare of Crohn’s Disease or ulcerative colitis [5-8] and to monitor disease activity and response to therapy. Fecal calprotectin can be raised due to a number of reasons other than inflammatory bowel disease (IBD) such as coeliac disease, diverticular disease, pancreatitis, the use of proton pump inhibitors or non-steroidal anti-inflammatory drugs, small intestinal bacterial overgrowth, bile acid malabsorption, colorectal cancer or polyps [6,9-11].
A fecal calprotectin test is negative for inflammation when ≤15 mcg/g (mcg/g=mg/kg) is found in a stool. The test is positive when the fecal calprotectin level >60 mcg/g which leaves a grey zone for interpretation between >15 and ≤60 mcg/g. Athreshold of 50 mcg/g in a stool has been agreed by NICE [5] in adults with IBD but 100 mcg/g is more precise when diagnosing IBD [12].
The sensitivity of the fecal calprotectin test to correctly identify individuals with inflammatory bowel disease in adults lies between 82% and 98% [13-17]. The ability to correctly exclude inflammatory bowel disease (specificity of the test) reported in the same studies ranged from 68% to 96%.
As there is no gold standard to define chronic radiotherapy-induced GI toxicity and no single biomarker, two studies in patients treated with radiotherapy (RT) for pelvic cancer showed a correlation between raised fecal calprotectin levels and gastrointestinal inflammation during treatment and only one longitudinal study, measuring fecal calprotectin a year and 40 months post treatment, also showed elevated levels in those with chronic gastrointestinal toxicity compared to levels below 60 mcg/g in those without chronic gastrointestinal toxicity [18-20]. These studies suggest that fecal calprotectin could still be a viable indicator of long-term GI toxicity as the need for investigation in clinical practice is indicated by patient reported GI symptom burden.
The mechanism which makes fecal calprotectin indicate gastrointestinal toxicity is assumed to be via the inflammatory pathway caused by radiation damage to healthy intestinal tissue in the acute phase. However, insight into the longer-term mechanism, beyond the acute inflammatory phase, is lacking.
The aims of this study were to assess changes in fecal calprotectin levels during and after RT, whether ascertain there was an association between GI symptoms and fecal calprotectin and to evaluate whether it can predict patients at risk.
METHODS
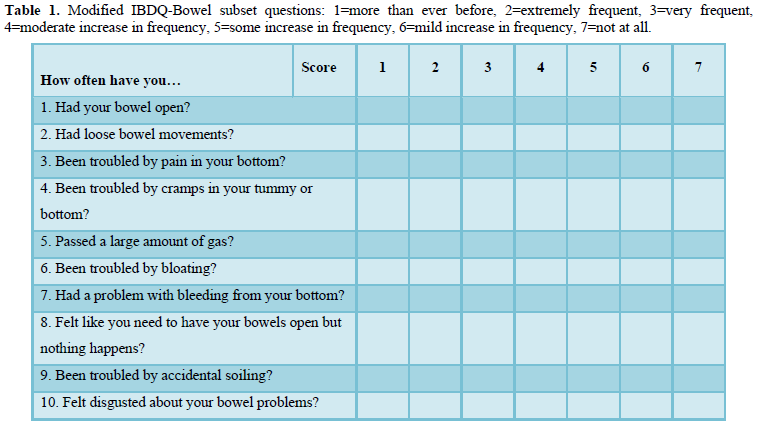
A prospective study including women treated with pelvic radiotherapy for gynecological cancer measured fecal calprotectin from baseline to 2 years after completion of treatment (baseline, beginning of RT, end of RT, and at six months, 12 months 24 months post RT) and GI symptom onset and burden using the modified Inflammatory Bowel Disease Questionnaire - Bowel subset (IBDQ-B) at the same time-points. A modified version of the IBDQ has been developed for oncology patients [21] and has been used in several studies assessing GI toxicity following treatment with radiotherapy [22-23]. The modified IBDQ-B [21] has 10 questions (Table 1) related to bowel function, each is measured on a 7-point scale (troublesome more than ever before-not troublesome at all) with a minimum total score of 10 (worst bowel function) and a maximum total score of 70 (best bowel function). There is an inverse relationship between modified IBDQ-B scores and fecal calprotectin levels as a high modified IBDQ-B score denotes good bowel function and low GI symptom burden, whilst a high fecal calprotectin level is related to inflammation in the intestinal tract.
Associations overall, and across time-points are described using the Pearson correlation coefficient. A general linear mixed model (GLMM) for repeated measures (nested within participants), a population-average model, was used to test the relationship between modified IBDQ-B and fecal calprotectin scores overall across time. The dependent variable was the modified IBDQ-B score and the independent variable the fecal calprotectin level. The model was fitted to account for the different time points. Random effects were omitted because, when fitted, an error message stated there was not enough variance. Population averaged models still account for correlations within multiple responses by individuals, and they have less strict mathematical requirements [24].
A time point and fecal calprotectin interaction was added to the model to determine whether this association varied across time. The model residuals were skewed to the left. To address this, fecal calprotectin, which was skewed to the right, was natural log transformed and modified IBDQ-B scores, which were skewed to the left, was natural log transformed after taking the modified IBDQ-B score for each patient from 71 (i.e. one more than the highest possible modified IBDQ-B score). The model was refitted using these transformed variables and the residuals were normally distributed. Pearson’s correlation between fecal calprotectin level at the end of treatment and modified IBDQ-B scores at 1 year and 2 years post RT were calculated. The Pearson correlation p-values were calculated using a bootstrap analysis of 10,000 samples [25]. Analysis was undertaken using IBM SPSS version 25.
This study was approved by the organization’s Research and Development department and obtained ethical approval (LREC 15/LO/0436).
RESULTS
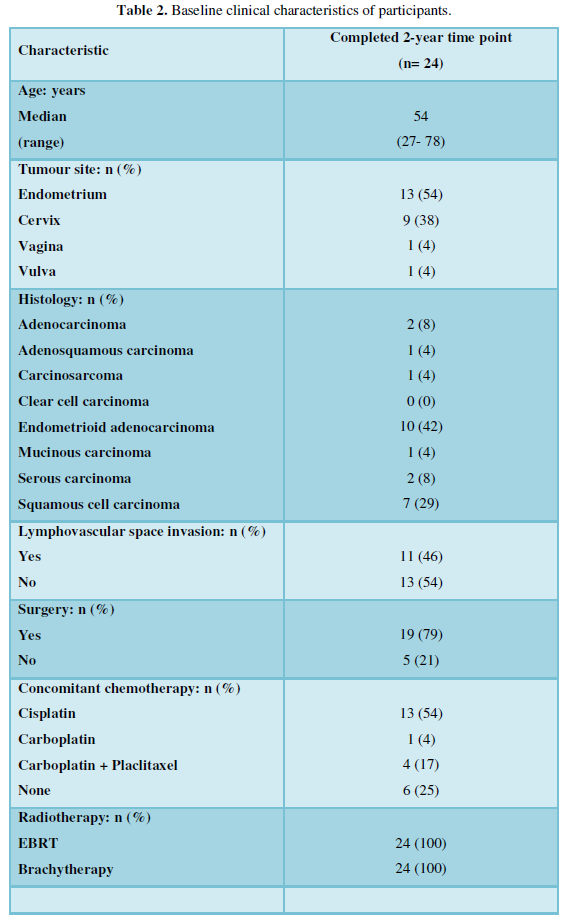
From November 2015 to November 2017, 41 women treated for gynecological cancers consented to participate in the PREDICT CCR 4201 study. This study explores potential indicators to predict long-term radiation-induced GI toxicity in women treated for a gynecological malignancy (CDRF-2014-05-004). Twenty-four participants went on to complete data collection two years after their radiotherapy treatment ended and are included in this analysis.
Of the 24 women included, the median age was 54 years (range: 27-78 years). The main cancer diagnoses were endometrial (n=13, 54%) and cervical (n=9, 38%) cancer. Just under a half (n=11, 46%) had lymphovascular space invasion. The majority had surgery (n=19, 79%) before further treatment with external beam radiotherapy (EBRT) and brachytherapy. Most had concurrent chemotherapy (n= 18, 75%). The median dose of EBRT was 45 Gy (range: 45-58Gy). The median dose of brachytherapy was 8 Gy (range: 6-20 Gy) (Table 2).
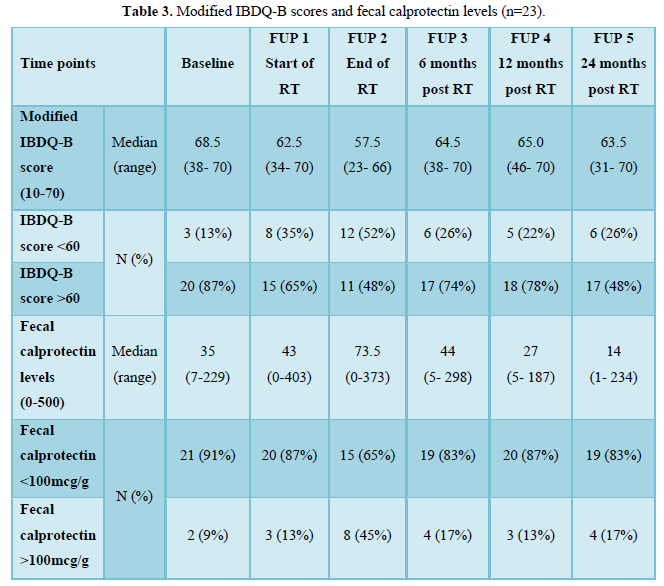
The median baseline modified IBDQ-B score was 68.5 (maximum score 70, range: 38-70). The median baseline fecal calprotectin level was 37 mcg/g which is within the normal limit. There was one outlier with known Crohn’s disease and fecal calprotectin level greater than 2000 mcg/g at baseline and all other time points who was excluded from the final analysis. Without this participant, the median baseline fecal calprotectin level was 35 mcg/g and 2 women (9%) had a fecal calprotectin level of >100 mcg/g and median baseline modified IBDQ-B score was 68 (range: 51-70) with 3 women (13%) having a modified IBDQ-B score below 60. At the end of treatment, the median IBDQ-B score had dropped to 57.5 (range: 23-66) and more than half of the participants (n=12,52%) had a modified IBDQ-B score less than 60 and the median fecal calprotectin level increased to 73.5 mcg/g (range: 0-373) with 8 women (45%) having a level above 100 mcg/g. Two years after radiotherapy the median fecal calprotectin decreased to 14 mcg/g and 4 women (17%) had a fecal caprotectin level above 100 mcg/g; the median modified IBDQ-B score was 63.5 (range: 31-70) and 6 women (26%) had a modified IBDQ-B score below 60 (Table 3).
Of the 8 women with a fecal calprotectin level above 100 mcg/g at the end of treatment, three women had a modified IBDQ-B score below 60 one-year post RT and two had a modified IBDQ-B score below 60 two years after radiotherapy. Only one of those participants had a high modified IBDQ-B score (66/70) at baseline, a raised fecal calprotectin level at the end of treatment (373 mcg/g) and modified IBDQ-B scores of 55/70 (one-year post RT) and 31/70 (two years post RT) (Figure 1).
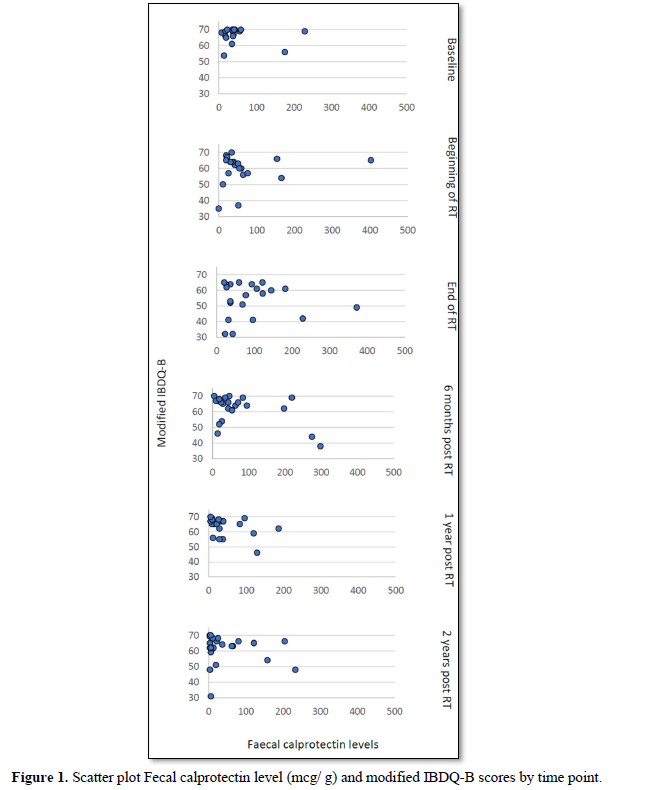
In the GLMM with fecal calprotectin as the independent variable the fecal calprotectin *time interaction was not statistically significant (F=0.69 [numerator df=5, denominator df=43.45], p=0.64) suggesting that if there was an effect of fecal calprotectin on IBDQ scores it did not vary across time. The fixed effect beta coefficient for fecal calprotectin was -0.02 overall (95% CI -0.04 to -0.01, p= 0.030) and was statistically significant. Correlations between fecal calprotectin and IBDQ-B scores were negative except at the beginning of RT (Pre-RT-0.15, Beginning of RT 0.14, End of RT -0.11, 6 months post RT-0.40,one-year post RT -0.18, two years post RT -0.35).
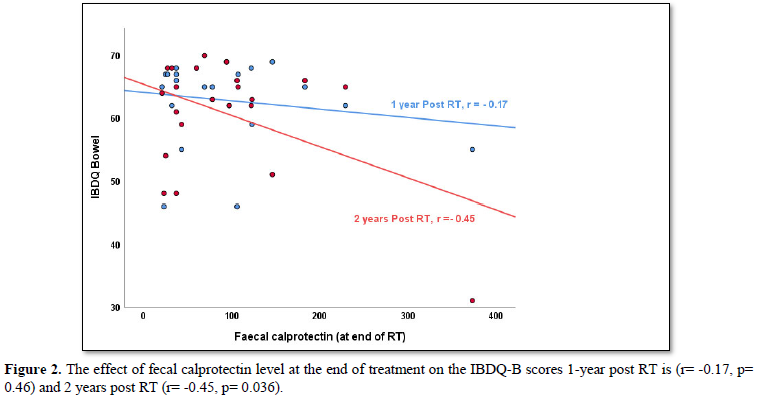
When looking at the correlation between fecal calprotectin levels at the end of treatment and modified IBDQ-B scores one-year post RT, the Pearson correlation was -0.17 (p=0.46) and two years post RT, the Pearson’s correlation was -0.45 (p=0.04). This does not take into account any of the other time points. More so, when applying the bootstrap of 10.000 samples, the statistical significance was lost 2 years post RT (p=0.36).
DISCUSSION
This study shows, in line with previous studies [18-20], that fecal calprotectin levels rise at the end of treatment and modified INDQ-B scores decrease. There was a weak association (fixed effect coefficient: -0.20) between the modified IBDQ-B scores and fecal calprotectin levels (p=0.03). Some heterogeneity across time was observed based on correlations at each time-point but this was not statistically significant (p=0.64).
The pathophysiology of GI injury involves dynamic interactions among the intestinal vasculature, epithelial stem cells and stromal elements including resident and infiltrating macrophages and mast cells. Microvascular injury plays a significant and early role in the development of the acute phase of the GI injury. Vascular ischemia contributes to secondary enterocyte depletion, mucosal barrier breach, bacterial translocation, and structural damage of the intestine and resulting in fibrosis [26-27]. The increase in fecal calprotectin levels seen at the end of radiotherapy are likely a result of acute inflammatory changes. However, with a fixed effect coefficient of -0.20, in clinical practice, fecal calprotectin is a weak indicator for chronic radiation-induced toxicity related to fibrosis 2 years post radiotherapy and fecal calprotectin testing in clinical practice has a limited role in determining radiation-induced toxicity as early fecal calprotectin levels do not predict long term GI toxicity. Long-term, mildly raised fecal calprotectin levels could be due to small intestinal bacterial overgrowth or bile acid malabsorption which are present in women treated for gynecological cancer with radiotherapy up in about 50% [28] and patients warrant further investigation into the potential contributing GI pathology.
As expected, there is an inverse relationship between modified IBDQ-B scores and fecal calprotectin levels detected in stool samples: high modified IBDQ-B scores (good bowel function) correlated with low fecal calprotectin levels (no inflammation present) and vice versa. However, visual inspection of the scatter plot of fecal calprotectin levels and modified IBDQ-B scores at each time point did not show a clear cut-off level where fecal calprotectin correlates more strongly with IBDQ-scores (Figure 2).Even though some correlation was found between fecal calprotectin level at the end of treatment and modified IBDQ-B scores 1-year post RT (r=-0.17, p=0.46) and 2 years post RT (r= -0.45, p=0.04) acknowledgement of the small number of participants is required when interpreting the p-values as the bootstrap analysis lost the statistical significance.
More so, as only one woman had a raised fecal calprotectin level at the end of treatment (373 mcg/g) and low modified IBDQ-B scores both one year and two years following treatment with radiotherapy raised fecal calprotectin levels above 100 mcg/g during treatment caution needs to be taken to explain chronic radiation-induced GI toxicity.
This study sample size was small, but data were collected over six time-points from pre-radiotherapy up to two years after radiotherapy. This small sample limits the conclusions that can be drawn. However, it is the largest cohort to date in women treated for gynecological cancer with follow-up data up to two years after the completion of radiotherapy.
CONCLUSION
There is no single biomarker for long-term radiation-induced GI toxicity, and this is an important area of concern in clinical practice for patients who experience long-term GI symptoms following treatment with radiotherapy. This study was only the second of its kind, evaluating fecal calprotectin as a potential biomarker for long-term radiation-induced GI toxicity. The results suggest that fecal calprotectin provides some evidence of correlation with modified IBDQ-B scores in this patient cohort but is a weak indicator of chronic radiation-induced GI toxicity as measured by the modified IBDQ-B score. Raised fecal calprotectin levels in the acute phase of radiotherapy do not fully explain long-term deterioration in modified IBDQ-B scores one or two years after treatment. Fecal calprotectin testing in patients with long-term radiotherapy-induced GI toxicity has limited value in clinical practice if no acute inflammatory pathology is suspected. In view of the small number of participants in this study, further testing on a larger sample would help to confirm these findings.
ETHICAL/LEGAL CONSIDERATIONS
This article is an original contribution not previously published and is not under consideration for publication elsewhere. Each author has contributed significantly to this research.
CONFLICT OF INTERESTS
The authors declare no conflict of interests.
FUNDING
Ann Muls is part-time funded for a clinical doctoral research fellowship (CDRF-2014-05-004) supported by the National Institute for Health Research and Health Education England. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, Health Education England, or the Department of Health.










