Review Article
Exploitable Options for Curbing the Danger of Covid-19
4526
Views & Citations3526
Likes & Shares
It is understood that the coronavirus is highly mutated, in December, 2019 a new strain of coronavirus emerged which originated in Wuhan, from seafood. The pathogen was named novel coronavirus, while the disease it causes is known as Covid-19, the 2019 coronavirus disease. Bat is the major reservoir host of the virus. Over 34 million Covid-19 cases were registered between 31st of December 2019 and October 1st 2020, specifically 34 029 923 cases. The continent with the highest reported cases is America, with over 40% of the global reported cases. Some of the exploitable options that can be adopted is curbing the Covid-19 menace are cell lines adaptation in vaccine production, proteomic analysis of the viral interactomes, treatment approaches using natural occurring compounds, which will provide cost effective options to low-income countries.
Background: Coronavirus belong to the family Coronaviridae, subfamily Coronavirinae, order Nidovirales and genus Betacoronavirus, it is an enveloped non-segmented, single stranded, positive-sense RNA virus. Coronavirus got its name from it crown-like surface projections. The 2019 coronavirus disease (COVID-19), now a global pandemic is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It started as pneumonia from unknown cause that occurred in Wuhan, Hubei province, China in December 2019. A novel coronavirus known as SARS-CoV-2 was detected in a patient’s throat swab sample on 7 January 2020. World Health Organization announced the disease caused by SARS-CoV-2 as coronavirus 2019 (COVID-19), which has since affected over 70 countries globally making it a pandemic. The virus can be transmitted mainly through droplets or close contact, and is generally susceptible to crowding. Covid-19 thrive better in immunosuppressed individuals, as increased mortality rate been reported in patients with compromised immune system. Patients with HIV, tumor, diabetics, asthma, and high blood pressure are at high risk for this pathogen because of impaired immune function and organ. Symptoms include dry cough, fever, and sore throat, loss smelling sensation, dyspnea, fatigue, body ache and pain. Early detection of patients with this underlying illness infected with the novel coronavirus disease Covid-19 and also understanding its dissemination characteristics will help increase patient cure rates and better control of the SARS-Cov-2 epidemic and growth.
Keywords: Novel Coronavirus, Viral interactomes, Coronaviridae, SARS-CoV-2, Dissemination characteristics
INTRODUCTION
Anatomy of the virus
On its outer surface, Coronavirus has a crown-like spike, it is an enveloped virus with a positive sense single stranded RNA [1], of all RNA viruses, they have the largest genome, with an average size of 32 kilo base pair and a diameter of approximately 125 nm [1]. The viral G-C content is about 43%. Within a helical capsid created by the nucleo-capsid protein (N) the viral genome is packed, which is further encircled by envelope [2]. The viral envelope is linked to at least 3 structural proteins, for the viral assembly, the envelope protein (E) and the membrane protein (M) are responsible, while the spike protein (S) mediates the entry of the virus into the host cells. SARS-CoV-2 binds through its spike to ACE2 (angiotensin-converting enzyme 2) and enables Covid-19 to enter the host cells. A hemagglutinin-esterase (HE) protein is also contained in certain coronaviruses [2].
THE COVID-19 MENACE
The patient characteristics of Covid-19 show many signs of disease vulnerability and severity. Both symptomatic and asymptomatic person in the same vicinity of positive case may get infected. This can occur through respiratory tract droplets transporting the virus or contact with infected surfaces and then using that contaminated hand to touch the mouth, nose and eyes. The virus is transmitted to everyone in close contact with the infected carrier without restrictions. Coronavirus pneumonia development is directly linked with old age, history of smoking, metabolic disorder, and cardiovascular diseases. The global Covid-19 outbreak triggered fear, vulnerability and depression due to its rapid rate of infection, people showing no symptoms with high transmission potential, unspecific symptoms and control mechanisms imposed on the public. Despites this, there is no specific vaccine or antiviral therapy for Covid-19 until now, at this point any effective therapeutic agent will do, despite the fact there is a need for reliable, and cost-effective drugs as an appropriate response to the outbreak.
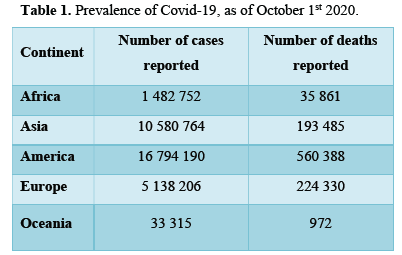
PREVALENCE OF COVID-19, AS OF OCTOBER 1st 2020
Over 34 million Covid-19 cases were registered between 31st of December 2019 and October 1st 2020, specifically 34 029 923 cases. The continent with the highest reported cases is America as shown in Table 1, with almost half the global reported cases, registering approximately 16.7 million cases. Oceania reported the least cases, as depicted in Figure 1, while Asia registered higher number of cases, they have reported better number of cases to death ratio. Europe has recorded a large number of deaths to cases ratio.


SOME TREATMENT OPTIONS USED IN MANAGEMENT OF COVID-19
Antiviral treatment
Some of the antiviral used includes: Oseltamivir, ganiciclovir, arbidol and ritonavir.
Antibiotics therapy
The most employed antibiotics used in Covid-19 management is Azithromycin which was at a time combined with the antimalarial hydroxychloroquine, other antibiotics used were vancomycin, moxifloxacin, meropenem, cefaclor, cefepime, and tazobactam.
Other administered medications
Most patients required respiratory assistance, ventilators, nasal cannula, immunoglobulin, antifungal, corticosteroids, and interferon.
SARS-CoV-2 Vaccines
To prevent and mitigate the morbidity and mortality caused by SARS-CoV-2 infection, SARS-CoV-2 vaccines that elicit protective immune response are crucial. The knowledge available suggests that for the defense from Covid-19 and the prevention of vaccine-enhanced disease, a balanced humoral and Th-1 directed cellular immune response may be essential [3]. Different candidate vaccines are being manufactured and evaluated, these includes vaccines for the viral nucleic acids, live attenuated vaccines, vaccines for protein or peptides subunits, vaccines for the viral vectors [4]. It is not worth discounting the function of mucosal immunity, and several formulations of intranasal vaccines are being investigated [5,6]. Several phase 3 clinical vaccine trials of tens of thousands of participants have been initiated, as at August 2020[7]. It is expected that preliminary results from these trials will be available by the end of 2020 [8]. though, in term of appropriate the numbers of participants, this model may be problematic. In the USA, guidance has been provided by the Food and Drug Administration (FDA), stated that to be deemed successful, a Covid-19 vaccine will have to protect at least 50 percent of vaccinated individuals [8]. At the present, all studies exclude pregnant women, a lot of SARS-CoV-2 mutation have been recognized [9]. Therefore, if the virus subsequently evades immunity to the spike glycoprotein used to create the vaccine, vaccine production could be obstructed [10].
SOME OF THE CURRENTLY PHASE 3 TRIALS VACCINE CANDIDATE
AstraZeneca
A chimpanzee adenovirus-vectored investigational vaccine (ChAdOx1/AZD1222) has been developed by AstraZeneca and Oxford University; the vaccine encodes the SARS-CoV-2 glycoprotein spike [11]. In non-human primates, the vaccine has shown to be highly immunogenic. Study showed that this vaccine elucidates humoral immune response in human. When a vaccine user developed symptoms associated with transverse myelitis, the phase 3 trial was halted and continued as of October 5th 2020, this vaccine needs cold chain system, which may be difficult for low-income countries to use.
Sinopharm
Sinopharm has developed and is evaluating 2 inactivated whole-viruses. The Wuhan Institute of Biological Products has created the first vaccine candidate [12]. Sinopharm researchers announced at the end of August 2020 that they had already started delivering the vaccine to health care workers and groups at elevated risk of infection. The Beijing Institute of Biological Products has produced the second vaccine candidate being evaluated by Sinopharm. In the UAE, a phase 3 trial is taking place. Emergency use of the vaccine was granted by the UAE to health care personnel. Hundreds of thousands of individuals were reportedly given these experimental vaccines by Sinopharm under emergency use condition approved by the government of China [13].
Gamelya
The findings of two phase 1/2 clinical trials of a Covid-19 vaccine which consists of recombinant adenovirus vector serotype 26 (rAd5) and recombinant adenovirus vector serotype 5 (rAD5) have been released by the Gamaleya National Research Centre for Epidemiology and Microbiology [14]. Concerns about the safety and effectiveness of the vaccine have been raised since the vaccine has not yet been evaluated in its phase 3 clinical trial [15].
Johnson & Johnson
A randomized, placebo-controlled, double-blind, phase 3 trial was conducted by the Janssen Pharmaceutical Companies of Johnson & Johnson of their Ad26.COV2. S which is a replication-defective vaccine that expresses glycoprotein spike full-length [16]. It was reported that with this vaccine, a single immunization in rhesus aged 6 to 12 years, induces strong neutralizing antibody responses and provides defense against SARS-CoV-2 challenges [17]. Details of the vaccine’s safety profile and effectiveness have not been officially released by the company yet. The phase 3 trial of this vaccine began on September 23, 2020.
Pfizer and Biotech
A series of Covid-19 vaccines based on mRNA has also been produced by Pfizer and Biotech. They reported BNT162b1, an mRNA vaccine formulated with lipid nanoparticle, nucleoside-modified, induced RBD-binding IgG and neutralizing antibodies, with mainly mild side effects [18]. Individuals vaccinated with BNT162b2 had higher CD4+ and CD8+ T-cell responses to spike glycoprotein and RBD than the participants with BNT162b1 [18]. The candidate chosen for evaluation in phase 3 trials was BNT162b2, although it requires storage at -800C, a fact that may pose logistical issues.
Moderna
An mRNA-based vaccine (mRNA-12733) has been jointly developed by Moderna and National Institutes of Health. Comprising of sequence optimized mRNA encoding the lipid nanoparticles encapsulated spike protein [19]. In non-human primates, the vaccine has shown to be highly immunogenic. This vaccine caused both spiked glycoprotein binding and virus-neutralizing antibody responses in recipients in a phase 1 dose-escalating study [20]. The humoral responses were identical to those found by patients recovering from Covid-19 in convalescent plasma. Cellular responses, primarily biased towards CD4+ Th1 cells, were also produced by the vaccine recipients. In August 2020, a phase 3 clinical trial of mRNA-1273 began in USA. For vaccine deployment, one potential problem is storage requirement of -20°C temperature is needed.
Sinovac Biotech
The CoronaVac is whole virus, inactivated chemically given in a two-dose regimen. An emergency use permit of the vaccine was granted by the Chinese authorities in July, 2020 prior to the start of phase 3 trials [21]. Anti-RBD antibodies were elicited from the vaccine. No data on cellular immune response measurements for this vaccine have been released. In Brazil and Indonesia, a phase 3 clinical trials have been initiated, with the experiment in Brazil aimed at enrolling 9,000 health care workers.
Casino Biologics
A recombinant adenovirus serotype 5 vectored Covid-19 vaccine has been engineered; it expresses the Wuhan-Hu-1 virus strain of SARS-CoV-2 full length spike glycoprotein [22]. On 25th of June 2020, prior to the initiation of phase 3 trials, Casino Biologics and Institute of Biology at the Academy of Military Medical Sciences announced the approval of their adenovirus serotype 5 vector vaccine [21]. For this vaccine no information on the storage condition yet, but it will most likely be cold chain, similar to those of other adenovirus vector-based vaccines and may require storage at -20°C.
EXPLOITABLE OPTIONS TO CURB THE MENACE
Coronavirus is been known to be highly mutated, studying the novel pathogenic protein will assist in drug designing and vaccine production, proteomic analysis of the protein is also paramount in preventing future pandemic. Some of the options to be exploited are:
- Production of vaccine through culture:
Viral isolation could be carried out both on embryonic chicken eggs and on continuous cell cultivation [23]. These circumstances were also associated with a culture-binding approach to enhanced improved biological products such as insulin remedy for xenotransplantation using the goats’ islets [24]. Covid-19 cultivation and isolation on Air liquid interface culture, Vero cell line, HEK-293 cells, Chinese hamster ovary for production vaccine. This good approach would help low-income countries also reduce the risk of Covid-19 in their region.
- Proteomic analysis:
The novel upstream Covid-19 regulator involved in the genesis of the viral pathogenesis must be recognized, it is very important to evaluate the upstream regulator. Intra-viral and virus-host interactomes can be identified following standard method of affinity mass spectrometry. Also, protein engineering for the design of potential drug for Covid-19 should be exploited.
- Phytotherapy:
In order to explore natural drugs with lesser side effect and cost, a natural compound purple coneflower, is one among the plant reported to have active components such as chicoric acid, polysaccharides and echinacoside. This plant extract is known to stimulate immune response. The aqueous fractions of the stems, leaves, and flowers of Echinacea purpurea possess potent antiviral activity against HSV1 and HSV2 and hemagglutinin of influenza virus, as coronavirus also possess a similar protein. This activity was attributed to the plant extract components, polysaccharide and cichoric acid. A potent antiviral photosensitizer was seen in the ethyl acetate and ethanol soluble fractions of the plants stem and leaves. Another molecular docking research in which one of the plants components, L-chicoric acid was docked against the protein HIV-1 integrase by, it shows a very good binding modes between the ligand and the viral integrase. This explains its reported potency which is consistent with the experimental data available. Exploring medicinal will give both option of producing an antiviral agent and immune stimulators. This will also serve as a preparedness approach for any possibility of future SARS-CoV-2 mutation. More plant extracts have shown both antiviral properties and ability to confer immunity in human, a good example of such plant is Asparagus africanus which has proven to be important pharmacologically.
CONCLUSION
This review highlighted the need to look at some approaches to curb the danger of the global pandemic Covid-19. Coronavirus is been known to be highly mutated, studying the novel pathogenic protein will assist in drug designing and vaccine production, proteomic analysis of the protein is also paramount in preventing future pandemic. In addition to these approaches, low-income countries will find it difficult to meet up with the demand of Covid-19 vaccines currently in phase 3 clinical trials, certain medicinal plants have some pharmacological potentials and can be used as cost effective chemotherapy for infectious agents such as SARS-CoV-2.
- Cui J, Li F, Shi ZL (2019) Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 17: 181-192.
- Su S, Wong G, Shi W (2016) Epidemiology, Genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 24: 490-502.
- Graham BS (2020) Rapid COVID-19 vaccine development. Science 368: 945-946.
- Poland GA, Ovsyannikova IG, Crooke SN, Kennedy RB (2020) SARS-CoV-2 vaccine development: Current status. Mayo Clin Proc 95(10): 2172-2188.
- Hassan AO, Kafai NM, Dmitriev IP, Fox JM, Smith BK, et al. (2020) A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell 183(1): 169-184.
- Bloomberg News China starts testing COVID-19 nasal spray vaccine. Accessed on: September 11, 2020. Available online at: https://www.bloomberg.com/news/articles/2020-09-11/china-starts-testing-covid-19-nasal-spray-vaccine-in-world-first
- MediciNova announces that its intranasal COVID-19 vaccine successfully induced systemic IgG and mucosal IgA neutralizing antibodies against SARS-CoV-2 in mice using BC-PIV vector technology. Accessed on: September 23, 2020. Available online at: https://finance.yahoo.com/news/medicinova-announces-intranasal-covid-19-103000673.html
- US Food and Drug Administration Coronavirus (COVID-19) update: FDA takes action to help facilitate timely development of safe, effective COVID-19 vaccines. Accessed on: June 30, 2020. Available online at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-action-help-facilitate-timely-development-safe-effective-covid
- Long SW, Olsen RJ, Christensen PA (2020) Molecular architecture of early dissemination and massive second wave of the SARS-CoV-2 virus in a major metropolitan area. Med Rxiv. Accessed on: September 23, 2020. Available online at: https://www.medrxiv.org/content/10.1101/2020.09.22.20199125v3
- Poland GA (2020) Tortoises, hares, and vaccines: a cautionary note for SARS-CoV-2 vaccine development. Vaccine 38: 4219-4220.
- van Doremalen N, Lambe T, Spencer A (2020) ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. Bio Rxiv. Accessed on: May 13, 2020. Available online at: https://www.biorxiv.org/content/10.1101/2020.05.13.093195v1
- Xia S, Duan K, Zhang Y, Zhao D, Zhang H, et al. (2020) Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: Interim analysis of 2 randomized clinical trials. JAMA 324: 951-960.
- Deng C (2020) China injects hundreds of thousands with experimental COVID-19 vaccines. Accessed on: September 12, 2020. Available online at: https://www.wsj.com/articles/china-injects-hundreds-of-thousands-with-experimental-covid-19-vaccines-11599834029
- Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatullin AI, Shcheblyakov DV, et al. (2020) Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomized phase 1/2 studies from Russia. Lancet 396: 887-897.
- Bucci E (2020) Note of concern. Accessed on: September 17, 2020. Available online at: https://cattiviscienziati.com/2020/09/07/note-of-concern/
- Loftus P (2020) For COVID-19 vaccine, J&J plans 60,000-subject pivotal trial. Accessed on: August 20, 2020. Available online at: https://www.wsj.com/articles/for-covid-19-vaccine-j-j-plans-60-000-subject-pivotal-trial-11597936496
- Mercado NB, Zahn R, Wegmann F, Loos C, Chandrashekar A, et al. (2020) Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 586(7830): 583-588.
- Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, et al. (2020) Phase 1/2 study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 586(7830): 589-593.
- Corbett KS, Flynn B, Foulds KE (2020) Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med 383(16): 1544-1555.
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, et al. (2020) An mRNA vaccine against SARS-CoV-2-preliminary report. N Engl J Med 383(20): 1920-1931.
- Wee SL, Simões M (2020) In coronavirus vaccine race, China strays from the official paths. Accessed on: July 16, 2020. Available online at: https://www.nytimes.com/2020/07/16/business/china-vaccine-coronavirus.html
- Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, et al. (2020) Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomized, first-in-human trial. Lancet 395: 1845-1854.
- Bala JA, Balakrishnan KN, Abdullah AA, Mohamed R, Haron AW, et al. (2018a) The re-emerging of Orf virus infection: A call for surveillance, vaccination and effective control measures. Microb Pathog 120: 55-63.
- Yusuf L, Bala JA, Aliyu IA, Kabir IM, Abdulkadir S, et al. (2020) Phytotherapy as an alternative for the treatment of human papillomavirus infections in Nigeria: A review. Afr J Clin Exper Microbiol 21: 175-184.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Oncology Clinics and Research (ISSN: 2643-055X)
- Dermatology Clinics and Research (ISSN:2380-5609)



