Research Article
The Preventive Effect of Artemisia Campestris Infusion Against Hepatotoxicity
5036
Views & Citations4036
Likes & Shares
Background: Hepatic diseases and intoxications are of great incidence and threat human lives. Some hepatic pathologies are till now untreatable but could be prevented.
Objective: This paper aims to evaluate the protective effect of Artemisia campestris as diet- supplementation against hepatic disorder.
Experimental Design: Hepatic toxicity was induced by CCl4 i.p. injection (0.5 ml/ rat) in three groups: G2 (non-treated), and G3 and G4(treated by oral intake of the plant infusion at doses of 10 g/L and 50 g/ L, respectively). A fourth group (G1) was kept untreated and served as sham negative group. The chemical properties and anti-oxidative potential of the infusion were determined in vitro. Selected hematological and biochemical parameters and the histological features were analyzed. The hepatic-MDA concentration was also determined.
Results: Chemical analyses showed the richness of the plant in flavonoids, polyphenols and tannins. The daily oral intake re-established CCl4-induced tissular damages and hepatic enzymes status. The infusion showed significant scavenging potential of DPPH and induced significant decrease of MDA levels in comparison to G2 group.
Conclusion: Our findings approve the preventive effect of A campestris against NAFLD and might be used as dietary supplement to treat the disease. Its mechanism of action involves anti-inflammatory and anti-oxidative pathways.
Keywords: Artemisia campestris, Infusion, Diet supplement, Liver intoxication
INTRODUCTION
The liver constitutes a cross-road functional organ that maintains the organism homeostasis; and ensures its metabolism processes and detoxification from xenobiotics and their toxic derivatives [1]. On beneath of its multidimensional functionality hepatic tissues are always impinged by several pathological events. Mostly, it is affected by viral infection, such as hepatitis viruses, and intoxication by chemicals, drugs and food derivatives. The chrono-biological processes of hepatic pathologies go from acute injury and fulminant hepatic failure to much more chronic diseases including viral hepatitis, cirrhosis, steatosis, fibrosis, and cancer. They rely on several risk factors, such nutritional quality, alcohol intake, age and obesity [2-4]. While there are great advances in managing liver diseases and intoxication, their fatality burden is still higher [5]. During the last decades, ethnomedicine is sought with great concern for its beneficial therapeutic effects against several diseases, low toxicity and cost. In such context, several natural phototherapeutic products exhibit important protective effects against liver disorders [6,7]. Huge plethora of studies bring proof for the health protecting potential of Artemisia herbs (Asteraceae family). They are dotted with several biological activities (anti-inflammatory, anti-nociceptive, anti- viral, anti-bacterial, anti-oxidative activities, etc.) [8]. In particular, these plants owed great interest since the discovery of anti-malaria Artemisinin component [9], that also exhibits anti-hepatitis viral effects [10-12]. This prompted us to investigate the anti-hepatotoxic effect of A. campestris, in a murin experimental model of carbone tetrachloride (CCl4)- induced hepatoxicity.
MATERIALS AND METHODS
- Artemisia campestris’ infusion
Artemisia campestris (Asteracae family) aerial parts have been collected from the South-East mountains (Matmata) of Tunisia, during the flowering-fructification period. They were left to dry at ambient temperature in dark and dry condition. The used infusions were freshly prepared each day of the experiment. they consisted of decoctions of 10 g or 50 g of the powdered aerial part of the plant that were boiled in 1 Lof water, for 5 minutes. The infusion was filtrated before its oral administration to rats. The obtained solution was of clear yellowish color. This method might prevent the loss of artemisinin derivatives that melts at about 150°C [13].
- Chemical characterization of Artemisia campestris’ infusion
- FTIR spectroscopy
The chemical composition of the plant extract was evaluated using the Fourier Transform Infra-Red (FTIR) spectroscopy (Shimadzu, FTIR-8400S, CE). To do so, a sample of the infusion (1mg of the plant dissolved into 1 ml of distilled water) was mixed with 0.2 g of Potassium Bromure and compressed to obtain a lozenge. The FTIR spectrum of this later was evaluated at wave lengths ranging from 500 to 4000 cm-1.
- Quantification of total polyphenols, flavonoids and tannins
The amounts of total polyphenols, flavonoids and tannins were spectrophotometrically determined according to the methods described respectively by Dallali [14], Zhishen [15] and Broadhurst and Jones [16]. The principle of polyphenols quantification method is based on the reaction of the sample with Folin-Ciocalteu that reacts with polyphenols in alkaline solution to give a blue color. The intensity of the color was determined at 725 nm. Gallic acid was used as standard and results were expressed as µg of gallic acid equivalent (EAG) per mg. Flavonoids react with aluminium trichloride (AlCl3) to result in a yellow color that was read at 510 µm. The quercetin was used as standard and results were expressed in µg quercetin equivalent per mg of extract. For condensed tannins quantification, samples of the extract were mixed with vanillin in acidified milieu. The intensity of the formed reddish color was then measured at 500 nm. The concentration of tannins was expressed in µg of tannic acid equivalent per mg of extract (µg ETA/mg).
- Animal housing and experimental design
Twenty male rats (white Wistar) weighing 220 ± 42 g were used in this study. Rats were caged, per five, in standard conditions (Temperature of 23 ± 3°C, air- humidity of 40 to 50 %, and light/ dark cycle of 12 hours). They received standard food pellets and tap water, free ad libitum, for one week, before the beginning of the experiment. Thereafter they were repatriated into four different groups of five rats each. The first group did not receive any treatment and served as normal control (G1). It was intraperitoneally injected with 0.5 ml of vehicle (olive oil). Hepatotoxicity was induced in the three other groups (G2, G3 and G4) of animal by a single intraperitoneal injection of 0.5 ml of CCl4 (dissolved into olive oil at 37%), per rat. G1 and G2 continued to receive tap water for drinking. In G3 and G4 the drinking water was replaced by the plant infusion, respectively at a dose of 1g/100 ml and 5g/100 ml. At the 15th day, rats were euthanized by overdose exposure to diethyl ether, and blood samples and organs were harvested. The blood was collected into ethylenediamine tetra acetic acid (for hematology) or heparin (for biochemistry) containing tubes. Heparinized blood was centrifuged for 15 minutes at 3000 rpm and plasma was aspirated and conserved at -20°C until the biochemical analysis. Our research was conducted following the guide for care and use of laboratory animals (2010) [17] and was approved by a local committee at our institution.
- Determination of the antioxidant activity of Ac-infusion
- DPPH test
The measurement of the anti- DPPH (1,1- diphenyl-2-picryl hydrazyl) scavenging potential of the extract was carried out according to the method described by Tailor and Goyal [18]. Briefly, solutions of dissolved extracting distilled water were prepared, in order to obtain different concentrations (ranging from 50 µg/ml to 500 µg/ml). 1 ml of DPPH was mixed with 3 ml from each solution, vigorously shacked and incubated for 30 min at room temperature. The absorbance of the resultant solution was then determined at 517 nm. The ascorbic acid was used as standard reference. The assay was made in triplicate and the IC50 of was calculated using log-dose inhibition curve.
- FRAP test
The ferric reducing antioxidant power (FRAP) was determined following the method described by Quisumbing [19], using BHT as standard. The reducing potential of the extract was expressed as percentage of which of the standard. Briefly, each sample was mixed with BPS (0.1 M, pH 6.6) and potassium ferricyanide K3[Fe(CN)6] (1% w/v) ; after vertexing and incubation at 50°C for 20 minutes, the solution was acidified using trichloroacetic acid (10%). The solution was centrifuged for 10 mn at 3000 rpm, and the supernatant was aspirated and mixed with Iron chloride (FeCl3, at 0.1%) and distillated water. The absorbance of the later solution was determined after 30 mn of incubation at 28°C, at 700 nm. Each assay was made in triplicate.
- Liver’s MDA determination
The level of malondialdehyde (MDA) was evaluated in homogenates of hepatic tissues following the method of Buege and Aust [20]. Briefly, homogenated fragments of liver in buffer saline solution (1g/1ml, w/v) were centrifuged at 9000 rpm for 15 minutes. the supernatant cytosolic fraction was then aspirated and used for MDA quantification. 200µl of each sample of the cytosolic fraction were mixed with 2 ml of thiobarbituric acid and heated (90°C) for 10 minutes. The resultant solution’s color intensity was measured at 530 nm. MDA was expressed as mmol/ml.
- Hematological and Biochemical analysis
Hematological and biochemical analyses were carried out using automated apparatus according to the manufacturer’s instructions (EasyRa ® Medica corporation Bedford, USA). White blood (WBC) red blood (RBC) and platelets (PLT) cell counts, lymphocytes percent (Lymph %), hemattocit (HCT) and hemoglobin concentration (HGB) were determined in whole blood collected into ethylenediamine tetra acetic acid containing tubes. Alanine transaminase (ALAT), Aspartate transaminase (ASAT), total bilirubin and blood nitrogen- urea were also quantified, in plasma.
- Histological study
Fragments of liver were fixed in 4 % formalin for 48 hours. Thereafter they were embedded into paraffine. Sections of 4 to 5µm thickness were subjected to routine hematoxylin- eosin staining. Slides were microscopically examined either at 100 x or 400 x magnification.
- Statistical analysis
Statistical analyses were carried out using SPSS program for Windows.20 (IBM corporation). Comparison between different groups was performed by ANOVA- test followed by LSD one, with significance considered at 5%.
RESULTS
- Chemical characterization
- FTIR- spectroscopy:
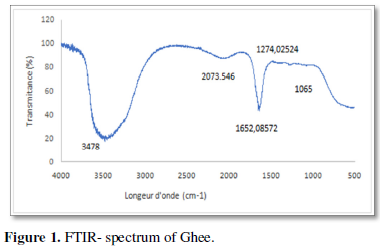
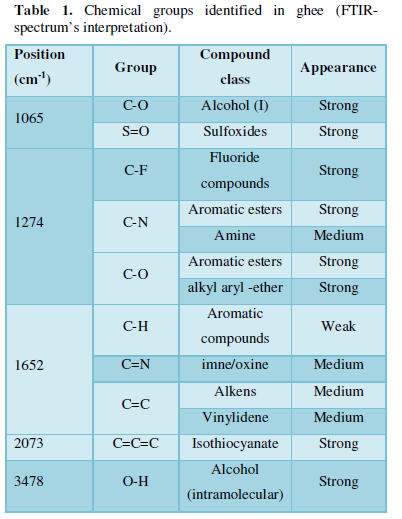
The FTIR spectrum shows the presence of various chemical compounds at length positions ranging from 1065 cm-1 to 3478 cm-1 (Figure 1). It strongly suggests the presence of primary and intramolecular alcohols, aromatic esters, sulfoxides, fluorides, alkyls and isothiocyanate (Table 1).
- Total polyphenols tannins and flavonoids
Spectrophotometric analyses revealed that A campestris aqueous extract contains important amounts of polyphenols (428.52± 92.37 µg EAG.mg-1), flavonoids (37.62±50,04 µg EQ.mg-1) and tannins (10.95±28.94 µg ETA.mg-1).


- Antioxidative stress activity
- In vitro assays:
The DPPH scavenging test revealed that A campestris ’infusion inhibits the DPPH radical with an IC50 % of 35.62 ± 4.75 µg/ml. The free radical scavenging potential was about three folds greater than which of the ascorbic acid (101.23± 6.35 µg/ml). In contrary, its reducing potential, as tested by the FRAP assay, was very weak (IC50 over 500 µg/ml) in comparison to BHT (47.33 ± 11.45 %).
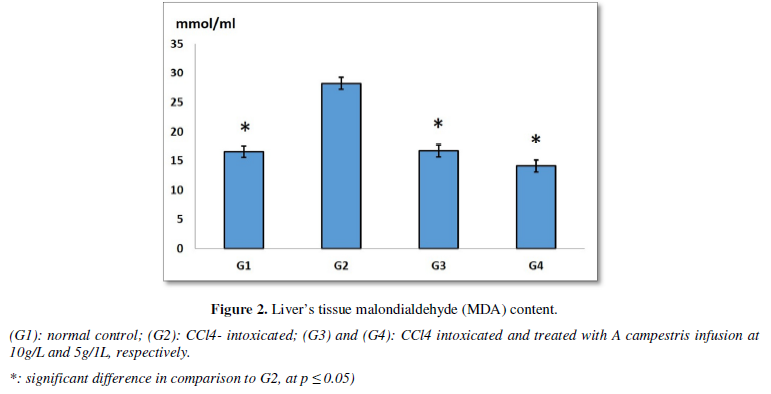
The in vivo study, showed that the daily oral intake of the plant infusion, for two weeks, induced significant decrease in MDA hepatic levels, both in G3 (16.66 ± 0.43 mmol/mL) and G4 (14.09 ± 0.35 mmol/mL), in comparison to CCl4-treated rats (28.18 ± 0.20 mmol/mL). However, it was unchanged when compared to normal control (G1: 16.50 ± 0.50 mmol/mL) (Figure 2).

- Hematological and biochemical effects
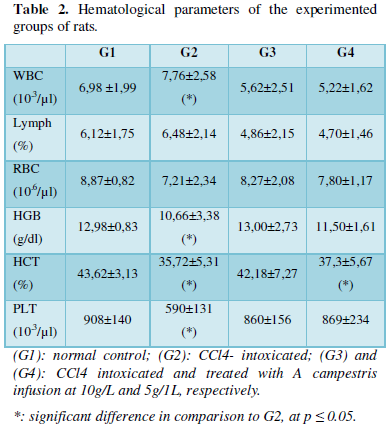
The hematological analyses showed that CCl4 single injection to rats induces significant increase in white blood cells count (7.76±2,58 103/µL), in comparison to sham group (6,98 ±1,99 103/µL). This augmented number of WBC was fully reestablished by the oral intake of the infusion (5,62±2,51 103/µL and 5,22±1,62 103/µL, respectively for G3 and G4). Furthermore, there was relevant decrease (p < 0.050) in hemoglobin concentration, platelets count and hematocrit following the intra-peritoneal injection of CCl4. Almost of these perturbations returned to the normal range when rats received the infusion by oral administrating at the two chosen doses (Table 2).
Similarly, the oral intake of the herbal infusion induced significant diminishing of the hepatic enzymes’ levels in comparison to CCl4- treated rats. In fact, ALAT and ASAT levels were of 63.24 ± 12.03 UI and 202.60 ± 15.07 UI in G3, respectively; and 59.04 ±09.37 UI and 155.08 ± 15.74 UI, respectively in G4. These values were comparable to which of the normal control, but they were Higher in G2 (78.22 ± 14.75 UI and 361.10 ± 24.86 UI, respectively). Blood nitrogen urea and total bilirubin concentrations were also elevated in CCl4 -intoxicated rats than in the other groups (Figure 3).
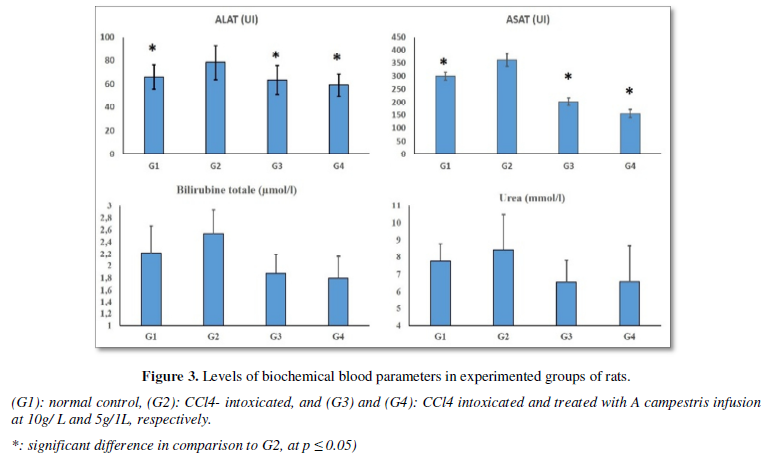


- Histological study
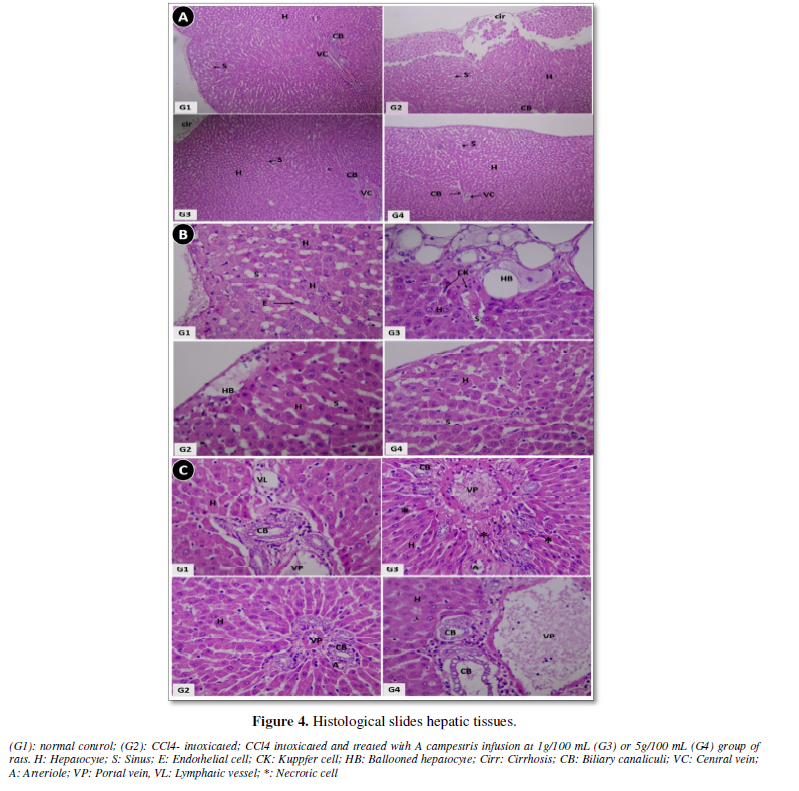
Figure 4A (x 100 magnification) shows cirrhosis with hepatocytes desegregates (cirr) in rats receiving only CCl4 by intraperitoneal injection (G2). The oral intake of the herb’s infusion significantly ameliorates the CCl4 induced damages, both at 10/L (G3) and 50 g/L (G4). These later presents nearly a normal hepatic feature as the control group (G1). At higher magnification (x400), the sinusoidal zone (Figure 4B) presents prominent ballooning of hepatocytes that originates from fat deposition in G2. This ballooning was diminished in G3 and approximately absent in G4. Furthermore, there was hepatocellular aplasia which associates to huge inflammatory cells infiltration and fibrosis around the blood vessels in CCl4- treated rat (G2), as shown in Figure 4C. A re-establishment of the conditions when rats are treated with A. campestris infusion.
DISCUSSION
Non- alcoholic fatty liver diseases (NAFLD) are among the most represented pathologies that affect about the quartile of adulthood, and associate to higher mortality [5,21]. NAFLD range from steatosis to much severe cirrhosis cases. They develop by excessive accumulation of fats in hepatic tissue that lately progress to fibrosis through inflammatory process resulting in scarring features. Subsequently a fibrosis stage could develop, and might be ranked into four different grades of severity. Untreated fibrosis leads to marked liver’s nodulation and appearance of the chronic state of cirrhosis. The etiology of NALFD is diverse and includes hepatitis viruses, ischemia, pregnancy, drug intake and intoxication [22]. Obesity, diabetes, hyperlipidemia and metabolic syndrome are associated to NALFD [21]. Non pharmacogical and complementary and alternative medicine are sought as determinant in preventing the disease development and progression. Herein, we investigated the possibility to use A campestris infusion as a nutritional supplement to counteract liver’s pathology induced by CCl4, in a murine experimental model. Our findings revealed that daily intake of A. campestris infusion at lower doses prevents the development and progression of the CCl4-induced cirrhosis. In fact, there was re-establishment of the normal histological features of the hepatic tissues which associated to hepatic enzymes return to the normal range. The lipid-peroxidation level was also diminished following the treatment of CCl4- intoxicated rats by the herb infusion. Similar investigations brought approval of such preventive effects of Artemisia herbs. They were shown to improve free fatty acids induced steatosis, and exert anti-lipidemic and antifibrotic activities [23-32]. Previous studies revealed that the anti-hyperlipidemic activity of Artemisia extracts involved the suppression of the expression of peroxisome proliferator activated receptor γ (PPARγ) and leptin release, thus inhibits the cytoplasmic accumulation of fats [33]. Great consensus is attributed to the anti-oxidative and anti-inflammatory [34-38] effects of the plant molecules in the preventive/ healing mechanism. The chemistry of the plant extract showed the presence of diverse biologically active molecules, such as flavonoids, polyphenols and tannins. These molecules are known to chelate the reactive oxygen species or inhibit their formation through modulation of anti-oxidative stress enzymes. They also modulate the inflammatory process [39-41]. In fact, flavonoids extracted from Artemisia herbs did inhibit IL-12, and nitric oxide, interleukin- 1β and 6 and prostaglandin E2 production by immune cells [42,43]. Artemisia extracts have been also shown to ameliorate the cholestatic liver fibrosis and faces the free fatty acids induced apoptosis in hepatic cells (HepG2) [23,26]. The anti-apoptotic activity of the extract is suggested to be mediated through the inhibition of caspases 3 and 8 in intoxicated hepatocytes [44] thus favorizes the regeneration and repair of damaged tissue. Recent studies focused on nicotinamide N methyltransferase control to manage obesity and hyperlipidemia [45,46]. In such context, extracts from artemisia were found to reduce the nicotinamide adenine dinucleotide phosphate induced lipid peroxidation in liver tissues [47].
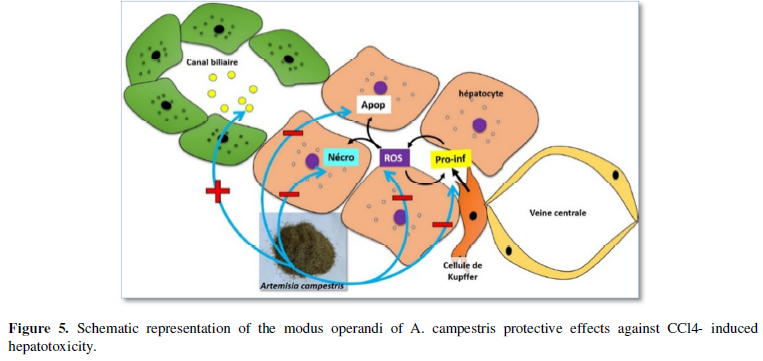
The daily intake of A campestris infusion, at low doses, might prevent NAFLD development and progression to severe cases. The herb’s molecules might prevent the reactive oxygen species (ROS) generation and pro-inflammatory factors (Pro-inf) release in hepatocytes and protect them from damages. Theses extracts could also regulate the fatty acids turn-over in biliary and inhibit their apoptotic effects in hepatocytes (Figure 5). Obviously, Artemisia plants have been used since the ‘antiquité’ to treat several diseases and might be considered as safe, except for people showing hypersensitivity against the plant, and could be envisaged as candidate therapeutic to relieve NAFLD in humans.
CONCLUSION
Our findings strongly suggest the preventive daily intake of Artemisia campestris infusion, at low doses, to prevent NAFLD development and pathogenesis progression. Thus, it could be used as nutritional supplement in order to manage and prevent hepatic diseases. Further investigations are in need to better outline the mechanistic scheme of the observed effects and to translate it for clinical usage.
CONFLICTS OF INTERESTS
None.
- Trefts E (2017) Hepatic Integrin-Linked Kinase Determines Glucose Homeostasis through a Specific Glucose Partitioning Program. Diabetes.
- Hoekstra LT, de Graaf W, Nibourg GAA, Heger M, Bennink RJ, et al. (2013) Physiological and biochemical basis of clinical liver function tests: A review. Ann Surg 257(1): 27-36.
- Gulati K, Reshi MR, Rai N, Ray A (2018) Hepatotoxicity: Its mechanisms, experimental evaluation and protective strategies. Am J Pharmacol 1(1): 1-9.
- Di Ciaula A, Baj J, Garruti G, Celano G, De Angelis M, et al. (2020) Liver steatosis, gut-liver axis, microbiome and environmental factors. A never-ending bidirectional cross-talk. J Clin Med 9(8): 2648.
- Abubakar I, Tillmann T, Banerjee A (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 385(9963): 117-171.
- Liu Q, Zhu L, Cheng C, Yi-Yang H, Feng Q (2017) Natural active compounds from plant food and Chinese herbal medicine for nonalcoholic fatty liver disease. Curr Pharm Des 23(34): 5136-5162.
- Subramoniam A, Pushpangadan P (1999) Development of phytomedicines for liver disease. Indian J Pharmacol 31(3): 166.
- Dib I, Angenot L, Mihamou A, Ziyyat A, Tits M (2017) Artemisia campestris L.: Ethnomedicinal, phytochemical and pharmacological review. J Herbal Med 7: 1-10.
- Tu Y (2011) The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med 17(10): 1217-1220.
- Han J, Yan-ling Z, Li-mei S, Feng-jiao H, Xiao-he X (2005) An experiment on standardized cell culture assay in assessing the activities of Composite Artemisia Capillaris Tablets against hepatitis B virus replication in vitro. Chin J Integr Med 11(1): 54-56.
- Chang-An G, Tong-Hua Y, Xiao-Yan H, Yang J, Yun-Bao M, et al. (2018) Anti-hepatitis B virus effects of the traditional Chinese herb Artemisia capillaries and its active enzymes. J Ethnopharmacol 224: 283-289.
- Zhao Y, Chang-An G, Chang-Li S, Yun-Bao M, Xiao-Yan H, et al. (2014) Polyacetylenes and anti-hepatitis B virus active constituents from Artemisia capillaries. Fitoterapia 95: 187-193.
- Liao F (2009) Discovery of artemisinin (qinghaosu). Molecules 14(12): 5362-5366.
- Dallali S, Aloui F, Selmi H, Sebei H (2018) Comparison of the chemical composition and the antioxidant activity of the leaves of Carob tree (Ceratonia siliqua L.) collected in three sites of Djebel Zaghouan (Tunisia). J New Sci Agric Biotechnol 21: 3429-3438.
- Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64(4): 555-559.
- Broadhurst RB, Jones WT (1978) Analysis of condensed tannins using acidified vanillin. J Science Food Agric 29(9): 788-794.
- National Research Council (2010) Guide for the care and use of laboratory animals. National Academies Press.
- Tailor CS, Goyal A (2015) Isolation of phytoconstituents and in vitro antilithiatic by titrimetric method, antioxidant activity by 1, 1-diphenyl-2-picryl hydrazyl scavenging assay method of alcoholic roots & rhizomes extract of Hedychium coronarium J. Koenig plant species. Asian J Pharm Clin Res 8(4): 225-229.
- Quisumbing E (1978) Medicinal Plants of the Philippines. Quezon City, Philippines. Katha Publishing Co., Inc.
- Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52: 302-310.
- Le MH, Devaki P, Ha NB, Jun DW, Te HS, et al. (2017) Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PloS One 12(3): e0173499.
- Abeysekera KWM, Fernandes GS, Hammerton G, Portal AJ, Gordon FH, et al. (2020) Prevalence of steatosis and fibrosis in young adults in the UK : A population-based study. Lancet Gastroenterol Hepatol 5(3): 295-305.
- Jang E, Min-Hee S, Ki-Suk K, Kim Y, Yun-Cheol N, et al. (2014) Anti-lipoapoptotic effect of Artemisia capillaris extract on free fatty acids-induced HepG2 cells. BMC Complement Altern Med 14(1): 253.
- Wang ZQ, Zhang XH, Yu Y, Tipton RC, Raskin I, et al. (2013) Artemisia scoparia extract attenuates non-alcoholic fatty liver disease in diet-induced obesity mice by enhancing hepatic insulin and AMPK signaling independently of FGF21 pathway. Metabolism 62(9): 1239-1249.
- Wang JH, Choi MK, Shin JW, Hwang SY, Son CG (2012) Antifibrotic effects of Artemisia capillaris and Artemisia iwayomogi in a carbon tetrachloride-induced chronic hepatic fibrosis animal model. J Ethnopharmacol 140(1): 179-185.
- Han JM, Kim HG, Choi MK, Lee JS, Park HJ, et al. (2012) Aqueous extract of Artemisia iwayomogi Kitamura attenuates cholestatic liver fibrosis in a rat model of bile duct ligation. Food Chem Toxicol 50(10): 3505-3513.
- Saravanakumar A, Periyasamy P, Jang HT (2019) In vitro assessment of three different artemisia species for their antioxidant and anti-fibrotic activity. Biocatal Agric Biotechnol 18: 101040.
- Kim KE, Ko KH, Heo RW, Yi CO, Shin HJ, et al. (2016) Artemisia annua leaf extract attenuates hepatic steatosis and inflammation in high-fat diet-fed mice. J Med Food 19(3): 290-299.
- Liu L, Zhao J, Li Y, Wan Y, Lin J, et al. (2016) Artemisia capillaris formula inhibits hepatic steatosis via an miR‑122‑induced decrease in fatty acid synthase expression in vivo and in vitro. Mol Med Rep 13(6): 4751-4758.
- Pan J, Liu G, Liu H, Qiu Z, Chen L (1998) Effects of Artemisia capillaris on blood glucose and lipid in mice. Zhong Yao Cai. J Chinese Med Mater 21(8): 408-411.
- Sefi M, Fetoui H, Makni M, Zeghal N (2010) Mitigating effects of antioxidant properties of Artemisia campestris leaf extract on hyperlipidemia, advanced glycation end products and oxidative stress in alloxan-induced diabetic rats. Food Chem Toxicol 48(7): 1986-1993.
- Zhang Y, Zhao J, Zhong X, Zheng H, Li Y, et al. (2015) Ameliorative potential of Artemisia capillaris formula on nonalcoholic fatty liver disease in rats through regulation of fat metabolism. Afr J Tradit Complem Alternative Med 12(6): 151-161.
- Hong JH, Hwang EY, Kim HJ, Jeong YJ, Lee IS (2009) Artemisia capillaries inhibits lipid accumulation in 3T3-L1 adipocytes and obesity in C57BL/6J mice fed a high fat diet. J Med Food 12(4): 736-745.
- Sekiou O, Boumendjel M, Taibi F, Boumendjel A, Messarah M (2019) Mitigating effects of antioxidant properties of Artemisia herba alba aqueous extract on hyperlipidemia and oxidative damage in alloxan-induced diabetic rats. Arch Physiol Biochem 125(2): 163-173.
- Ghlissi Z, Sayari N, Kallel R, Bougatef A, Sahnoun Z (2016) Antioxidant, antibacterial, anti-inflammatory and wound healing effects of Artemisia campestris aqueous extract in rat. Biomed Pharmacother 84: 115-122.
- Ahmad F, Khan RA, Rasheed S (1992) Study of analgesic and anti-inflammatory activity from plant extracts of Lactuca scariola and Artemisia absinthium. J Islamic Acad Sci 5(2): 111-114.
- Moscatelli V, Hnatyszyn O, Acevedo C, Megías J, Alcaraz MJ, et al. (2006) Flavonoids from Artemisia copa with anti-inflammatory activity. Planta Med 72(1): 72-74.
- Yoon WJ, Moon JY, Song G, Lee YK, Han MS, et al. (2010) Artemisia fukudo essential oil attenuates LPS-induced inflammation by suppressing NF-κB and MAPK activation in RAW 264.7 macrophages. Food Chem Toxicol 48(5): 1222-1229.
- Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB (1995) The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res 22(4): 375-383.
- Kim EJ, Choi JY, Yu MR, Kim MY, Lee SH, et al. (2012) Total polyphenols, total flavonoid contents, and antioxidant activity of Korean natural and medicinal plants. Korean J Food Sci Technol 44(3): 337-342.
- Zhang H, Tsao R (2016) Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci 8: 33-42.
- Messaoudene D, Belguendouz H, Ahmedi ML, Benabdekader T, Otmani F, et al. (2011) Ex vivo effects of flavonoïds extracted from Artemisia herba alba on cytokines and nitric oxide production in Algerian patients with Adamantiades-Behçet's disease. J Inflamm 8(1): 35.
- Chung EY, Shin SW (2009) In Vitro Anti-Inflammatory Effects of the Essential Oil of Artemisia iwayomogi and Its Main Component, Vulgarone B. Nat Prod Sci 15(4): 229-233.
- Yuan HD, Jin GZ, Piao GC (2010) Hepatoprotective effects of an active part from Artemisia sacrorum Ledeb. against acetaminophen-induced toxicity in mice. J Ethnopharmacol 127(2): 528-533.
- Kraus D, Yang Q, Kong D, Banks AS, Zhang L, et al. (2014) Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature 508(7495): 258-262.
- Mortezaee K (2018) Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) and liver fibrosis: A review. Cell Biochem Funct 36(6): 292-302.
- Kimura Y, Okuda H, Okuda T, Hatano T, Agata I, et al. (1985) Studies on the activities of tannins and related compounds from medicinal plants and drugs. VII. Effects of extracts of leaves of Artemisia species, and caffeic acid and chlorogenic acid on lipid metabolic injury in rats fed per oxidized oil. Chem Pharm Bull 33(5): 2028-2034.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- Journal of Pathology and Toxicology Research
- Advance Research on Alzheimers and Parkinsons Disease
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)







