4003
Views & Citations3003
Likes & Shares
Context: E. jambolana (EJ) has been traditionally used for the management of diabetes. Even after the isolation of the herbal anti-diabetic compound (FIIc) from the fruit pulp of EJ has been done, yet it is not commercialized due to unavailability of the fruit throughout the year and due to the low yield of the isolation process of FIIc. The structure elucidation of FIIc has been done and it was found to be α-hydroxy succinamic acid (α-HSA). The goal of the present study is to chemically synthesize α-HSA and further to investigate its anti-diabetic and antioxidant potential in streptozotocin (STZ) and nicotinamide (NAD) induced type 2 diabetic rats.
Materials & methods: The synthesis of α-HSA has been done in a step wise manner. Male albino wistar rats were made diabetic by injecting NAD and STZ (230 and 45 mg/kg body weight i.p with a time interval of 15 min). α-HSA was orally administered to STZ+ NAD induced diabetic rats (20mg/kg body weight).
Results: The structure confirmation of α-HSA was done by spectral studies like 1H NMR, 13C NMR, & HRMS. α-HSA treated rats at week 6 of the study showed marked improvement in FBG levels, HbA1c levels, serum insulin levels, malondialdehyde (MDA) and total antioxidant capacity(TAC) levels in comparison to diabetic control rats.
Conclusions: Above findings suggest that α-HSA possesses anti-hyperglycemic and antioxidant activity which makes it a suitable candidate for the management of T2DM. However, to establish its role as potent anti-diabetic agent in STZ+NAD induced type 2 diabetic rats, its mechanism of action needs to laid down.
Keywords: E. jambolana, Succinamic acid derivative, T2DM, STZ+ NAD, Antidiabetic, Antioxidant
INTRODUCTION
Type-II diabetes mellitus (T2DM) is a carbohydrate metabolic disorder which is recognized by decreased insulin sensitivity or there may be partial destruction of pancreatic β cells. Presently, it is one of the leading causes of mortality and morbidity [1,2]. The global burden of diabetes was about 465 million in 2019 and its projected to rise to 700 million by 2045 [3]. There are many synthetic drugs (metformin & sulphonylureas) available in the market for the management of T2DM. However, they have limited efficacy, tolerability and mechanism-based side effects like weight gain, itching, hypoglycemia, edema, hepatotoxicity etc., which are not normally associated with herbal drugs. Many medicinal plants which are known to have anti-diabetic activity like E. jambolana, Cassia auriculata, Glycine max and have been studied in our lab [4-6]. E. jambolana (Botanical name-Syzgium cumini) is one such plant and its role in the management of diabetes is well mentioned in the scientific literature [7-10].
In the previous studies done by Sharma et. al, the isolation of anti-hyperglycemic compound (FIIc) from the fruit pulp of EJ has been done and granted Product Patent (No. 2,30,753)
for isolation and identification of herbal compound [10,11]. The chemical structure of FIIc was elucidated (α-hydroxysuccinamic acid, α-HSA) by spectral analysis. Therefore, it was contemplated that chemically synthesized α-HSA will also possess anti-diabetic and antioxidant properties due to its identical structure with FIIc. Hence, the objective of the present study was to synthesize and assess the anti-diabetic & antioxidant potential of α-HSA in STZ+NAD induced diabetic rats.
The structure of α-HSA is displayed in Figure 1.
MATERIALS & METHODS
Design and developing a synthetic strategic for the synthesis of α-HSA
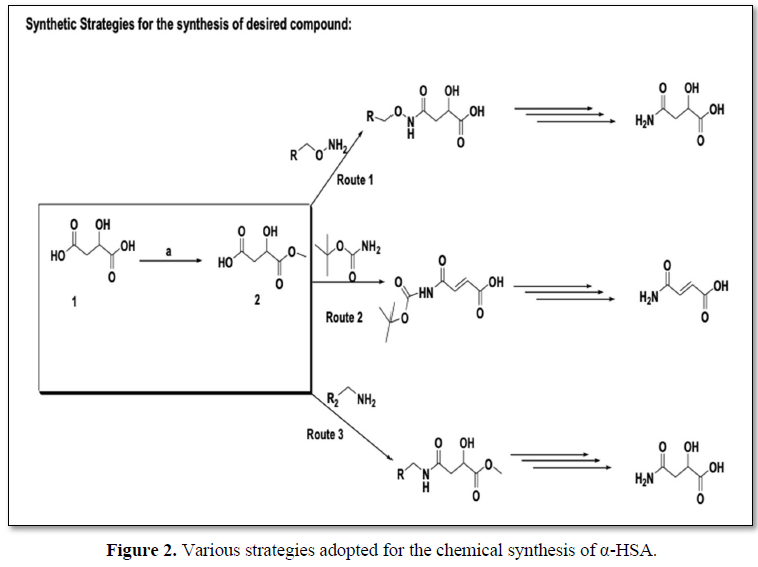
Three different routes have been used to synthesize the main compound 4-amino-2-hydroxy-4-oxobutanoic acid (α-HSA) to standardize the experimental conditions. However, route no. 1 has been found to be successful and best yields were obtained (Figures 2 and 3).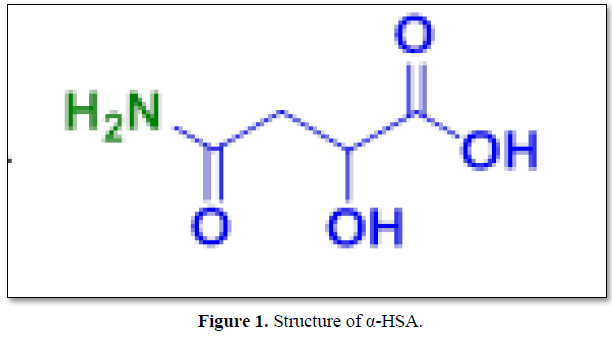
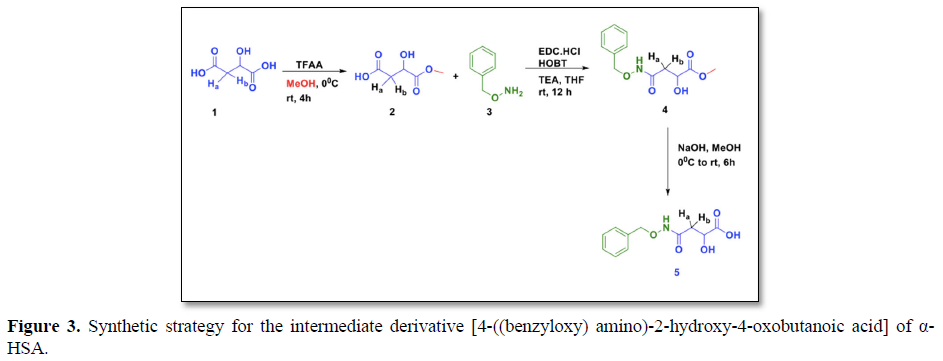
We have already synthesized the intermediate compound (5) 4-((benzyloxy) amino)-2-hydroxy-4-oxobutanoic acid in previous study [12]. In the present study, the herbal anti-diabetic compound4-((benzyloxy) amino)-2-hydroxy-4-oxobutanoic acid (α-HSA) (6) has been chemically synthesized from the intermediate compound 5 (Figure 4).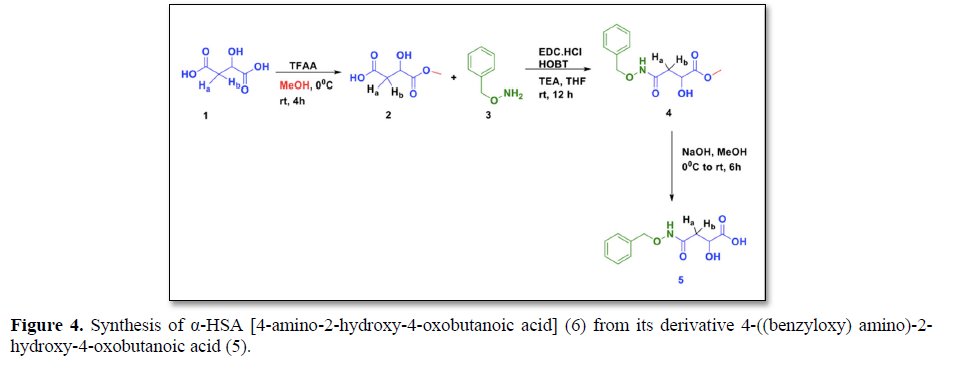
Synthesis of 4-amino-2-hydroxy-4-oxobutanoic acid (6) (α-hydroxysuccinamic acid)
Pd/C (0.1 eq) was added to intermediate 5 (0.5 g, 1 eq) in MeOH and was continuously stirred at room temp, under H2 atm for 6 hrs. After the completion of reaction, the progress was evaluated by TLC, the reaction mixture was filtered through sintered funnel, washed with MeOH and concentrated under reduced pressure. The crude compound was purified by prep HPLC. Under high vacuum, the concentrated compound was dried to give the target compound 6 as white solid. Melting-point: 110-1140C; Yield: 30 % ;1H NMR (400 MHz, DMSO-d6): 11.81 (br s, 1H, -COOH), 8.314 (br s, 1H, NH), 4.12 (t, 1H), 2.46 (dd, J=14.20 Hz. 1H, Ha), 2.36 (dd, J=14.21 Hz. 1H, Hb); 13C NMR (100 MHz, DMSO) δ: 173.63, 171.64, 78.019, 36.79.; HRMS (ESI) (M+H)+Calcd for C11H13NO5: 133.0375, found 133.0454.
ANIMALS EXPERIMENTAL SETUP AND ETHICAL CLEARANCE
The animal study was conducted at Central Animal Service Unit, University College of Medical Sciences (UCMS) & GTB Hospital, Delhi, India. All the animals were acclimatized under standard lab conditions (25°C to 30°C). They are fed with standard pellet diet and water ad libitum. The experimental protocol was approved by the Institutional Animal Ethical Committee (IAEC), University College of Medical Sciences & GTB Hospital, Delhi, India (UCMS/IAEC/26/13). All experimental procedures conducted in the study complied with the relevant guidelines on the care and use of laboratory animals.
Induction of T2DM in Experimental Animals
The induction of diabetes was done by injecting a single dose of freshly made STZ in citrate buffer (0.1 M; pH 4.5) and NAD (45 and 230 mg/kg b.wt.) [13]. Diabetes was confirmed after 72 h by measuring the fasting blood glucose levels. Wistar rats showing FBG levels 200 mg/dl or more were used for the experimental setup.
ANIMAL EXPERIMENT DESIGN
For the experimental setup, six group of male albinowistar rats having six rats in each group (n=6) were used.
Group I: Control (healthy non diabetic) given normal saline
Group II: Untreated diabetic given normal saline
Group III: Diabetic- administered withFIIc (20 mg/kg b.wt)
Group IV: Diabetic- administered with α-HSA (20 mg/kg b.wt.)
Group V: Diabetic- administered withglibenclamide (600 μg/kg b.wt.)
- The dosage of FIIc (20 mg/kg b.wt) was standardized from the previous studies done in our lab [10].
- The therapeutic dosage of α-HSA (20 mg/kg b.wt.) was calculated after performing the LD50
- The dosage of glibenclamide (600 μg/kg b.wt.) was decided from the previous studies done by Stanley et al. [14].
ACUTE TOXICITY STUDIES AND DETERMINATION OF LD50
Acute toxicity studies on α-HSA were done by following the guidelines given by Organization for the Economic Co-operation and Development (OECD) (Section 4.Test No. 425). Determination of LD50 was carried out by the method given by Affifi et al. [15].
For the experimental setup, six group of male albinowistar rats having five rats in each group (n=5) were used. Increasing gradual dose (200, 400, 600, 800, 1000 and 1200 mg/ kg b. wt.) of α-HSA were orally administered to each group respectively. The no of dead animals were recorded 48 hours after the administration of α-HSA. LD50 was calculated by applying the following formula.
LD50=The average lethal dose in which all animals are killed – Σ (z.d)/n
z=the mean of dead animals in two progressive doses
d=the steady factor between two progressive doses
n=number of animals in each group
COLLECTION OF SAMPLES
Micro-capillary technique was used to draw blood from retro orbital plexus [16]. For plasma separation, blood was collected in vials containing anti-coagulant (sodium fluoride potassium oxalate) for estimation of fasting blood glucose levels and for serum separation, blood was collected in plain vials for the estimation of insulin levels, MDA & TAC levels. Whole blood was collected in EDTA vials for glycosylated Hb.
BIOCHEMICAL & ANTIOXIDANT ACTIVITIES ASSESSMENTS
Fasting blood glucose levels (Centronic, GmbH, Gemany), serum insulin levels (MercodiaRat ELISA kit, Sweden) and Hb1Ac levels (Biosystems S.A., Costa Brava, Spain) were measured according to the instruction provided by the manufacturer.
TAC & MDA levels using plasma was estimated according to manufacturer’s instruction. The kits were provided by BioAssay, USA and ELAB Biosciences, China respectively.
STATISTICAL ANALYSIS
Two-way analysis of variance (ANOVA) followed by Turkey’s test were used to perform the comparison of parameters between different groups. P
RESULTS
- Acute oral toxicity test
LD50 of α-HSA was determined by giving single increasing gradual doses to different groups of normal male albino wistar rats. LD50 of α-HSA was found to be 867 mg /kg b.wt. (Table 1). The therapeutic dose chosen for the subsequent study was 20 g/kg b.wt. which is around 1/50 of LD50 and considered safe.
LD50 = Median lethal dose which kill all animals - Σ(z.d)/n = 1200-2000/6 =867mg /kg b. w.
1/50 of LD50 is about 20 mg/kg b. w. which was considered as sub lethal dose that was used as therapeutic dose in the subsequent studies.
- Effect of α-HSA on Glycemic index
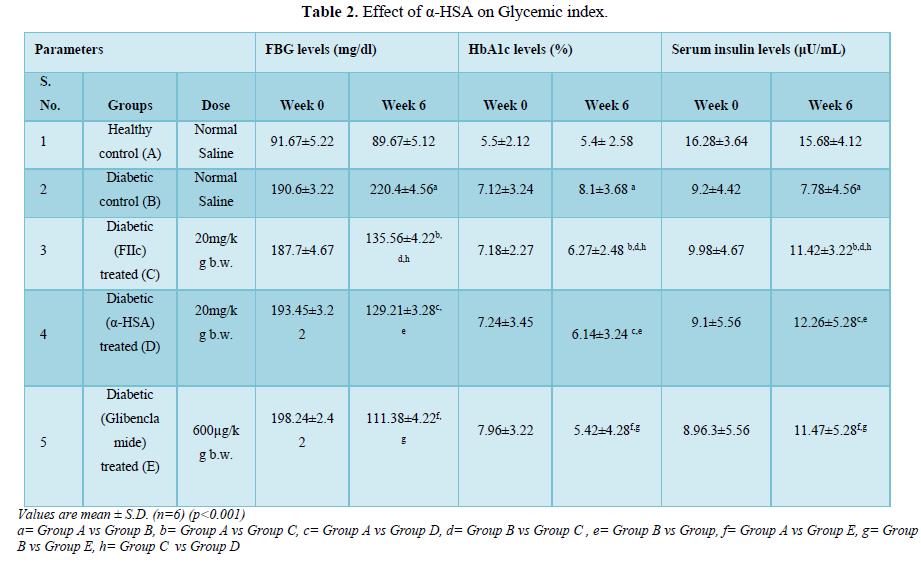
- i) Effect of α-HSA on FBG levels
Blood glucose levels were estimated in the overnight fasted experimental rats. After the administration of α-HSA and FIIc to diabetic treated groups (C & D), a significant fall in FBG levels were observed as compared to diabetic control (group B). However, an insignificant difference in FBG levels was observed in group C & D (Table 2).
- ii) Effect of α-HSA on HbA1c levels
After the induction of diabetes, the levels of HbA1c increased significantly in diabetic control rats (group B) when compared with normal control rats (group A) (p<0.005). After administration of α-HSA, there was significant improvement in HbA1c level α-HSA and FIIc treated groups (group C and D). However, a statistically insignificant difference in group C & D in HbA1c levels was observed (Table 2).
iii) Effect of α-HSA on insulin levels
In diabetic control rats (group B), the serum insulin levels were found to be significantly decreased (p<0.001) as compared to healthy control rats (group A). However, after administration of α-HSA and FIIc for 6 weeks to group C and D animals respectively, a significant increase in serum insulin levels was found when compared to diabetic control rats (group B). The serum insulin levels were slightly higher in group D in comparison with group C. However, the difference was statistically insignificant (Table 2).
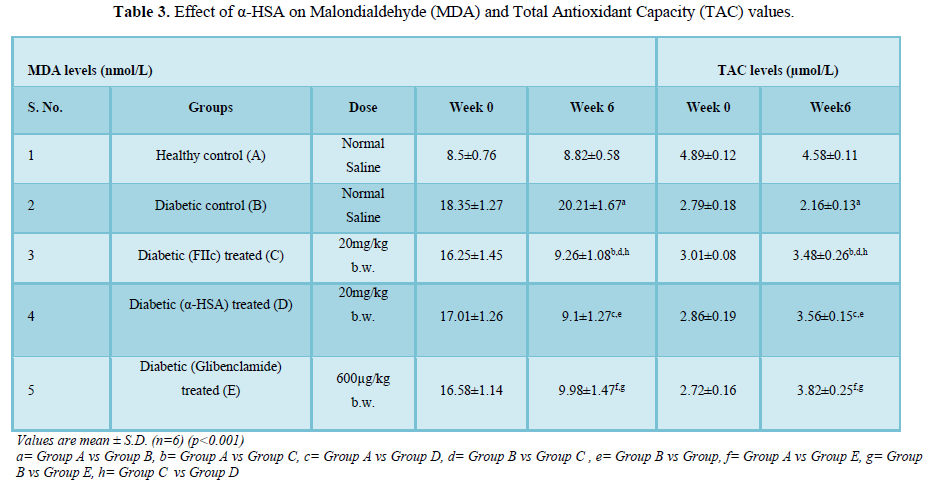
- Effect of α-HSA on Malondialdehyde (MDA) and Total Antioxidant Capacity (TAC) values
A significant increase in MDA levels and a decrease in TAC levels (p<0.001) was observed in diabetic control rats (group B) when compared to normal control rats (group A). After administration of α-HSA and FIIc to group C and D respectively for 6 weeks showed a significant decrease in the MDA levels and increase in TAC levels when compared to diabetic control rats (group B) (p<0.005). The difference in MDA & TAC values of group C & D was insignificant (Table 3).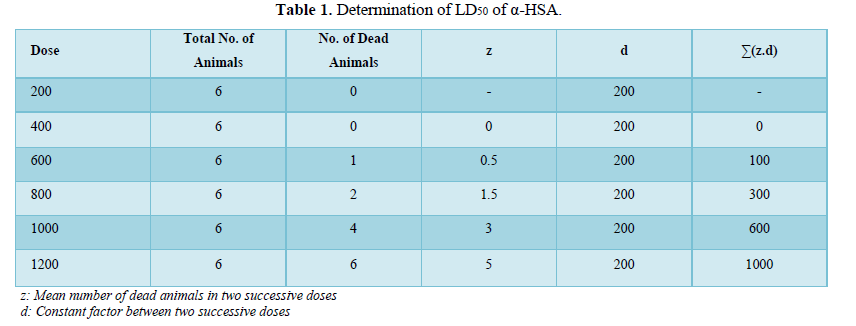
DISCUSSION
Type 2 Diabetes mellitus is considered to be one of the most common chronic diseases and is associated with hyperglycemia and hyperlipidemia. The metabolism of carbohydrates, proteins including lipids and lipoprotein are altered during diabetes.
In the present study, α-HSA was evaluated in vivo for its anti-hyperglycemic and antioxidant properties in STZ+NAD induced diabetic rats. Blood glucose, HbA1c, serum insulin and antioxidant/oxidative parameters (TAC & MDA) were analyzed.
In the pancreas, there is partial destruction of β-cells in STZ+NAD induced diabetic rats due to which the levels of serum insulin in the diabetic rats were reduced as compared to normal healthy control rats. After administration of α-HSA to diabetic rats, the serum insulin levels were increased significantly in comparison to diabetic control rats.
The fasting blood glucose levels of diabetic rats after the administration with α-HSA were significantly reduced as compared to diabetic control rats. The anti-diabetic activity of α-HSA is either due to improved insulin secretion or due to enhanced transport of blood glucose to the peripheral tissue [17].
The possible mechanism of action which can be correlated with the way sulphonylureas work i.e. promoting insulin secretion by membrane depolarization, closure of K+ ATP channels and opening of Ca2+ channels [18,19]. Glycosylated hemoglobin (HbA1c) is as an excellent marker for overall glycemic control and diabetic complications. α-HSA has significantly reduced the glycosylated level in α-HSA treated diabetic rats indicating its efficiency in glycemic control.
The role of lipid peroxidation in the development of diabetes complications is well mentioned in the literature. According to previous studies, the pace of development of MDA, a significant final result of lipid peroxidation, is altogether higher in diabetic rats contrasted with non-diabetic controls [20].
In T2DM there is a direct correlation between levels of MDA and the severity of the disease [21]. The role of free radicals in the onset of lipid peroxidation during T2DM is well mentioned in several scientific reports [21].
In our study, it has been found that α-HSA possess potent antioxidant properties as it decreases the serum MDA levels in α-HSA treated diabetic rats.
Total antioxidant capacity (TAC) is a widely used method that uses antioxidants as reductants in a redox-linked colorimetric reaction, wherein Cu2+ is reduced to Cu+. This ability of plasma to scavenge free radicals can be used to exploit its potential to counter the excess RONS observed in diabetes [22,23]. In our study, we have found that after administration of α-HSA, the plasma antioxidant levels of diabetic rats were significantly enhanced as compared to diabetic control rats.
Hence, chemically synthesized α-HSA has shown beneficial effects on hyperglycemia &oxidative stress. α-HSA improves insulin secretion through β-cells restoration in the pancreas and promotes insulin utilization capability in various peripheral tissues and its free radical scavenging property has potential to prevent diabetes associated complications.
CONCLUSION
In the present study, we have concluded that the α-HSA plays a vital role in the management of Type 2 diabetes. Molecular studies need to be done for elucidating the therapeutic action of α-HSA and before administrating it as possible replacement of insulin therapy or as an adjuvant.
ACKNOWLEDGMENT
The financial support given by Indian Council of Medical Research, New Delhi is acknowledged by the authors.
AUTHOR DISCLOSURE STATEMENT
The authors declare that they have no conflict of interests to disclose.
-
- Moller DE (2000) New drug targets for type 2 diabetes and the metabolic syndrome. Nature 1414: 821-827.
- Ciechanowski PS, Katon WJ, Russo JE, Walker EA (2001) The patient-provider relationship attachment theory and adherence to treatment in diabetes. Am J Psychiatr 158: 29-35.
- Farag YM, Gaballa MR (2011) Diabesity: An overview of a rising epidemic. Nephrol Dial Transplant 26: 28-35.
- Peetrson KF, Shulman GI (2006) Etiology of Insulin Resistance. Am J Med 119: 10-16.
- Samuel VT, Shulman GI (2012) Integrating mechanisms for insulin resistance: Common threads and missing links. Cell 148(5): 852-871.
- Tyalor DA (2004) Botanical supplements weeding out the health risks. Environ Health Perspects 112: 750-753.
- Sharma B, Balomajumder C, Roy P (2008) Hypoglycemic and hypolipidemic effects of flavonoid rich extract from Eugenia jambolana seeds on streptozotocin induced diabetic rats. Food Chem Toxicol 46: 2376-2383.
- Ravi K, Sivagnanam K, Subramanian S (2004) Anti-diabetic activity of Eugenia jambolana seed kernels on streptozotocin induced diabetic rats. J Med Food 7: 187-191.
- Rizvi SI, Mishra N (2013) Traditional Indian medicines used for the management of diabetes mellitus. J Diabetes Res 712092.
- Tanwar RS, Sharm SB, Singh UR, Gupta S, Shukla SK, et al. (2011) Anti-atherosclerotic potential of active principle isolated from Eugenia jambolana in streptozotocin induced diabetic rats. Evid Based Complement Alternat Med 127641.
- Tanwa RS, Sharma SB, Prabhu KM (2017) In vivo assessment of antidiabetic and antioxidative activity of natural phytochemical isolated from fruit pulp of Eugenia jambolana in streptozotocin induced diabetic rats. Redox Rep 22: 301-307.
- Khurana N, Sharma P, Bhagat S, Sharma SB (2018) Effect of a novel succinamic acid derivative as potential anti diabetic agent in experimental diabetic rats. J Drug Deliver Therapeut 8: 57-62.
- Szkudelski T (2012) Streptozotocin-nicotinamide induced diabetes in the rat: Characteristics of the experimental model. Exp Biol Med 237: 481-490.
- Stanely P, Menon VP (2003) Hypoglycaemic and hypolipidaemic action of alcohol extract of Tinosporacordifolia roots in chemical induced diabetes in rats. Phytother Res 17: 410-413.
- Afifi NA, Ramadan A, El-Kashoury EA, El Banna HA (1994) Some pharmacological activities of essential oils of certain umbelliferous fruits. Vet Me J Giza 42: 85-92.
- Sorg DA, Buckner BA (1964) Simple method of obtaining venous blood from small laboratory animals. Proc Soc Exp Biol Med 115: 1131-1132.
- Sen S, Kar M, Roy A, Chakraborti AS (2005) Effect of nonenzymatic glycation on functional and structural propertiesof hemoglobin. Biophys Chem 113: 289‐298.
- Zawalich WS, Zawalich KC (1992) Biochemical mechanisms involved in monomethyl succinate-induced insulin secretion. Endocrinology 131: 649-654.
- Ladriere L, Louchami K, Vinamber C, Kadiata MM, Jijakli H, et al. (1998) Insulinotropic action of the monoethyl ester of succinic acid. Gen Pharm 31: 377-383.
- Hamadi N, Mansour A, Hassan MH, Touhami KF, Badary O (2012) Ameliorative effects of resveratrol on liver injury in streptozotocin-induced diabetic rats. J Biochem Mol Toxicol 26: 384-392.
- Peerapatdit T, Sriratanasathavorn C (2010) Lipid peroxidation and antioxidantenzyme activities in erythrocytes of type 2 diabetic patients. J Med Assoc Thai 93: 682-693.
- Oyenihi AB, Ayeleso AO, Mukwevho E, Masola B (2015) Antioxidant strategies in the management of diabetic neuropathy. BioMed Res Int 15.
- Katalinic V, Modun D, Music I, Boban M (2005) Gender differences in antioxidantcapacity of rat tissues determined by 2,20-azinobis 3-ethylbenzothiazoline6-sulfonate ABTS and ferric reducing antioxidant power FRAP assays. Comp Biochem Physiol Toxicol Pharmacol 140: 47-52.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Rheumatology Research (ISSN:2641-6999)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- Journal of Pathology and Toxicology Research
- BioMed Research Journal (ISSN:2578-8892)






