3202
Views & Citations2202
Likes & Shares
Abbreviations: AF-MSCs: Amniotic Fluid Mesenchymal Stem Cells; aGVHD: Acute Graft-vs-Host Diseases; ALF: Acute Liver Failure: BM-MSCs: Bone Marrow Mesenchymal Stem Cells; EPCs: Endothelial Progenitor Cells; HCC: Hepatocellular Carcinomas; HE: Hepatic Encephalopathy; HGF: Hepatocyte Growth Factor; HLA-G: Human Leucocyte Antigen-G; HLCs: Hepatocyte Like Cells; hP-MSCs: Human Placenta Mesenchymal Stem Cells; IDO: Indole Amine 2,3-Dioxigenase; IGF-1: Insulin-Like Growth Factor; IL: Interleukin; iNOS: Inducible Nitric Oxide Synthase: LC: Liver Cirrhosis; MenSCs: Menstrual Stem Cells; MMPs: Matrix Metalloproteinases; MSCs: Mesenchymal Stem Cells; NLRP: Nod- Like Receptor Protein; PDGF: Platelet-Derived Growth Factor; PGE2: Prostaglandin E2; TGF: Transforming Growth Factor; TNF: Tumor Necrosis Factor; UC-MSCs: Umbilical Cord Mesenchymal Stem Cells; XBP: X-Box Binding Protein; YAP: Yes-Associated Protein
MSCs are described as pluripotent cells having the ability to self-renew and give birth to a variety of distinct kinds of differentiated mesenchymal cells [7]. The first stromal cells from bone marrow were extracted and recognized by Friedenstein [8] in 1966. MSCs (Mesenchymal stem cells) were recovered from a variety of tissues, including dental pulp, adipose tissue, amniotic fluid, and the umbilical cord [9-18]. MSCs are the perfect candidates for therapeutic applications in a variety of disorders because of their immunomodulatory capabilities, the constrained ability for self-renewal, and multi-lineage development [19]. MSCs' low intrinsic immunogenicity ensures the safety of transplants [20]. Even MSCs that are HLA-mismatched might be employed in several therapeutic settings, particularly for stem cell-based treatments [19]. Additionally, the main therapeutic effect of MSC transplantation is the capacity of these cells to localize to certain lesions and organs [20].
The major factor in surgical failure and patient postoperative mortality is subsequent rejection, particularly acute graft-vs-host disease (aGVHD), which is the only known therapeutic therapy for end-stage liver disorders [21]. The onset and progression of rejection are mediated by a number of immunological cells including Natural Killer cells, Tregs, T cells, and dendrite cells (DCs) [22]. A growing body of research has shown that transplantation of MSC can greatly lessen the problem of aGVHD because MSCs have immunomodulatory effects [22-24]. The cytological and molecular processes, however, still need to be investigated. This review's objective is to explain the therapeutic immuno-modulatory pathways that MSCs use to treat liver disease.
Role of Mesenchymal Stem Cell in Repair and Regeneration of Liver
Mesenchymal Stem Cells (MSCs) may considerably enhance regeneration of liver cell in a variety of liver disorders, according to clinical and laboratory studies. Amniotic fluid mesenchymal stem cells (AF-MSCs) increased survival and regeneration of liver following mice with 80% hepatectomy, as demonstrated by Despeyroux [25].
However, Chen [26] showed that MSCs from menstrual blood were attracted to damaged liver locations in liver fibrosis brought on by carbon tetrachloride (CCl4) mice models, but that only a small number of transplanted cells transformed into hepatocyte-like cells (HLCs). Shi [27] found that following infusion of BM-MSCs (3x 106 cells/kg) through intraportal vein in D-gal (D-galactosamine) induced pigs’ model, the proportion of human-derived hepatocytes to all pig hepatocytes was only 4.5% [27]. Even little-expanded BM-MSCs demonstrated no ectopic tissue growth and only little long-term engraftment upon intravascular injection, according to research by von Bahr [28]. Accordingly, MSCs may primarily stimulate liver regeneration by methods other than their differentiation into HLCs. Through the secretion of several immunomodulatory substances, MSC can in vivo develop into hepatocytes and serve a significant therapeutic function in the cure of liver fibrosis. Following MSC treatment, hepatocyte growth factor (HGF) and Insulin-like growth factor (IGF-1) levels increased along with stimulation of angiogenetic and mito-genetic factors [29].
MSCs have been shown in in vitro investigations to decrease collagen formation and encourage the death of hepatic stellate cells. The PDGF and Notch signaling pathways were responsible for the improved cell proliferation and angiogenic capability that resulted from endothelial progenitor cells directly cocultured (EPCs) with MSCs in vitro [30]. Through the release of inflammatory factors such as growth-related oncogene, IL-8, HGF, interleukin-6 (IL-6), and osteoprotegerin, MSCs prevented the proliferation of LX2 (a hepatic stellate cell line) in an indirect coculture experiment [26]. Through their paracrine activity, MSCs took part in cell communication either directly or indirectly.
Immunomodulatory Mechanisms of Mesenchymal Stem Cell Transplantation for the Treatment of Liver Disease
The majority of earlier investigations have demonstrated that MSCs may enhance or restore damaged tissue by direct cell-to-cell communication or paracrine secretion by influencing tissue immune responses. MSCs could alter immune responses, both innate and adaptive. For MSC-mediated immunomodulation to occur, regulatory T cells (Tregs) must be induced to become CD4+CD25+FoxP3+. Previous research suggested that alcohol-induced liver disease and chronic hepatitis B, and autoimmune hepatitis may all have a link with an imbalance in Treg/T17 cells. According to a random study, the IL-17 (interleukin-17), TNF (tumor necrosis factor)), and IL-6 (interleukin-6) blood levels were lesser in the transplanted group compared to those in the control group. Further evidence that BM-MSCs demonstrate effective anti-inflammatory and immunosuppressive actions via the modulation of the inflammatory cytokines levels in serum was found in the transplanted group, including a large rise in Tregs and a noticeably decreased number of T17 cells [31]. Shi [32] conducted pilot research employing MSCs for curing liver transplant patients, which is consistent with the earlier work. The findings demonstrated that T17 cells were downregulated and Tregs were increased in the liver following MSC infusion. Additionally, they discovered that after receiving UC-MSC infusions, the number of HLA-DR+ CD4+ T cells significantly reduced, which may help to suppress alloreactive reactions. Ex-vivo immunologic investigations have only sometimes been incorporated into clinical regimens up to this point in order to better understand the molecular consequences of MSC treatment in liver disease. The immunoregulatory ability of transplanted MSCs in rodent models of liver problems is being supported by a growing body of research. Prostaglandin E2 (PGE2), inducible nitric oxide synthase (iNOS), Hepatocyte growth factor (HGF) and transforming growth factor (TGF)- are just a few of the chemicals released by MSCs that have been shown to have an immunomodulatory influence on T-cell activity in nonclinical tests [33,34]. A different investigation found that MSCs can release MMPs (matrix metalloproteinases) (MMPs), including MMP-2 and MMP-9, which inhibit T cell activation by cleaving the T cells surface CD25 molecules [35]. MSCs suppress T cells by preventing CD25 translation via the LKB1-AMPK-mTOR pathway, as demonstrated by Yoo [36]. Furthermore, Zhang et al. discovered the potential for the human placenta (hP)-MSCs to control the interaction between Nrf2 and NF-B signaling pathways to limit PD-1 expression in IL-10 +CD4+T cells and reduce liver injury in a mouse model of graft versus host disease [37]. Additionally, it has been demonstrated that MSCs stimulate the production and expansion of Tregs by possibly producing transforming growth factor-ß (TGF-ß). Notably, the immunosuppressive properties of TGF- ß reduces inflammation of liver [38], but it can also hasten the development of liver fibrosis [39,40]. According to Yan et al. research's MSCs produced IL-10 may have a role in the higher immunosuppression that was generated when Tregs and MSCs were co-cultured [41]. Through the Notch signaling pathway, Toll-like receptors 3 and 4 which are extensively expressed in MSCs can trigger the development of Tregs [42]. Additionally, it has been shown that MSCs have immunomodulatory effects on macrophages, which are essential for both liver fibrosis and fibrotic resolution. B lymphocytes also contribute to the pathophysiology of liver fibrosis. MSCs may prevent B cell proliferation by halting the cell cycle to the G0/G1 phase. Additionally, B cell’s ability to differentiate and produce chemotactic cytokines was suppressed [43]. The key players in adaptive immunity are T and B cells. As previously mentioned, MSCs prevent DC maturation, which lowers T cell activation. By inhibiting T cells in the G0/G1 phase of the cell cycle instead of causing T cell death, the MSCs significantly reduce the proliferation of activated T cells as well [44]. A number of soluble molecules, including chemokine ligand 2 (CCL2), heme oxygenase-1, galectin (Gal), HLA-G (human leucocyte antigen-G), IDO, HGF, IL-10, and PGE2 (HO-1), and TGF-1, have been implicated in studies as mediating the inhibitory effect of MSCs on the proliferation and activation of T cells [45-48]. Additionally, MSCs' released PD-L1 and PD-L2 can prevent CD4+ T cell activation and cause permanent T cell hypo-reactivity [49]. Watanabe [50] discovered that MSCs might induce an M2 anti-inflammatory phenotype in macrophages, which involves the release of several anti-inflammatory substances, such as CCL-1 (chemokine ligand 1) and IL-10. It stimulates the phagocytosis of hepatic cell debris (during which macrophages raise the levels of pro-regenerative substances) and the increased synthesis of matrix metalloproteinases to reduce ECM [50]. Similar to the previous work, it was shown that murine adipose-derived MSCs dramatically increased the number of M2-like cells by boosting Arginase 1 activity and IL-10 [51]. We discovered that adoptive transfer of MSCs decreased hepatocellular damage and changed the polarization of macrophages from the M1 to M2 phenotype of ischemia/reperfusion (IR)-induced sterile inflammatory damage occurs in mice's liver of the liver. MSCs often suppress M1 (a pro-inflammatory subtype) and activate M2 (an anti-inflammatory subtype), which promotes tissue regeneration and the resolution of inflammation. Through the actions of prostaglandin E2, indoleamine-2, cyclooxygenase 2, 3-dioxygenase (IDO), TGF-1, and IL-6, activated MSCs help polarize monocytes (M0) into the M2-type [52-54]. By encouraging the Hippo signaling pathway, Li [55] demonstrated that BM-MSCs promote the reprogramming of macrophage polarization to an anti-inflammatory M2 phenotype [55]. The primary elements of the innate immune system, dendritic cells (DCs), process antigens before presenting them to T cells. By secreting soluble factors such as PGE2, IDO, HGF, TGF-, and nitric oxide (NO), the MSCs prevent DCs from differentiating, maturing, and migrating [56-58]. Natural killer (NK) cells are essential for the activation of circulating lymphocytes, controlling hepatic inflammation, and providing the first line of defense against invasive infections. MSCs can suppress NK cells by secreting IDO and PGE2, according to Spaggiari [59]. Further investigation revealed that MSCs boost the activity of the macrophage Hippo pathway, which in turn regulates XBP1-mediated NLRP3 activation and controls NLRP3 activation through direct communication between YAP and catenin [55]. This reprogramming of macrophage polarization toward an anti-inflammatory M2 phenotype occurs Figure 1.
T lymphocytes, Monocytes, and B cells are infiltrated along with chronic liver damage brought on by inflammation [60]. According to reports, immunosuppressive medications can help the liver regenerate both after and before liver transplantation [61,62]. In this way, liver disease may benefit from the immunomodulatory abilities of MSCs. First, through producing a variety of soluble substances such as Interleukin (IL)-6, IL-10, prostaglandin E (PGE)-2, nitric oxide, and indoleamine 2, 3-dioxygenase, and human leukocyte antigen G, MSCs can downregulate T cells. These elements have the power to affect Treg cell activity and immune cell proliferation [63]. MSCs can also stop the growth of T cells by interacting with T-lymphocytes directly. Interferon, IL-1, and tumor necrosis factor (TNF) are cytokines that work together to give MSCs their immunosuppressive properties [64]. These cytokines aid immune cells and certain chemokines in maintaining contact with MSCs and controlling immunological responses.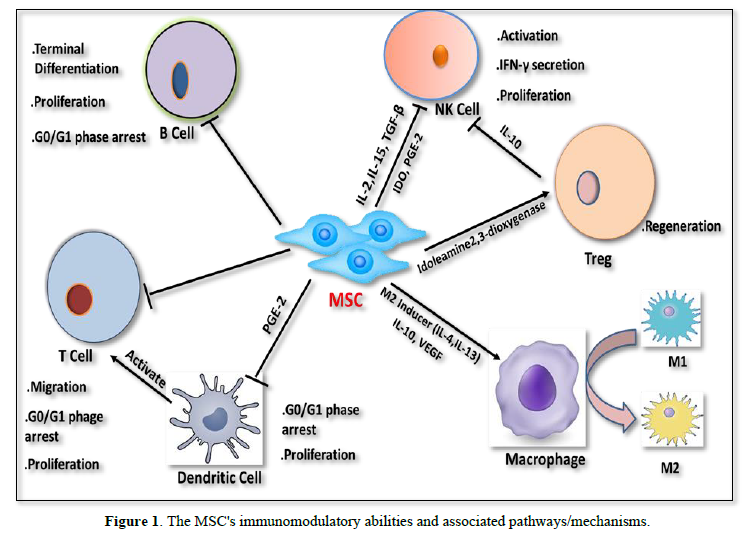
Additionally, MSCs have the ability to prevent B cell activation, which lowers immunoglobulin levels. Chemokine receptors (XCR5, XCR4, and XCR7) have been shown to express much less on their surface when co-cultured with MSCs [45]. Additionally, natural killer (NK) cells play a well-established role in immunological responses to cancer and viral infections [65]. By either cell-to-cell contacts or the release of soluble substances like PGE2 and transforming growth factor (TGF)-, MSCs cause the production of IL-2, which reduces the secretion of IL-15 from IL-2-induced NK cells [66]. Finally, it has been demonstrated that MSCs cause inflammatory macrophages to polarize toward alternative macrophages. This change improves liver damage by releasing soluble factors (IL-10 and IL1Ra) [67].
CONCLUSIONS AND FUTURE PROSPECTIVE
In conclusion, it was established that MSCs had a potential therapeutic impact in the treatment of liver disease. Clarification of the underlying principle of stem cell therapeutic benefits will require more research. According to the ideal time interval and appropriate treatment dosage, standard protocols should be devised. The therapeutic use of MSCs needs to better establish cell kind, injection method, and observation time points. Through its immunomodulation capabilities, MSC regenerative therapy has been demonstrated to be useful in the treatment of chronic liver disease. Numerous clinical trials have shown how MSCs may effectively heal damaged hepatocytes by reducing tissue fibrosis and enhancing liver function. There are still a number of issues to be addressed, including limited migration, poor cell viability, the possibility of cancer development, and the spread of viruses. Additionally, MSC-derived EVs appear to offer therapeutic advantages over cell-free cell therapy in MSC-based transplantation by maintaining at least part of the cells and its immunomodulatory characteristics. Standardization of the cell source, growing conditions, method of administration, and results of upcoming large-scale clinical studies will define the future of MSC-based cell treatment for chronic liver disease.
SOURCE OF FUNDING
This work did not receive any grant from funding agencies.
CONFLICT OF INTEREST
None.
- Bernal W, Wendon J (2013) Acute liver failure. N Engl J Med 369: 2525-2534.
- Bernal W, Auzinger G, Dhawan A, Wendon J (2010) Acute liver failure. Lancet 376: 190-201.
- Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, et al. (2000) High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 192: 565-570.
- Fitzmaurice C, Allen C, Barber RM, et al. (2017) Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 3: 524-548.
- El-Serag HB, Rudolph KL (2007) Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 132: 2557-2576.
- Kang SH, Kim MY, Baik SK (2018) Novelties in the pathophysiology and management of portal hypertension: New treatments on the horizon. Hepatol Int 12(Suppl 1): 112-121.
- da Silva Meirelles L, Caplan AI, Nardi NB (2008) In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26: 2287-2299.
- Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV (1966) Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol 16: 381-390.
- Ji W, Chen Y, Wang L, Xu Z, Ahmed J, et al. (2020) Differentiation of human umbilical cord mesenchymal stem cells into Leydig-like cells with defined molecular compounds. Hum Cell 33: 318-329.
- Lelek J, Zuba-Surma EK (2020) Perspectives for future use of extracellular vesicles from umbilical cord and adipose tissue-derived mesenchymal stem/stromal cells in regenerative therapies-synthetic review. Int J Mol Sci 21: 799.
- Abumaree MH, Abomaray FM, Alshabibi MA, AlAskar AS, Kalionis B (2017) Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta 59: 87-95.
- Wang Z, Sun D (2018) Adipose-derived mesenchymal stem cells: A new tool for the treatment of renal fibrosis. Stem Cells Dev 27: 1406-1411.
- Gaur M, Dobke M, Lunyak VV (2017) Mesenchymal stem cells from adipose tissue in clinical applications for dermatological indications and skin aging. Int J Mol Sci 18: 208.
- Joerger-Messerli MS, Marx C, Oppliger B, Mueller M, Surbek DV, et al. (2016) Mesenchymal stem cells from Wharton’s Jelly and amniotic fluid. Best Pract Res Clin Obstet Gynaecol 31: 30-44.
- Loukogeorgakis SP, De Coppi P (2016) Stem cells from amniotic fluid-Potential for regenerative medicine. Best Pract Res Clin Obstet Gynaecol 31: 45-57.
- Ulrich D, Muralitharan R, Gargett CE (2013) Toward the use of endometrial and menstrual blood mesenchymal stem cells for cell-based therapies. Expert Opin Biol Ther 13: 1387-1400.
- Chen L, Qu J, Cheng T, Chen X, Xiang C (2019) Menstrual blood-derived stem cells: Toward therapeutic mechanisms, novel strategies, and future perspectives in the treatment of diseases. Stem Cell Res Ther 10: 406.
- Cui D, Li H, Wan M, Peng Y, Xu X, et al. (2018) The origin and identification of mesenchymal stem cells in teeth: From Odontogenic to Non-odontogenic. Curr Stem Cell Res Ther 13: 39-35.
- Klingemann H, Matzilevich D, Marchand J (2008) Mesenchymal stem cells - sources and clinical applications. Transfus Med Hemother 35: 272-277.
- Karp JM, Teo LSG (2009) Mesenchymal stem cell homing. The devil is in the details. Cell Stem Cell 4: 206-216.
- Hu C, Li L (2019) The immunoregulation of mesenchymal stem cells plays a critical role in improving the prognosis of liver transplantation. J Transl Med 17: 412.
- Amorin B, Alegretti AP, Valim V, Pezzi A, Laureano AM, et al. (2014) Mesenchymal stem cell therapy and acute graft-versus-host disease: A review. Hum Cell 27: 137-150.
- Yang D, Wang LP, Zhou H, Cheng H, Bao XC, et al. (2015) Inducible co-stimulator gene-transduced bone marrow-derived mesenchymal stem cells attenuate the severity of acute graft-versus host disease in mouse models. Cell Transplant 24: 1717-1731.
- Zhao K, Lou R, Huang F, Peng Y, Jiang Z, et al. (2015) Immunomodulation effects of mesenchymal stromal cells on acute graft-versus-host disease after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 21: 97-104.
- Despeyroux A, Duret C, Gondeau C, Perez-Gracia E, Chuttoo L, et al. (2018) Mesenchymal stem cells seeded on a human amniotic membrane improve liver regeneration and mouse survival after extended hepatectomy. J Tissue Eng Regen Med 12: 1062-1073.
- Chen L, Zhang C, Chen L, Wang X, Xiang B, et al. (2017) Human menstrual blood-derived stem cells ameliorate liver fibrosis in mice by targeting hepatic stellate cells via paracrine mediators. Stem Cells Transl Med 6: 272-284.
- Shi D, Zhang J, Zhou Q, Xin J, Jiang J, et al. (2017) Quantitative evaluation of human bone mesenchymal stem cells rescuing fulminant hepatic failure in pigs. Gut 66: 955-964.
- von Bahr L, Batsis I, Moll G, Hagg M, Szakos A, et al. (2012) Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 30: 1575-1578.
- Gorji SM, Malekshah AAK, Hashemi-Soteh MB, Rafiei A, Parivar K, et al. (2012) Effect of mesenchymal stem cells on Doxorubicin-induced fibrosis. Cell J 14: 142-151.
- Liang TZ, Zhu L, Gao WL, Gong M, Ren J, et al. (2017) Coculture of endothelial progenitor cells and mesenchymal stem cells enhanced their proliferation and angiogenesis through PDGF and Notch signaling. FEBS Open Bio 7: 1722-1736.
- Xu L, Gong Y, Wang B, Shi K, Hou Y, et al. (2014) Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: Regulation of Treg/Th17 cells. J Gastroenterol Hepatol 29(8): 1620-1628.
- Shi M, Liu Z, Wang Y, Xu R, Sun Y, et al. (2017) A pilot study of mesenchymal stem cell therapy for acute liver allograft rejection. Stem Cells Transl Med 6(12): 2053-2061.
- Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, et al. (2004) Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 103(12): 4619-4621.
- Zafranskaya M, Nizheharodava D, Yurkevich M, Ivanchik G, Demidchik Y, et al. (2013) PGE2 contributes to in vitro MSC-mediated inhibition of non-specific and antigen-specific T cell proliferation in MS patients. Scand J Immunol 78(5): 455-462.
- Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, et al. (2009) Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes 58(8): 1797-1806.
- Yoo HS, Lee K, Na K, Zhang YX, Lim HJ, et al. (2017) Mesenchymal stromal cells inhibit CD25 expression via the mTOR pathway to potentiate T-cell suppression. Cell Death Dis 8: e2632.
- Zhang A, Zhang J, Li X, Zhang H, Xiong Y, et al. (2021) hPMSCs inhibit the expression of PD-1 in CD4(+) IL-10(+) T cells and mitigate liver damage in a GVHD mouse model by regulating the crosstalk between Nrf2 and NF-kappaB signaling pathway. Stem Cell Res Ther 12: 368.
- Zhang Y, Cai W, Huang Q, Gu Y, Shi Y, et al. (2014) Mesenchymal stem cells alleviate bacteria-induced liver injury in mice by inducing regulatory dendritic cells. Hepatology 59(2): 671-682.
- Li J, Wang Y, Ma M, Jiang S, Zhang X, et al. (2019) Autocrine CTHRC1 activates hepatic stellate cells and promotes liver fibrosis by activating TGF-beta signaling. EBioMedicine 40: 43-55.
- Wu N, Meng F, Invernizzi P, Bernuzzi F, Venter J, et al. (2016) The secretin/secretin receptor axis modulates liver fibrosis through changes in transforming growth factor-beta1 biliary secretion in mice. Hepatology 64(3): 865-879.
- Yan Z, Zhuansun Y, Chen R, Li J, Ran P (2014) Immunomodulation of mesenchymal stromal cells on regulatory T cells and its possible mechanism. Exp Cell Res 324: 65-74.
- Rashedi I, Gomez-Aristizabal A, Wang XH, Viswanathan S, Keating A (2017) TLR3 or TLR4 activation enhances mesenchymal stromal cell-mediated Treg induction via Notch signaling. Stem Cells 35: 265-275.
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V (2006) Human mesenchymal stem cells modulate B-cell functions. Blood 107: 367-372.
- Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F (2005) Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 105: 2821-2827.
- Kuca-Warnawin E, Olesinska M, Szczesny P, Kontny E (2021) Impact and possible mechanism(s) of adipose tissue-derived mesenchymal stem cells on T-cell proliferation in patients with rheumatic disease. Front Physiol 12: 749481.
- Yang S, Wei Y, Sun R, Lu W, Lv H, et al. (2020) Umbilical cord blood-derived mesenchymal stromal cells promote myeloid-derived suppressor cell proliferation by secreting HLA-G to reduce acute graft-versus-host disease after hematopoietic stem cell transplantation. Cytotherapy 22: 718-733.
- Cao M, Liu H, Dong Y, Liu W, Yu Z (2021) Mesenchymal stem cells alleviate idiopathic pneumonia syndrome by modulating T cell function through CCR2-CCL2 axis. Stem Cell Res Ther 12: 378.
- Jiang W, Xu J (2020) Immune modulation by mesenchymal stem cells. Cell Prolif 53: e12712.
- Davies LC, Heldring N, Kadri N, Le Blanc K (2017) Mesenchymal stromal cell secretion of programmed death-1 ligands regulates T cell mediated immunosuppression. Stem Cells 35: 766-776.
- Watanabe Y, Tsuchiya A, Seino S, Kawata Y, Kojima Y, et al. (2019) Mesenchymal stem cells and induced bone marrow-derived macrophages synergistically improve liver fibrosis in mice. Stem Cells Translat Med 8(3): 271-284.
- Anderson P, Souza-Moreira L, Morell M, Caro M, O’Valle F, et al. (2013) Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut 62(8): 1131-1141.
- Le Blanc K, Mougiakakos D (2012) Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol 12: 383-396.
- Liu F, Qiu H, Xue M, Zhang S, Zhang X, et al. (2019) MSC-secreted TGF-beta regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res Ther 10: 345.
- Wang J, Liu Y, Ding H, Shi X, Ren H (2021) Mesenchymal stem cell-secreted prostaglandin E2 ameliorates acute liver failure via attenuation of cell death and regulation of macrophage polarization. Stem Cell Res Ther 12: 15.
- Li C, Jin Y, Wei S, Sun Y, Jiang L, et al. (2019) Hippo signaling controls NLR family pyrin domain containing 3 activation and governs immunoregulation of mesenchymal stem cells in mouse liver injury. Hepatology 70: 1714-1731.
- Liu WH, Liu JJ, Wu J, Zhang LL, Liu F, et al. (2013) Novel mechanism of inhibition of dendritic cells maturation by mesenchymal stem cells via interleukin-10 and the JAK1/STAT3 signaling pathway. PLoS ONE 8: e55487.
- Lu Z, Chang W, Meng S, Xu X, Xie J, et al. (2019) Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell Res Ther 10: 372.
- Li X, Dong Y, Yin H, Qi Z, Wang D, et al. (2020) Mesenchymal stem cells induced regulatory dendritic cells from hemopoietic progenitor cells through Notch pathway and TGF-beta synergistically. Immunol Lett 222: 49-57.
- Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, et al. (2008) Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 111: 1327-1333.
- Kisseleva T, Brenner DA (2012) The phenotypic fate and functional role for bone marrow-derived stem cells in liver fibrosis. J Hepatol 56: 965-972.
- Dmitrewski J, Hubscher SG, Mayer AD, Neuberger JM (1996) Recurrence of primary biliary cirrhosis in the liver allograft: The effect of immunosuppression. J Hepatol 24:253-257.
- Manousou P, Arvaniti V, Tsochatzis E, Isgro G, Jones K, et al. (2010) Primary biliary cirrhosis after liver transplantation: Influence of immunosuppression and human leukocyte antigen locus disparity. Liver Transpl 16: 64-73.
- Sharma RR, Pollock K, Hubel A, McKenna D (2014) Mesenchymal stem or stromal cells: A review of clinical applications and manufacturing practices. Transfusion 54: 1418-1437.
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, et al. (2008) Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2: 141-150.
- Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M (2006) Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 24: 74-85.
- Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, et al. (2005) T cell responses to allogeneic human mesenchymal stem cells: Immunogenicity, tolerance, and suppression. J Biomed Sci 12: 47-57.
- Lee KC, Lin HC, Huang YH, Hung SC (2015) Allo-transplantation of mesenchymal stem cells attenuates hepatic injury through IL1Ra dependent macrophage switch in a mouse model of liver disease. J Hepatol 63: 1405-1412.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Dermatology Clinics and Research (ISSN:2380-5609)
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- Journal of Spine Diseases
- International Journal of AIDS (ISSN: 2644-3023)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)



