6629
Views & Citations5629
Likes & Shares
PROCEDURE
Tools
We use a drill with adjustable speed that could be modified according to each indication or condition, from 5000 to a maximum of 22,000 revolutions per minute (rpm).We use granite, diamond, and tungsten tips, with several shapes and diameters, to remove the impaired portion of the nail plate and reach the affected nail bed.
Technique
Previous reports have described good tolerance to nail abrasion without anesthesia [2]; however, since in our technique we affect the nail bed, hyponychium, and nail folds, which are highly sensitive structures, we prefer to perform it under local anesthesia with digital block and/or sedation, which allows a comfortable and safe procedure for both, the patient and physician.
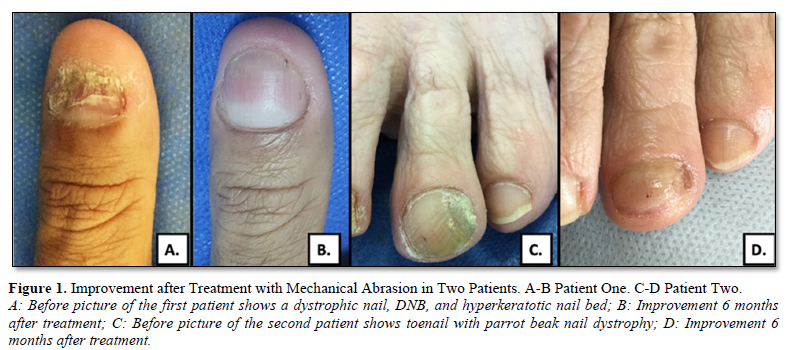
We use cylindrical tips to remove the affected nail plate and the nail bed hyperkeratosis (Figure 2A), the treatment endpoint is to achieve punctate bleeding at this level (Figure 2B). If the periungual folds are also altered, we treat them with a triangular tip (Figure 2C).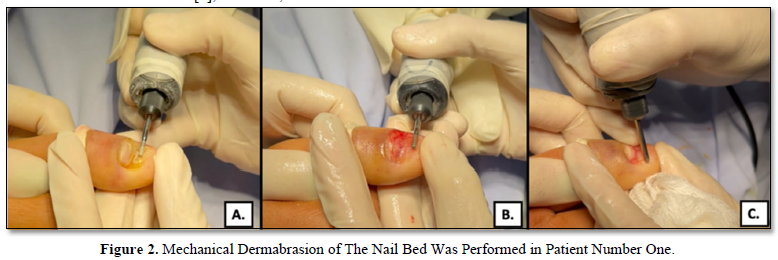
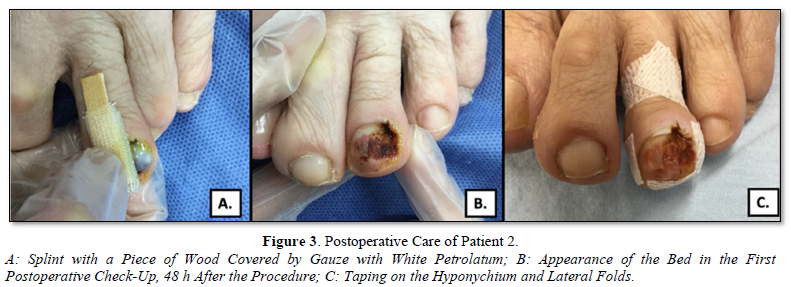
If the nail bed is deformed or displaced to one side, we can use part of the excess nail plate as a splint or a wooden splint covered with Vaseline gauze dress, this allows the nail bed to remain flattened and not retract. (Figures 3A-B).
During the procedure, alcohol is used to keep the treated area clean and wet and to control the temperature generated by the procedure.
Post Procedure Care
After the procedure, the area is covered with a Vaseline gauze dress, which is removed after 48 h (Figure 3B). Subsequently, according to the inflammation and pain, once the patient tolerates it, complementary techniques, such as taping, are started. Taping on the nail folds allows adequate growth of the nail plate over the treated nail bed (Figure 3C). This therapy is maintained until the nail completes its growth.
- Removal of nail bed hyperkeratosis with a cylindrical tip.
- Pinpoint bleeding from the nail bed, treatment includes the area of the nail bed that has disappeared.
- Remodeling of the nail folds using a triangular tip.
Biosecurity
There are not many studies that evaluate the biosecurity of this procedure in terms of exposure to debris generated during its performance. A study carried out in 1984 studied the effects of the aerosols generated during this procedure in podiatrists chronically exposed to them, and found symptoms of rhinitis, conjunctivitis, and cough, among others [10].
A more recent study evaluated fungal viability by collecting nail dust from gloves and masks used in 9 patients with onychomycosis who underwent this procedure. They found hyaline hyphae; however, they were morphologically deformed and there was no growth of any fungus in cultures in the Mycosel medium [11].
With current evidence, no cutaneous or systemic mycoses associated with exposure to these aerosols have been demonstrated. However, the use of gloves, protective glasses, and a mask, ideally N95, is recommended during procedure [11].
MECHANISM OF ACTION
Mechanical Forces
Mechanical dermabrasion allows removing hyperkeratosis from the nail bed so that it becomes flattened; The advantage of this change lies in the fact that there are stem cells mainly in the most proximal portion of the nail bed and it has been shown that under normal conditions the cells that make up the nail bed move distally towards the hyponychium [12]. The rectification of the bed that is achieved with dermabrasion could restore this normal migration of the nail bed.
In addition to this, the nail plate that slides longitudinally “pushes” and continues flattening the nail bed to fit its corresponding space, supporting the adhesion and prolongation of the new plate.
Heating and Tissue Debridement
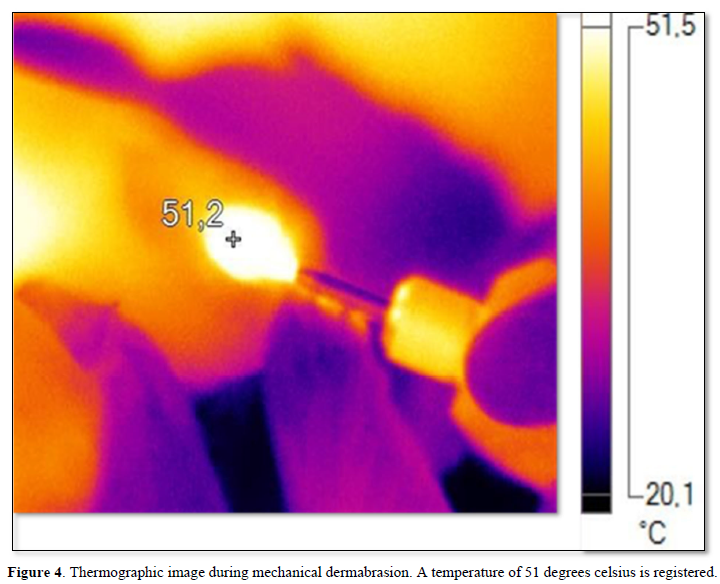
During our procedures, we have made thermographic readings to evaluate the temperatures reached during its realization (Figure 4). Depending on the speed of the drills and the material of the tip (granite, diamond, and tungsten), the device and consequently the tissue can reach high temperatures between 40 and 55 degrees Celsius (104- and 131 degrees Fahrenheit).
The Heating makes easy the abrasion of the hyperkeratosis, the debridement of biofilms (in cases of onychomycosis) [13,14] and the receding of the DNB, inducing slight bleeding.
CONCLUSION
The use of mechanical dermabrasion in the nail unit should not be limited solely to the control of diseases confined to the nail plate. The nail bed is a dynamic tissue; its manipulation through this procedure can allow the management of entities such as the DNB.
- Di Chiacchio N, Kadunc BV, de Almeida AR, Madeira CL (2003) Nail abrasion. J Cosmet Dermatol 2(3-4): 150-152.
- Gupta S, Jangra RS, Gupta S, Mahendra A, Kumar A (2017) A painless, minimally invasive technique for debulking onychomycotic nails. J Am Acad Dermatol 76(1): e17-e19.
- Mochizuki T, Kawasaki M, Tanabe H, Ishizaki H (2005) A Nail Drilling Method Suitable for the Diagnosis of Onychomycosis. J Dermatol 32(2): 108-113.
- Maeda N, Mizuno N, Ichikawa K (1990) Nail Abrasion: A New Treatment for Ingrown Toenails. J Dermatol 17(12): 746-749.
- Lin K, Lipner SR (2020) Painless technique for debridement of onychodystrophic nails. J Am Acad Dermatol 82(2): e39-e40.
- Zaias N, Escovar SX, Zaiac MN (2014) Finger and toenail onycholysis. J Eur Acad Dermatol Venereol 29(5): 848-853.
- Daniel CR 3rd, Tosti A, Iorizzo M, Piraccini BM (2005) The disappearing nail bed: A possible outcome of onycholysis. Cutis 76(5): 325-327.
- Filisio F, Busch S, Wickramage DJH, Hill R, Kabadi S, et al. (2021) Disappearing Nail Bed: Review of Etiology, Grading System, and Treatment Options. Clin Podiatr Med Surg 38(4): 521-527.
- Avila-alvarez AM, Gomez-Vargas LM, Velez-Pelaez MC (2022) The Nail Bed: Theories of Its Functioning and Therapeutic Mechanisms of Dermabrasion. J Skin Stem Cell 9(1): e123012.
- Abramson C, Wilton J (1992) Nail dust aerosols from onychomycotic toenails. Part II. Clinical and serologic aspects. 1984. J Am Podiatr Med Assoc 82(2): 116-123.
- Di Chiacchio N, de Sá Menezes Carvalho G, Di Chiacchio NG, de Mello CDBF, Veasey JV (2021) Fungal Viability of Nail Dust from Onychomycosis Abrasion: A Pilot Study. Skin Appendage Disord 7(5):66-369.
- Momin MA, Honda K, Yosue T (2014) The nail bed, part I. The normal nail bed matrix, stem cells, distal motion, and anatomy. J Dermatol Clin Res 2(1): 1008.
- Sen CK, Roy S, Mathew-Steiner SS, Gordillo GM (2021) Biofilm Management in Wound Care. Plast Reconstr Surg 148(2): 275e-288e.
- Gupta AK, Foley KA (2019) Evidence for biofilms in onychomycosis. Giornale Italiano Di Dermatologia e Venereologia 154: 1.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Journal of Spine Diseases
- Journal of Alcoholism Clinical Research






