Research Article
Mesenchymal Stem Cells Regulate Mi1gration of Dendritic Cells via Paracrine Hepatocyte Growth Factor in Lipopolysaccharide-Induced Acute Lung Injury
3729
Views & Citations2729
Likes & Shares
Background: Mesenchymal stem cells (MSCs) have been shown to reduce the proportion and maturity of lung dendritic cells and ameliorate acute lung injury (ALI) via the paracrine hepatocyte growth factor (HGF), but the mechanism remains unclear.
Methods: Flow cytometry (FCM) was used to evaluate the effects of recombinant mouse-HGF (rmHGF) or HGF-expressing MSCs on the migration, CCR7 or HIF-1α of immature DCs (imDCs) or regulatory DCs (DCregs) under LPS and CCR7 ligands (CCR7L, CCL19 and CCL21) stimulation. Then, a HIF-1α inhibitor was used to observe their effects on HGF-promoted DC migration and/or CCR7 expression. In vivo, DCs from lung to Para bronchial lymph nodes (PBLNs) were detected by FCM at different times after ALI modeling and the expression of CCR7 or CD86 of DCs in the lung or migrating to PBLNs treated with MSCs with different HGF expression were compared.
Results: The migration of imDCs increased under the induction of CCR7L and LPS, while rmHGF promoted the migration, accompanied by increased CCR7 expression. MSCs inhibited the migration of imDCs to CCR7L and decreased the expression of CCR7 and HIF-1α. High HGF expression weakened the inhibition of DC migration by MSCs but promoted the expression of CCR7 and HIF-1α. rmHGF- promoted DC migration was significantly suppressed by the HIF-1α inhibitor. In vivo, the number of CFSE+CD11c+DCs migrating to the PBLNs peaked at 6 h after ALI modeling. High HGF gene expression weakened the inhibition of DC migration by MSCs but enhanced the suppression of DC maturation (considered as DCregs) by MSCs. MSCs decreased lung DC aggregation and CCR7 expression in ALI mice, while high or low HGF gene expression decreased or increased the lung DC ratio, respectively, but did not affect CCR7 expression. Further in vitro experiments confirmed that rmHGF increased DCreg migration without affecting CCR7 expression.
Conclusions: MSCs activate the CCR7/HIF-1α pathway via paracrine HGF to enhance imDC migration triggered by CCR7L and LPS. MSCs inhibited the migration of DCs to the PBLNs by decreasing CCR7 expression in lung DCs, but high HGF expression weakened the inhibition of DC migration by MSCs in a CCR7-independent manner.
Keywords: Acute respiratory distress syndrome (ARDS), Acute lung injury (ALI), Dendritic cells, Migration, Hepatocyte growth factor (HGF), Mesenchymal stem cell (MSC), HIF-1α
Abbreviations: ARDS: Acute respiratory distress syndrome; MSC: Mesenchymal stem cell; DCs: dendritic cells; ALI: acute lung injury; PBLN: Para bronchial lymph node; HGF: hepatocyte growth factor; CCR7L: CCR7 ligands; BM: bone marrow; GM-CSF: granulocyte-macrophage colony-stimulating factor; IL-4: interleukin-4; imDCs: immature dendritic cells; LPS: lipopolysaccharide; DMEM/F12: Dulbecco’s modified Eagle’s media/nutrient mixture F-12; EGFP: enhanced Green Fluorescent Protein; BSD: blasticidin; Abs: antibodies; DCregs: regulatory DCs; CFSE: carboxyfluorescein diacetate succinimidyl ester; PBS: phosphate-buffered saline; ANOVA: analysis of variance; SD: standard deviation; Fig: figure. Tregs: regulatory T cells; RDCs: respiratory DCs
INTRODUCTION
Background
Acute respiratory distress syndrome (ARDS) is the major cause of acute respiratory failure in critically ill patients, with an incidence of 26.3 cases/100,000 people and mortality rates of 40% despite significant advances in pharmacological interventions and respiratory support throughout the decades [1-4]. Excessive inflammatory reactions, particularly the activation of dendritic cells (DCs), have been implicated in the pathogenesis of ARDS [5-7]. Migration of DC to the PBLNs is a prerequisite for activating and differentiating initial CD4+ and CD8+ T cells and for initiating an adaptive immune response to abnormal pulmonary antigens [8-10]. However, aberrant DC trafficking and accumulation are implicated in the pathogenesis of various inflammatory diseases [11]. Therefore, the migration of DCs may play an important role in the pathophysiology of acute lung injury (ALI), and the regulation of DC migration may have clinical significance for the treatment of ARDS.
Bone marrow mesenchymal stem cells (MSCs), with high self-renewal and multipotent differentiation potential, have been widely used in the treatment of pulmonary diseases. In addition to homing to lung tissues to repair damaged alveolar epithelium and pulmonary vascular endothelial cells in ARDS [12,13], MSCs have immunomodulatory properties and functions toward immune cells, including B cells, NK cells, T cells and macrophages [14-18]. Previous studies have shown that MSCs induce the generation of regulatory dendritic cells through different mechanisms and inhibit the proliferation/function of T cells in adaptive immunity in the ARDS animal model [19,20]. The migration of DCs from tissues to lymph nodes is essential for antigen presentation and triggering tolerance or adaptive immune responses, while MSCs transplantation has been demonstrated to impair the migration of dendritic cells to the draining lymph nodes [21,22]. However, it remains unclear whether MSCs regulate dendritic cell migration to the Para bronchial lymph node (PBLN) in ARDS.
Previous in vivo and in vitro studies provided reliable evidence for the protective effect of hepatocyte growth factor (HGF) secreted by MSCs in ALI [13,23]. However, the reported effect of HGF on DC migration is different from that on MSCs [24,25]. Studies have shown that MSCs can inhibit the migration of DCs and reduce the expression of CCR7 [25], while blocking the Akt/HIF-1 pathway inhibits the CCR7-mediated migration of DCs [26, 27]. HGF can promote the migration of DCs by activating the c-Met pathway [26], which is essential for DC metastasis to the draining lymph nodes after inflammatory activation [24,27]. Similarly, activation of the CCR7 downstream pathway Akt/HIF-1α also promotes DC migration and antigen presentation [28-31]. Our previous studies have also demonstrated that HGF can induce regulatory DC production by activating the Akt pathway [19]. It is unclear whether HIF-1α is also an important link in the regulation of DC migration by HGF, and the effect of HGF expression in MSCs on DC migration in ARDS remains unknown. Therefore, the aim of this study was to assess regulatory effects of MSCs on DCs migration via paracrine HGF in ALI.
Previous in vivo and in vitro studies provided reliable evidence for the protective effect of hepatocyte growth factor (HGF) secreted by MSCs in ALI [13,23]. However, the reported effect of HGF on DC migration is different from that on MSCs [24,25]. Studies have shown that MSCs can inhibit the migration of DCs and reduce the expression of CCR7 [25], while blocking the Akt/HIF-1 pathway inhibits the CCR7-mediated migration of DCs [26,27]. HGF can promote the migration of DCs by activating the c-Met pathway [26], which is essential for DC metastasis to the draining lymph nodes after inflammatory activation [24,27]. Similarly, activation of the CCR7 downstream pathway Akt/HIF-1α also promotes DC migration and antigen presentation [28-31]. Our previous studies have also demonstrated that HGF can induce regulatory DC production by activating the Akt pathway [19]. It is unclear whether HIF-1α is also an important link in the regulation of DC migration by HGF, and the effect of HGF expression in MSCs on DC migration in ARDS remains unknown. Therefore, the aim of this study was to assess regulatory effects of MSCs on DCs migration via paracrine HGF in ALI.
In this study, we found that high HGF expression attenuates the inhibitory effect of MSCs on DC migration but promotes the expression of CCR7 and HIF-1α, while low HGF expression shows the opposite effect. In the LPS environment, recombinant mouse HGF (rmHGF) promotes the migration of immature DCs (imDCs) triggered by CCR7 ligands CCL19 and CCL21 (CCR7L) by activating CCR7/HIF-1α. In vivo, the migration of DCs from the lung to the PBLNs increased significantly within 12 h of ALI. MSC transplantation reduced the ratio and maturity of migrating DCs. High HGF gene expression weakened the inhibition of DC migration by MSCs but enhanced the suppression of DC maturation (considered as regulatory DC (DCreg) subpopulation) by MSCs; however, low HGF expression showed the opposite trend. LPS induced lung DC aggregation and increased CCR7 expression in ALI mice, while MSCs decreased lung DC aggregation and CCR7 expression in ALI mice. The high or low HGF gene expression decreased or increased the lung DC ratio, respectively, but did not affect the expression of CCR7. Further in vitro experiments confirmed that rmHGF could increase the migration of DCregs without affecting CCR7 expression. Our study sheds additional light on the mechanism of MSC‐secreted HGF affecting DC migration and weakens its immune activation function in the early stage of ALI, providing new insights into DC-based immunotherapy for ALI.
MATERIALS & METHODS
Mice
Specific pathogen‑free male C57BL/6 mice (aged 6-8 weeks) were obtained from the Laboratory Animal Center of Yangzhou University (Yangzhou, China). All the animal experiments performed in this study were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Southeast University Ethics Committee.
Generation of mouse BM-derived DCs
Mouse bone marrow (BM)-derived DCs were generated as previously described with minor modifications [32,33]. Briefly, BM cells were extracted from the medullary cavity of the femur and tibia. The erythrocytes were lysed using Lysing Buffer (BD Pharm Lyse™, USA), washed three times in phosphate-buffered saline(PBS) and cultured in 100-mm dishes with 5×106 cells(per dish) containing 10ml RPMI-1640 (Wisent, Nanjing, China) medium supplemented with 10% FBS (Wisent, Nanjing, China), 40 ng/ml recombinant murine granulocyte–macrophage colony stimulating factor (GM-CSF; NOVUS), and 40 ng/ml recombinant murine interleukin-4 (IL-4, NOVUS) in a humidified 5% CO2 incubator at 37°C. On day 3, 10 ml of fresh medium containing 10% FBS, GM-CSF and IL-4 (both 40 ng/mL) was added directly to each dish for further culture. On day 5 of culture, loosely adherent cells were harvested for flow cytometry or were collected and purified using anti-CD11c micro magnetic beads (Miltenyi Biotec). The purity of the cells was greater than 90%. The purified immature DCs (imDCs) were cultured in fresh medium or induced into mature DCs using 50 ng/ml of bacterial lipopolysaccharide (LPS, Sigma-Aldrich) for 24 h.
Mesenchymal stem cell transfection and culture
MSCs derived from the bone marrow of C57BL/6 mice were purchased from Cyagen Biosciences, Inc. (Guangzhou, China). The supplier identified mesenchymal stem cells based on cell surface phenotypes (CD29, CD44, CD117, SCA-1, CD31 and CD45) and multipotent differentiation potential (adipogenic, osteogenic, and chondrogenic lineages). MSCs were cultured in a 1:1 mixture of Dulbecco’s modified Eagle’s media/nutrient mixture F-12 (DMEM/F12) (Wisent, Canada) containing 10% fetal bovine serum (FBS) (Wisent, Canada) and 1% antibiotic-antimycotic solution (streptomycin, penicillin and amphotericin B; Wisent, Canada) in a humidified 5% CO2 incubator at 37°C.
The details of the transfection of MSCs mediated by lentiviral vectors have been previously described in our work [19]. In brief, HGF gene under-expression and over-expression were achieved using the recombinant lentivirus vectors, and the lentiviruses for under-expression and over-expression specific for EGFP were used as empty vector controls. The lentiviruses were packaged in 293T cells using multiple packaging plasmids to obtain higher or lower lentivirus titers, and then, the recombinant lentivirus was used to transfect the MSCs. MSCs carrying enhanced Green Fluorescent Protein (EGFP), namely, normal control MSC (NC-HGF-MSCs, NC-shHGF-MSCs) or MSCs carrying both the high/low HGF genes and EGFP (HGF-MSCs, shHGF-MSCs) were harvested after selection using blasticidin (BSD, 3μg/mL, InvivoGen). Subsequently, NC-HGF-MSCs, NC-shHGF-MSCs, HGF-MSCs and shHGF-MSCs were cultured in DMEM/F12 containing 15% FBS,1% antibiotic-antimycotic solution and 3μg/mL BSD were incubated at 37°C in a humidified atmosphere with 5% CO2. We verified in our previous study that the HGF gene of MSCs is overexpressed or knocked down at the mRNA and protein levels [19].
Trans well migration assay
The migration assay was performed using 24-well Trans well plates containing 8-mm-pore size polycarbonate filters (Corning, Life Science). CCL19, CCL21, and LPS (all 50 ng/ml; total volume in 600 μl of RPMI-1640 containing 1% BSA) were added to the lower chamber. ImDCs were added to the upper chamber (1×105 cells in a total volume of 100 μl) and incubated for 3h at 37°C. In some experiments, DCs or DCregs were simultaneously treated with vehicle or recombinant murine HGF (0-120 ng/mL; R&D Systems, USA), HIF-1α antioxidant inhibitor PX-478 (5 μmol/L; Selleck), or Akt inhibitor MK-2206 (5 μmol/L; Selleck). The number of migrated DCs in the lower chamber was determined by flow cytometry; the number of spontaneously migrated DCs in the absence of chemokine was used as the background.
To detect the influence of MSCs with different HGF expression levels on DC migration, 5×105 MSCs, NC-HGF-MSCs, HGF-MSCs, NC-shHGF-MSCs, and shHGF-MSCs were seeded into the lower chambers of 24-well Trans well plates containing 8-mm-pore size polycarbonate filters (Corning, Life Science). After 24 h, the old culture medium was removed, the cells were washed gently with PBS, and CCL19 and CCL21 (50 ng/ml; total volume, 600 µl) with 50 ng/ml of LPS were added to the lower chambers. DCs were added to the upper chambers (1×105 cells in a total volume of 100 µl) and incubated for 3 h at 37℃. The number of migrated DCs in the lower chamber was determined by flow cytometry.
Induction of regulatory DCs
MSCs (5×106 cells/well) were cultured in 6-well plates in DMEM/F12 medium containing 10% FBS. When MSC density increased to 50%, the old medium was replaced by 5 mL of RPMI 1640 containing 5% FBS. mDCs (5×105 cells/well) were inoculated into 6-well plates for coculture for 72 h and suspended regulatory DCs (DCregs) were used for migration experiments.
ALI model
The ALI model was induced as previously described with minor modifications [12]. Briefly, mice were intraperitoneally injected with 50 mg/kg of pentobarbital. LPS (5 mg/kg) (Sigma-Aldrich) was delivered to the lungs through a tracheostomy, and the incision was sutured. The mice were returned to the cage until fully awake.
Experimental groups and sample acquisition
To investigate the changes in DCs in lymph nodes transplanted from the lung at different times after ALI modeling, the mice were randomly assigned to one of the following 6 groups (n=6 mice per group): 24 h, 12 h, 6 h, 3 h, 1 h and 0 h. Mice in the 24, 12, 6, 3, 1 and 0 h (control) groups were sacrificed after intratracheal injection of carboxyfluorescein diacetate succinimidyl ester (CFSE) and 5 mg/kg of lipopolysaccharide (LPS). Lung tissue was collected for single-cell isolation and histological examination in accordance with slightly modified previous methods [5]. The PBLNs were collected and prepared into a single-cell suspension in a frozen milling machine (40 Hz×60 s).
Mice in each groups were injected with CFSE before LPS or PBS and were randomly assigned to one of the following groups (n=6 mice per group): control group (Con), mice were given the same amount of PBS at the corresponding time; ALI group (ALI), mice received 5 mg/kg of LPS to establish the ALI model; MSC+ALI group (MSCs), mice received MSCs (500,000 cells in 150 μl of PBS) via the tail vein immediately after LPS; NC-HGF-MSC+ALI group (NC-HGF-MSCs), mice received NC-HGF-MSCs (500,000 cells in 150 μl of PBS) via the tail vein immediately after LPS; HGF-MSC+ALI group (HGF-MSCs), mice received HGF-MSCs (500,000 cells in 150 μl of PBS) via the tail vein immediately after LPS; NC-shHGF-MSC+ALI group (NC-shHGF-MSCs), mice received NC-shHGF-MSCs (500,000 cells in 150 μl of PBS) via the tail vein immediately after LPS; shHGF-MSC+ALI group (shHGF-MSCs), mice received shHGF-MSCs (500,000 cells in 150 μl of PBS) via the tail vein immediately after LPS. The mice were sacrificed after 12 h, and the lung tissue and PBLNs were collected for single-cell isolation or histological examination.
PBLN histopathology
ImDCs were labeled with 0.5 mM CFSE. In total, 500,000 labeled cells were injected intratracheally into each wild-type recipient mouse before LPS or PBS. The PBLNs of ALI mice at 0 h, 1 h, 3 h, 6 h, 12 h and 24 h after modeling were removed to prepare frozen sections. DAPI nuclear staining was performed. Because CFSE showed green fluorescence, the number of migratory CFSE+ DC cells in the PBLNs was observed under a fluorescence microscope.
Flow cytometry
Lung and lymph node cell isolation and the measurement of the proportion and maturation of DCs were performed by flow cytometry as previously described with minor modifications [34]. For the phenotypic analysis of cell surface marker expression, the cells were harvested, resuspended in PBS, incubated for 15 min with FcR blocking reagent, and then incubated for 15 min with PE-, APC-, PE-Cy7-, PerCP-, or FITC-conjugated monoclonal antibodies (mAbs) on ice. In some experiments, FVS-780(BD Pharmingen) was incubated at 4℃from light for 10 minutes to detect the death and survival of cells. The DCs were stained with antibodies against CD11c, CD86, CD11b, or CCR7 (BD Pharmingen, USA). The T cells were stained with antibodies against CD4, CD44, or CD69 (BD Pharmingen, USA) Mouse IgG1 or Rat IgG isotype control antibodies were used in parallel as negative controls. CFSE (BD Pharmingen) was used in DCs, and the expression of DC HIF-1α (NOVUS, USA) was detected using the Alexa Fluor 488 conjugate. The stained cells were washed twice and resuspended in cold buffer and then were analyzed by flow cytometry (FACSCalibur; NovoCyte). The results were processed using Novo Express software and were expressed as the number or percentage of positive staining cells relative to the total number of cells.
Statistical analysis
All statistical analyses were performed using SPSS 26.0 software and GraphPad Prism 8.0. One-way analysis of variance (ANOVA) or two-tailed Student's t test was used to determine the significance between the groups. The data are expressed as means ± standard deviation (SD). P < 0.05 was considered statistically significant.
RESULTS
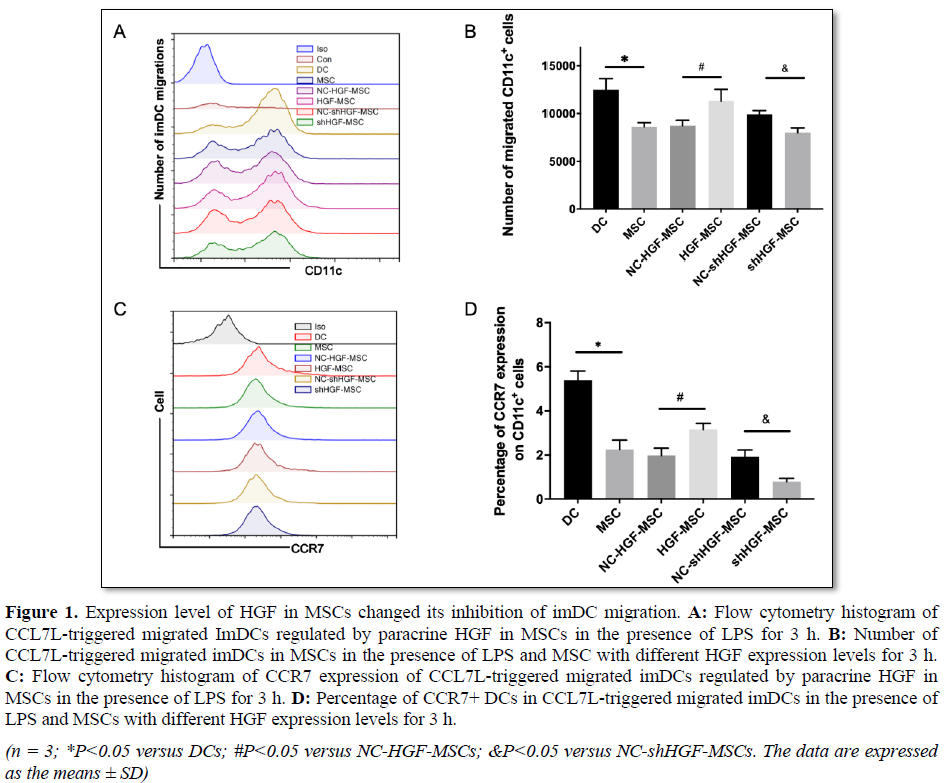
High HGF expression in MSC weakens its inhibition of DC migration
Previous studies have shown that MSCs can inhibit the migration of DC and reduce the expression of CCR7 [25], while HGF can promote the migration of DCs by activating the c-Met pathway [26]. By observing the influence of MSCs with different HGF expression levels on DC migration, we found that the migration of DC was significantly increased under the induction of CCL 19 and CCL21, but the number of migrated DCs was significantly reduced in the presence of MSCs. Compared with the empty vector control group, the number of migrated DCs in the HGF-MSC group increased significantly, while that in the shHGF-MSC group decreased markedly (Figures 1A and 1B). In addition to confirming that high HGF expression in MSCs weakened its inhibition of DC migration, we also found that the presence of MSCs inhibited CCR7 expression in DCs. The high HGF expression weakened the inhibition of CCR7 expression by MSCs in DCs, while HGF silencing enhanced the inhibition of DCs by MSCs (Figures 1C and 1D). Therefore, all the data indicated that high HGF expression in MSCs attenuated its inhibition of DC migration and CCR7 expression, while HGF down regulation showed the opposite effects.
HGF enhances CCL19- and CCL21-triggered DC migration via CCR7
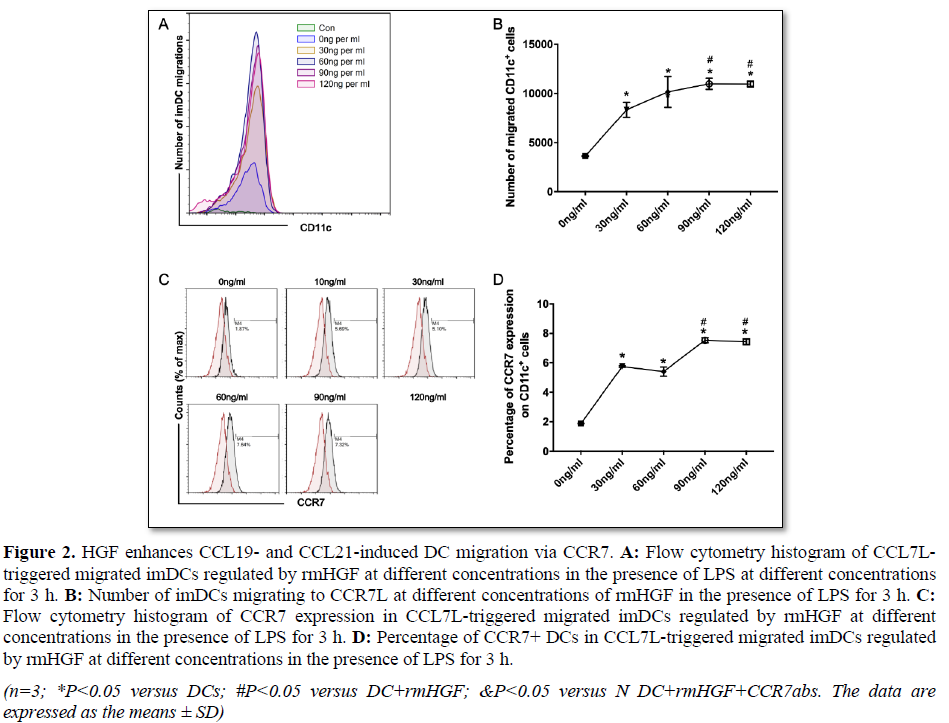
Previous studies have shown the continuous migration of DCs to an HGF gradient (45 ng/mL), but increased an HGF

concentration will eliminate this effect [24]. To observe the changes in DC migration in an in vitro ALI environment, we observed the influence of HGF on DC migration under LPS stimulation. We found that rmHGF can increase the number of migrated DCs without CCL19 and CCL21 stimulation, but the number was still small. However, under the stimulation of CCL19 and CCL21, rmHGF significantly increased the number of migrating DCs, and the dose-dependent growth trend occurred within a certain range; the number of migrated DCs reached a peak when the HGF concentration was 90 ng/mL (Figures 2A and 2B). Further detection of CCR7 expression in DCs showed that, with the increased HGF concentration, CCR7 expression also increased significantly, and the increase was more significant when the HGF concentration was 90 or 120 ng/ml (Figures 2C and 2B). From these data, we found that HGF promoted CCR7 expression while promoting the migration of DCs under LPS and CCR7 ligand stimulation.
HGF promotes DC migration via Hif-1 Pathway downstream of CCR7
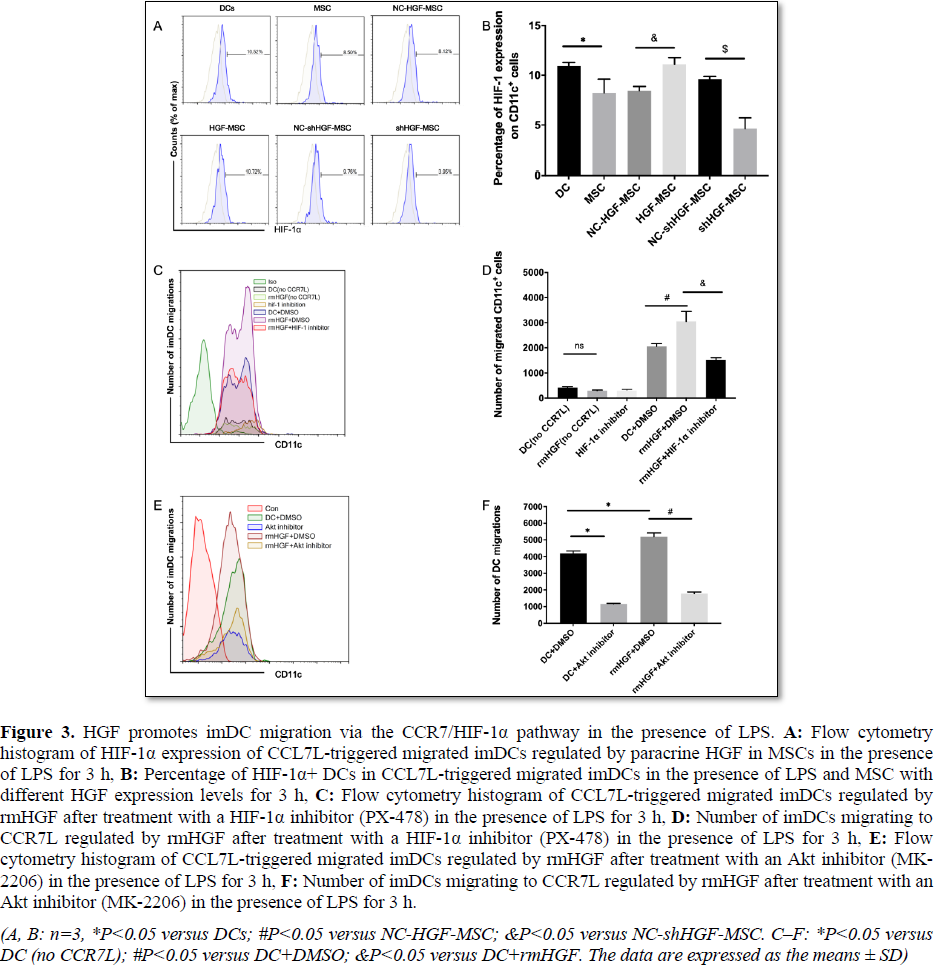
HGF treatment under hypoxia also leads to a significant increase in HIF-1α expression, and HIF-1α inhibition by siRNA leads to a decrease in HGF-mediated migration of HTR-8/SVneo cells [35]. Previous studies have also shown that hypoxia leads to increased migration of dendritic cells by CCL19 in a manner dependent on HIF-1α [36]; however, weakened CCR7-induced HIF-1α activity results in the inhibition of DC migration [28]. In the CCR7 L-triggered DC migration experiment, we detected HIF-1α expression changes in DCs in the presence of MSCs with different HGF expression levels. The results showed that HIF-1α expression in DCs was significantly decreased in the MSC group compared with that in the DC group. However, high HGF expression in MSCs increased HIF-1α expression in DCs, while low HGF expression in MSCs resulted in significantly decreased HIF-1α expression in DCs (Figures

3A and 3B). Therefore, these data revealed that the effect of MSCs on HIF-1α expression in migrated DCs was regulated by the HGF expression level in MSCs.
To further clarify the role of HIF-1α in HGF enhancement of CCR7L-triggered DC migration, a HIF-1α inhibitor (PX-478) was added to block the effect of HGF. The number of migrated DCs decreased significantly after PX-478 administration compared with that in the rmHGF+DMSO group (Figures 3C and 3D). These data indicate that HIF-1α plays an important role in the promotion of DC migration by HGF.
Liu et al. [5] showed that the inhibition of Akt or the downstream transcription factor HIF-1α significantly blocked CCR7-dependent DC migration. We added an Akt inhibitor (MK-2206) to the rhHGF and DC migration systems and found that the migration effect of DCs was significantly weakened in the Akt inhibitor group (Figures 3E and 3F). These data further confirmed that HGF promotes DC migration by activating the CCR7/Akt/HIF-1α pathway.
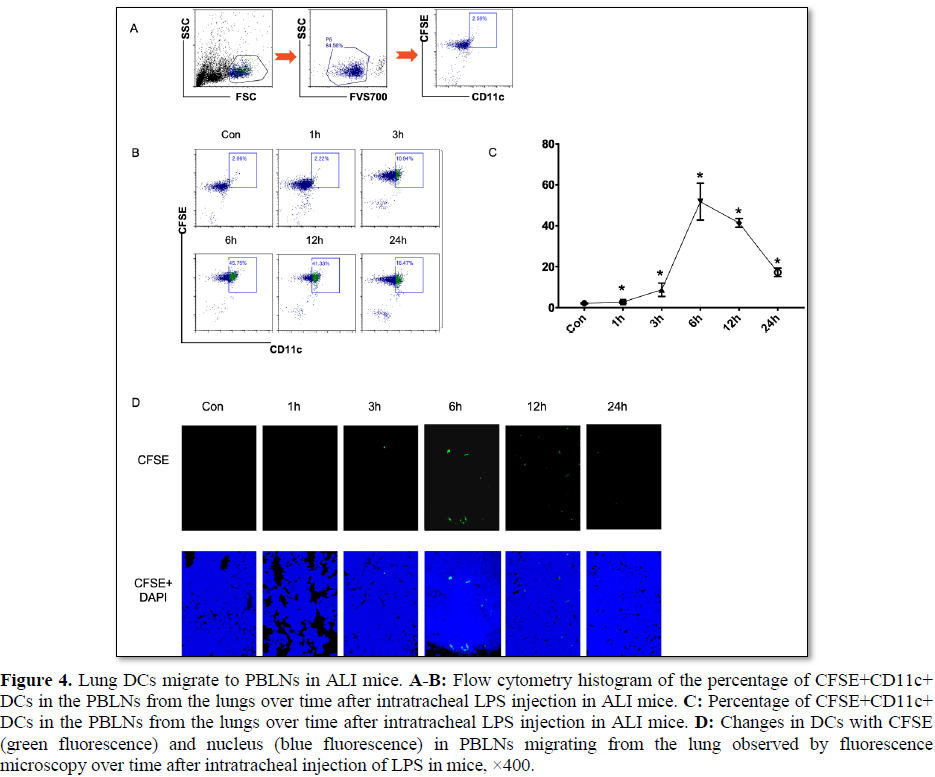
Lung DCs migrate to PBLNs in ALI mice
We investigated the percentage of CFSE+CD11c+ DCs in PBLNs from the lungs of LPS-induced ALI mice by flow cytometry, and found that the percentage of CFSE+CD11c+ DCs migrated to the PBLNs after intratracheal LPS injection markedly increased over time (after 1 h) and reached a peak at 6 h (Figure 4A-4C). CFSE-labeled DCs were injected into the airway, and then the ALI model was constructed. Fluorescence microscopy detected migratory DCs in the PBLNs 3 h after LPS injection, and the increase was more significant 6‒12 h after LPS injection (Figure 4D). The results indicated that the migration of DCs occurred mainly in the early stage of ALI, during which DCs might differentiate into mature DCs to promote the immune response. Therefore, in the follow-up study, we observed the effect of MSC transplantation on DC migration 12 h after ALI modeling.
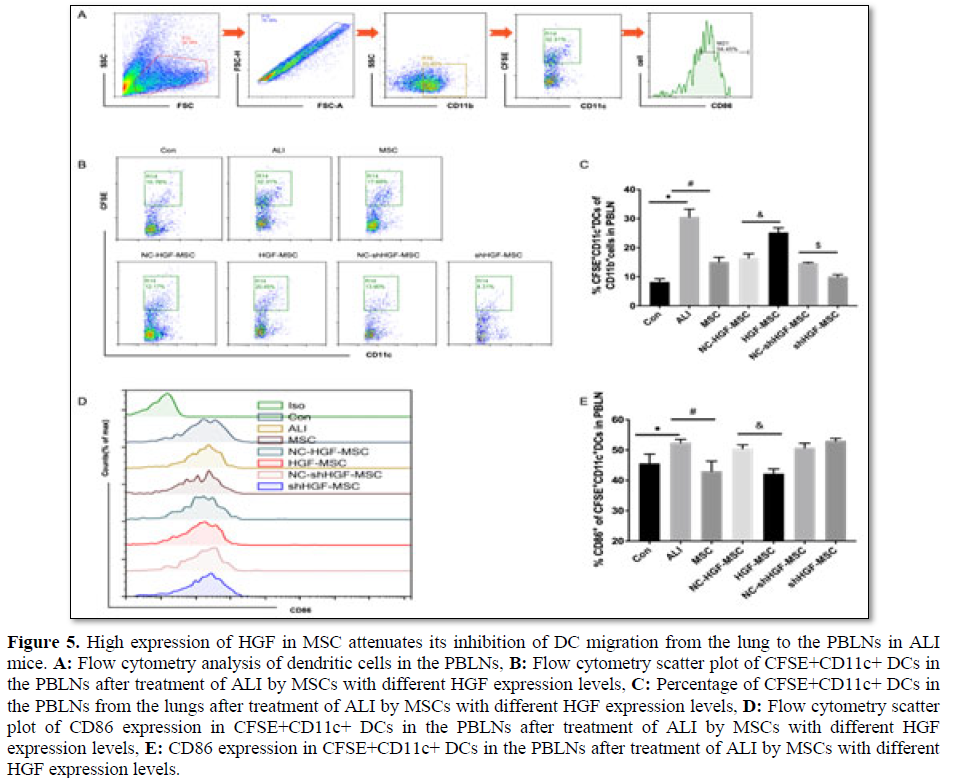
High HGF expression in MSCs attenuated its inhibition of DC migration from the lung to PBLNs in ALI mice
To determine the effect of HGF expression in MSCs on the migration of DCs in ALI mice, we treated mice with MSCs, HGF-MSCs and shHGF-MSCs immediately after the intratracheal injection of CFSE and establishment of the ALI model, and compared their effects on the migration of DCs to the PBLNs.
The proportion of CD11c+CFSE+ DCs was low in the PBLNs of unchallenged mice, significantly increased 12 h after LPS airway injection, but decreased after MSC treatment (Figures 5A and 5B). The percentage of CD11c+CFSE+ DCs in the PBLNs of ALI mice treated with shHGF-MSCs was further reduced, while the percentage of DCs in the PBLNs of ALI mice treated with HGF-MSCs was significantly increased compared with that in the empty vector-MSC group (Figures 5A and 5B). These results indicated that lung DC migration to the PBLNs increased in

The migration of dendritic cells to draining lymph nodes is a prerequisite for initiating an immune response or tolerance, and the migratory DC subtype determines the direction of immune regulation. Previous studies have shown that promoting the production of mature DCs in ALI lung promotes the immune response and aggravates lung injury, while inducing the production of DCregs in the lung reduces lung injury [37]. Mature DCs are characterized by the further increased expression of surface-stimulating molecules CD40, CD80, CD86 and MHCII, which are less expressed in DCregs [19,27,38]. We analyzed the maturity status of CD11c+CFSE+ DCs through the expression of CD86 in PBLNs. At baseline, CD11c+CFSE+ DCs in PBLNs expressed a relatively low level of CD86 in the control group (Figures 5C and 5D). In the PBLNs of ALI mice, CD11c+CFSE+ DCs were significantly increased in their surface expression of CD86. Notably, treatment with MSCs led to a marked reduction in CD86 expression of CD11c+CFSE+ DCs in the PBLNs. Treatment with HGF-MSCs resulted in a significant reduction in the CD86 expression of CD11c+CFSE+ DCs in the PBLNs compared with treatment with the empty vector control group, but CD86 expression was increased after treatment with shHGF-MSCs (Figures 5C and 5D). We speculate from the data that, in ALI mice, high HGF expression in MSCs reduced the inhibitory effect of DC migration from the lung to the PBLNs relative to MSCs, and the migrated DCs may be mainly DCregs with a negative immunomodulatory effect.
Mesenchymal stem cells inhibit lung DC-to-lymph node migration in a CCR7-dependent manner in ALI mice
We verified the effect of MSCs on the migration of DCs in ALI mice and determined whether the mechanism was related to CCR7 expression. We detected and compared the changes in classic DCs (cDCs) and CCR7+cDCs in the lungs of ALI mice treated with MSCs, HGF-MSCs, and shHGF-MSCs. In normal control mice, the proportion of cDCs in lung tissue was lower, but that in lung tissue was

significantly increased 12 h after the tracheal injection of LPS (Figures 6A and 6B). However, the proportion of cDCs in lung tissue was decreased after MSC treatment (Figures 6A and 6B). We detected CCR7 expression in pulmonary DCs after ALI treatment and found that, in normal control mice, the proportion of CCR7+cDCs in lung tissue was lower, but the proportion of CCR7+cDCs in lung tissue was significantly increased 12 h after the tracheal injection of LPS. However, the proportion of CCR7+cDCs in lung tissue was decreased after MSC treatment. Based on the results in section 3.5 of this study and these data, the total amount of cDC recruitment in LPS-induced ALI mice lungs increased rapidly and continued to migrate to lymph nodes. Additionally, MSC therapy reduced the number of DCs in the lung and inhibited the migration of DCs to regional lymph nodes by inhibiting CCR7 expression in DCs (Figures 6C and 6D).
LPS-induced ALI mice, whereas MSC treatment inhibited this migration. High HGF expression weakens the inhibitory effect of MSCs on DC migration in ALI mice, while low HGF expression strengthens the effect of MSCs.
High HGF expression in MSCs affects lung DC-to-lymph node migration independent of CCR7 in ALI mice
The vitro results showed that the promotion of DC migration in MSCs with high HGF expression might depend on the activation of CCR7 and its downstream pathways compared with that in the control group. We found that, compared with the empty vector control group, the proportion of lung DCs in ALI mice treated with HGF-MSCs further decreased, while that of shHGF-MSCs increased (Figures 6A and 6B). Treatment with HGF-MSCs did not increase CCR7 expression in lung cDCs compared with treatment with the empty vector control group, and CCR7 expression in the shHGF-MSC group was not decreased (Figures 6C and 6D). These findings indicate that high HGF expression in
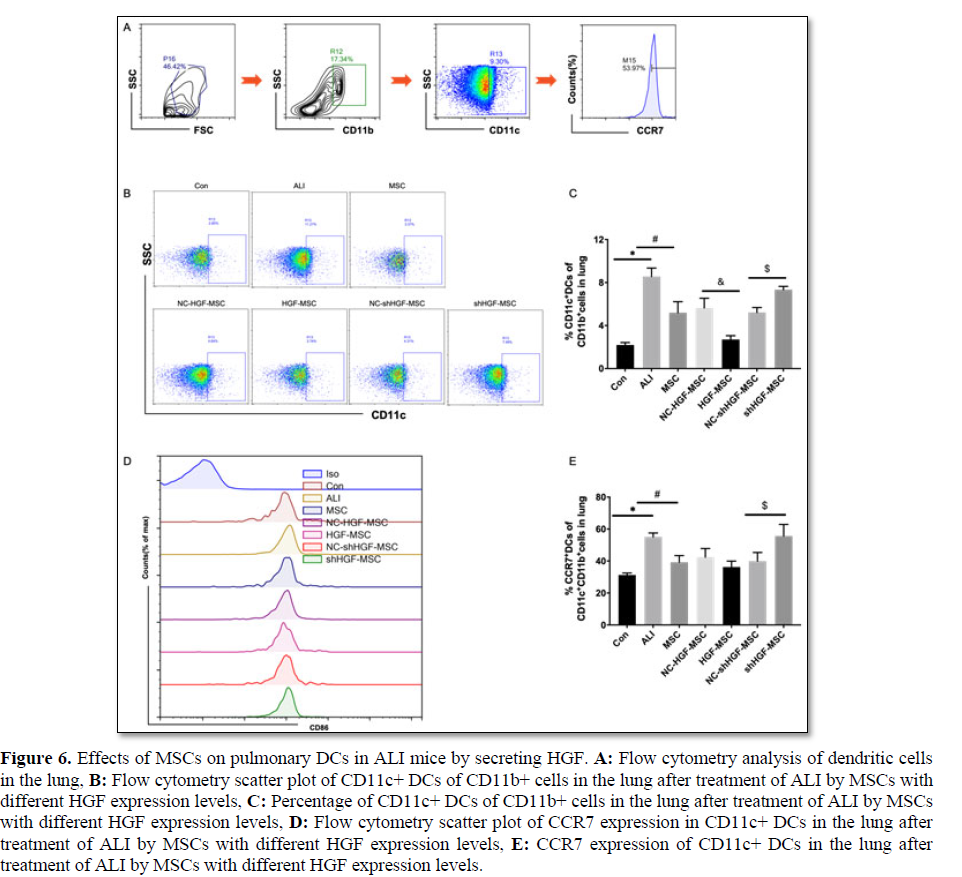

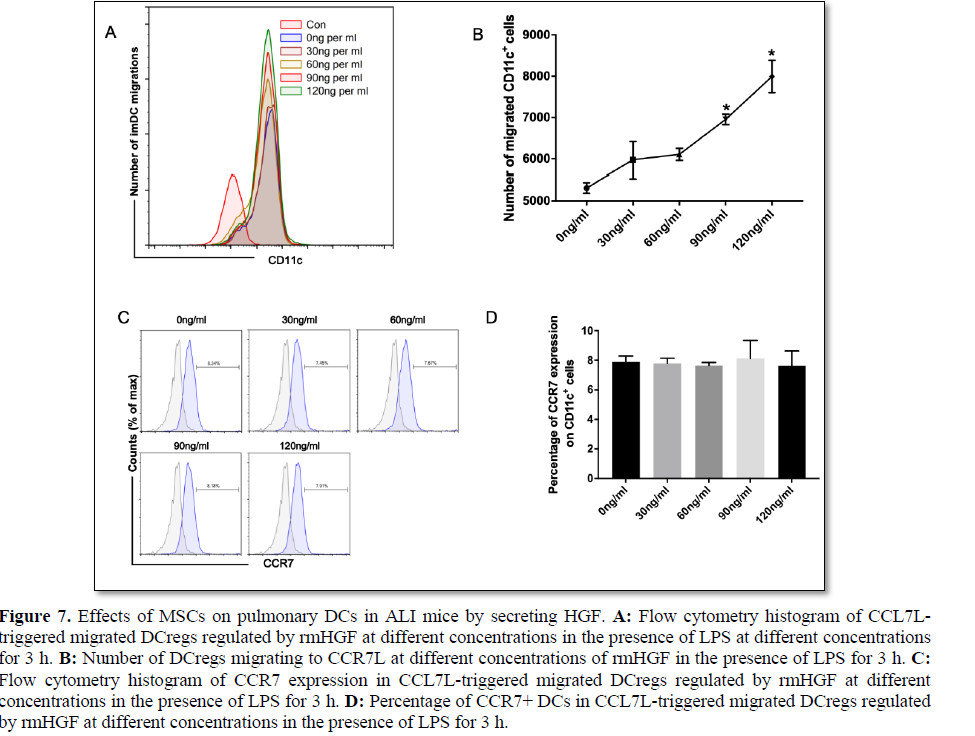
MSC affects the migration of lung DCs to lymph nodes in ALI mice, an effect that may be independent of CCR7.
We further clarified whether HGF can promote DCreg migration in vitro and whether this process is related to CCR7 expression. DCregs were obtained by extracting suspended DCs from mature DCs and MSC coculture for 3 days. In vitro simulation of the ALI environment by CCL19 and CCL21 was used to induce the migration of DCregs in an LPS environment, and rmHGF with different concentrations was administered to block the migration of DCregs. HGF at a concentration of 30 ng/ml or higher significantly promoted the migration of DCregs in the microenvironment and showed a concentration-dependent trend (Figures 7A and 7B), while CCR7 expression showed no significant difference among the groups (Figures 7C and 7D). Thus, HGF may promote the migration of DCregs by CCR7L and does not depend solely on CCR7.
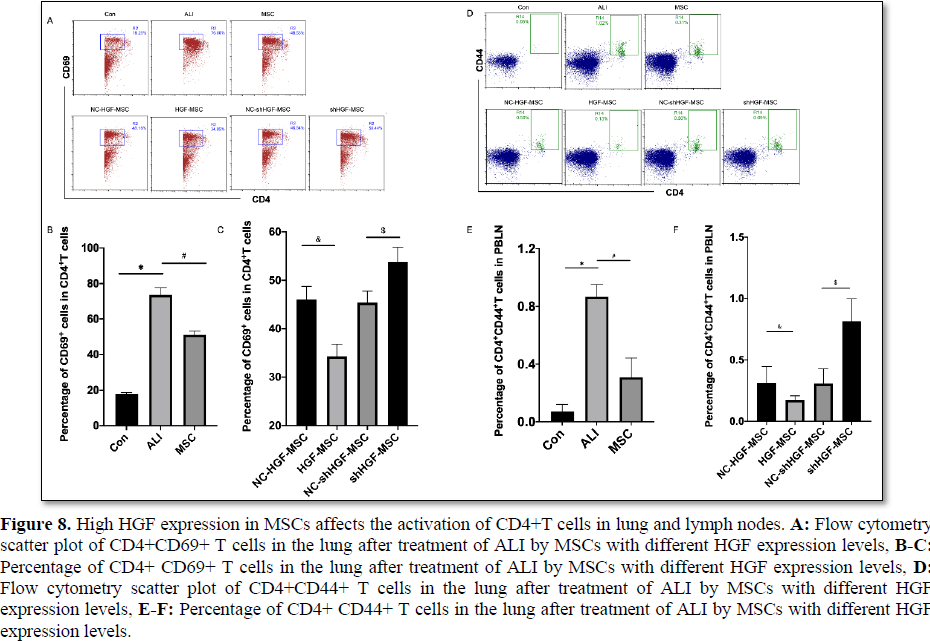
High HGF expression in MSCs affects the activation of CD4+T cells in lung and lymph nodes
Increased expression of CD44 in activated T cells promotes the targeted migration of T cells to target tissues. The activation-induced molecule CD69 is the earliest expression after T cell activation and promotes T cell activation and proliferation [39]. Studies have reported that inhibiting the activation of CD4+T cells and inducing the production of regulatory T cells (Tregs) can improve the inflammatory

significantly after 12 h of ALI, which was manifested as increased expression of CD44 or CD69, while MSC treatment inhibited their expression (Figure 8A-8F). Compared with the empty vector control group, the percentage of CD44+CD4+T cells in PBLN or CD69+ CD4+T cells in lung was significantly decreased in the HGF-MSC group, while that in the shHGF-MSC group increased markedly (Figure 8A-8F). Therefore, all data showed that MSCs treatment attenuated the activation of CD4+T cells, while high expression of HGF enhanced the inhibitory effect of MSC on T cells.
DISCUSSION
The mechanism underlying MSC-mediated immune regulation in ARDS remains unclear. Herein we found that, under an in vitro LPS environment, MSCs activated CCR7/HIF-1α pathway via paracrine HGF to regulate imDC migration. In vivo experiments revealed that, 1 h after intratracheal injection of LPS, lung DC migration to PBLN was significantly increased, and MSCs inhibited DC migration by suppressing the expression of CCR7 in lung DCs. However, compared with empty vector-MSCs, MSCs overexpressing HGF promoted the migration of lung DCs to PBLNs, which are immunomodulatory DCregs independent of CCR7 migration rather than imDCs dependent on CCR7.
MSCs inhibit the migration of dendritic cells and expression of CCR7 in dendritic cells during acute lung injury. Previous studies have shown that lung DC-to-PBLN migration is impaired, resulting in reduced T-cell activation and blunt airway inflammation [41]. Our previous study also found that, after endotracheal administration of LPS, the proportion of lung DCs in mice significantly increased and lung injury was aggravated, while MSC treatment reduced the proportion of lung DCs and alleviated lung injury [19,37]. Whether this effect is related to the influence of MSCs on the migration of DCs remains unclear. This study simulated the ALI environment in vitro and observed the effect of MSCs on the migration of immature DCs in the presence of LPS. We found that MSCs significantly inhibited the migration of DCs and decreased the expression of CCR7 in

DCs. During the migration of DCs triggered by CCL19, CCL21 and LPS, the expression of CCR7 was decreased and the migration effect of DCs disappeared after intervention with the CCR7-neutralizing antibody. The expression of CCR7 is critical to the migration of DCs, and MSCs inhibit the migration of imDCs by potentially reducing CCR7 expression. ImDCs can be differentiated into mature DCs under LPS stimulation, and both can be directionally migrated under CCR7L induction [28]. MSCs can reduce CCR7 expression in mature DCs and inhibit the migration to CCL19, which has been reported previously [25]. Our in vivo studies revealed that the migration of CFSE-labeled DCs from the lung to the PBLNs during ALI was significantly increased with CCR7 high expression, while the reduction of CFSE-labeled DCs migrating to lymph nodes after MSC treatment was accompanied by a decrease in CCR7 expression. The lung is an important source organ of DCs for the PBLNs. Through detection of classic DCs in the lung, we also found that the percentage of lung DCs and expression of CCR7 increased in the ALI group, while the proportion of DC and expression of CCR7 decreased significantly after MSC treatment, a finding that is consistent with previous study findings [19]. In addition to inhibiting migration to reduce the percentage of migrated DCs in the PBLNs, MSCs also reduced the number of lung DCs at the previous site of PBLN-DCs. The decreased number of DCs in the lung may be related to the decreased number of DCs in peripheral blood by MSCs therapy. The number of DCs in the lung is also affected by the number of DCs in the blood because previous studies have reported that MSCs may reduce the number of DCs in the blood to reduce its migration to the lung [37]. Therefore, the regulation of pulmonary DCs by MSCs is an important link to realize ALI immunotherapy. However, the regulation of MSC function to enhance the regulation of DCs in ALI has important clinical value in alleviating lung injury. Further studies are warranted.
The paracrine mechanism is mainly used by MSCs to mediate immune regulation and injury protection [42]. Studies have shown that more than 80% of the therapeutic effects of adult MSCs are realized via paracrine mediation [43]. In vitro and in vivo, HGF plays an important role in bone marrow MSC therapy for ALI and MSCs can alleviate ALI alveolar epithelial and endothelial injury via paracrine hepatocyte growth factor through a paracrine mechanism [23,44]. We previously found that, after treating ALI with MSCs with high HGF expression, the proportion of lung DCs can be reduced better than that of MSCs with empty vectors [19], a finding that may be related to the migration of DCs regulated by HGF. However, many studies have found that HGF and MSCs have opposite effects on DC migration [24,25], and the effect of high HGF expression on the inhibition of DC migration by MSCs remains unclear. This study found that, compared with empty vector-MSCs, HGF-MSC overexpression increased the migration of DCs to CCR7L in the LPS environment. Surprisingly, CCR7 expression in migrated DCs was also significantly increased. We have confirmed that the secretion of HGF in overexpressed HGF-MSC is significantly higher than that of the empty vector group [19]. Thus, we speculate that the paracrine HGF alleviated the inhibition of imDC migration by MSCs to CCR7L in the LPS environment. Whether HGF enhances the migration of imDCs to CCL19/CCL21 in the presence of LPS is related to the promotion of CCR7 expression remains to be further clarified.
HGF enhances the migration of imDCs to CCL19/CCL21 in the presence of LPS, which is dependent on promoting the expression of CCR7. We found that, in the presence of LPS, with an increasing rmHGF concentration, the migration of imDCs to CCL19/CCL21 gradually increased. This finding was not completely consistent with previous studies: DCs continuously migrate to the HGF gradient (45 ng/mL), and this effect is eliminated by the continuous increase in the HGF concentration [24]. This difference may be related to the different induced microenvironments. In the absence of CCL19/21, this study found that the effect of HGF in promoting DC migration was very weak. We also found that the CCR7 expression in migrated DCs increased in a gradient manner with the increase in HGF concentration, further confirming that the migration of DCs promoted by HGF may be related to the increase in CCR7 expression in DCs.
HGF can enhance the migration of imDCs to CCR7L in the LPS environment by activating the CCR7/Akt/HIF-1α pathway. Paracrine HGF in MSCs was reported to inhibit DC activation through the Akt pathway [19,45], and blocking the Akt-HIF-1α pathway can inhibit the glycolysis of dendritic cells, leading to the inhibition of CCR7-mediated DC migration, which has been verified in different studies [29,46]. It remains unclear whether the CCR7/HIF-1α pathway is an important link in the regulation of DC migration by HGF. This study found that, in the presence of MSCs, HIF-1α expression decreased in migrating DCs, increased with the up regulation of the HGF gene in MSCs, and decreased with the down regulation of the HGF gene. After inhibiting HIF-1α, the promotion effect of HGF on CCL19- and 21-triggered DC migration was also significantly weakened. Consistent with previous studies, activation of HIF-1α in the downstream pathway of CCR7 also promotes DC migration and antigen presentation [28-31]. Similarly, by inhibiting the upstream Akt of the HIF-1α pathway, the promotion effect of HGF on induced DC migration showed similar results as the inhibition of HIF-1α. Thus, HGF enhances the migration of imDCs to CCR7L in an LPS environment by activating the CCR7/Akt/HIF-1α pathway, findings that are consistent with the earlier published observation that CCR7 stimulation enhances HIF-1α activity both under normoxia and hypoxia conditions in an Akt-dependent manner to mediate DC migration [28].
Early accelerated DC migration is necessary for the rapid induction of immune defense to eliminate invading pathogens. Aberrant DC trafficking and accumulation need timely regulation to prevent excessive inflammation, which has been confirmed to be related to the pathogenesis of various inflammatory disorders [11,47]. Previous studies have shown that the migration of activated respiratory DCs (RDCs) from inflamed lungs to the draining lymph nodes mainly occurs within 6-18 h following a pulmonary virus stimulus [48]. We found that the migrated-to-PBLN CFSE-labeled CD11c+DCs population represents 1.10% of the total dendritic cell population in lymph nodes, suggesting that the normal respiratory tract of DCs is the absence of any stimulus to PBLN migration. Compared with previous reports on viral tracheal stimulation [48], DC migration occurred earlier and peaked earlier after LPS respiratory stimulation. The mobility of dendritic cells at 3 h after intratracheal injection of LPS was nearly 3 times that of the normal group and reached its peak at 6-12 h. Therefore, the migration of DCs during ALI mainly occurred in the early stage, and regulating the migration of DCs to maintain immune homeostasis during acute lung injury may also be more valuable within 12 h after ALI. In clinical practice, a balance is needed between regulating DC migration to avoid organ damage caused by excessive inflammatory responses to bacterial ARDS and eliminating pathogens.
Previous data have demonstrated that pulmonary influenza infection or intratracheal injection of other inducers of lung DC maturation/migration results in the rapid and augmented trafficking of mature RDCs to the PBLNs to exhibit a mature phenotype with high expression of CD80, CD86 and CD4 [37-48]. Consistent with this finding, we showed that pulmonary DC migration to PBLNs increased after intra-airway injection of LPS, and the migrated CFSE+ DCs showed high CD86 expression. However, after MSC treatment, the number of PBLN-migrated CFSE+ DCs was significantly reduced with a decrease in CD86 expression, indicating that the migrated CFSE+ DCs were no longer mature phenotypes. Our previous studies have shown that, in LPS-induced ALI mice, lung DCs may be induced as immunotolerant DCregs with low CD86 expression following MSC treatment [37] that is enhanced with increased HGF expression in MSCs and weakened with decreased HGF expression [19]. We showed that the proportion of lung DCs in MSC-treated ALI mice decreased with low CCR7 expression, but CCR7 expression did not increase with elevated HGF expression in MSCs and decreased with reduced HGF expression, a finding that was inconsistent with the in vitro experiment. Therefore, we hypothesized that the high HGF expression in MSCs promoted lung DCreg-to-lymph node migration in a CCR7-independent manner in ALI therapy compared with empty vectors-MSCs. Surprisingly, we found that the number of migrated DCregs obtained from coculture with MSCs increased with the increased rmHGF concentration in CCL19, CCL21 and LPS environments, but no difference was found in CCR7 expression. DCregs induced by prolonged mature DC coculture with MSCs reduced CCR7 expression, an observation that has been reported in previous studies [25]. Further in vitro experiments also confirmed that increasing the HGF stimulation concentration could not increase CCR7 expression but significantly promoted DCreg migration. Because DCreg has a negative immunomodulatory function and inhibits T cell proliferation and activation [19,37], which is consistent with this result. In the ALI group, CD4+T cell activation ratio increased in the lungs or PBLN, MSC therapy inhibited T cell activation, while high expression of HGF enhanced the inhibition of MSC on T cell activation. Therefore, HGF promotion of DCreg migration is not dependent on CCR7, which may be related to other signaling pathways or molecules such as matrix metalloproteinase 9 (MMP-9) [27], further explaining the reason why high HGF expression enhances the immunotherapeutic effect of MSCs on ALI [19].
CONCLUSIONS
This study showed that MSCs activated the CCR7/HIF-1α pathway via paracrine HGF to enhance imDC migration to CCR7L in the presence of LPS in vitro. The migration and maturation of RDCs to the PBLNs during ALI mainly occurred in the early stage of ALI. MSCs inhibited the migration of DCs to the PBLNs by reducing CCR7 expression in lung DCs, but high HGF expression weakened the inhibition of DC migration by MSCs and promoted DCreg migration in a CCR7-independent manner.
ACKNOWLEDGEMENTS
We would like to thank Feng Liu and Fei Peng for their assistance in caudal intravenous drug injection or endotracheal injection and lymph node extraction in mice, as well as Dr. Zhu Cuilin for their guidance in this manuscript.
- Kido T, Muramatsu K, Asakawa T, Otsubo H, Ogoshi T, et al. (2018) The relationship between high-dose corticosteroid treatment and mortality in acute respiratory distress syndrome: a retrospective and observational study using a nationwide administrative database in Japan. BMC Pulm Med 18(1): 28.
- Potapov AL, Novikov N, Tumanskii VA, Babanin AA (2013) Surfactant replacement therapy increases life-span of patients with acute respiratory distress syndrome. Klin Khir (2): 57-59.
- Gebistorf F, Karam O, Wetterslev J, Afshari A (2016) Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Sys Rev 2016(6): Cd002787.
- Villar J, Blanco J, Kacmarek RM (2016) Current incidence and outcome of the acute respiratory distress syndrome. Curr Opin Crit Care 22(1): 1-6.
- Liu J, Zhang PS, Yu Q, Liu L, Yang Y, et al. (2012) Losartan inhibits conventional dendritic cell maturation and Th1 and Th17 polarization responses: Nuovel mechanisms of preventive effects on lipopolysaccharide-induced acute lung injury. Int J Mol Med 29: 269-276.
- Bantsimba-Malanda C, Marchal-Somme J, Goven D, Freynet O, Michel L, et al. (2010) A role for dendritic cells in bleomycin-induced pulmonary fibrosis in mice? Am J Respir Crit Care Med 182(3): 385-395.
- Dong L, He HL, Lu XM, Yang Y, Qiu HB (2012) Modulation of FLT3 signaling targets conventional dendritic cells to attenuate acute lung injury. APMIS: Acta pathologica, microbiologica, et immunologica Scandinavica 120(10): 808-818.
- Sokol CL, Camire RB, Jones MC, Luster AD (2018) The chemokine receptor CCR8 promotes the migration of dendritic cells into the lymph node parenchyma to initiate the allergic immune response. Immunity 49(3): 449-463.
- Leon B, Lund FE (2019) Compartmentalization of dendritic cell and T-cell interactions in the lymph node: Anatomy of T-cell fate decisions. Immunol Rev 289(1): 84-100.
- Radtke AJ, Kastenmuller W, Espinosa DA, Gerner MY, Tse SW, et al. (2015) Lymph-node resident CD8alpha+ dendritic cells capture antigens from migratory malaria sporozoites and induce CD8+ T cell responses. PLoS Pathol 11(2): e1004637.
- Han Y, Li X, Zhou Q, Jie H, Lao X, et al. (2015) FTY720 Abrogates collagen-induced arthritis by hindering dendritic cell migration to local lymph nodes. J Immunol 195(9): 4126-4135.
- Cai SX, Liu AR, Chen S, He HL, Chen QH, et al. (2015) Activation of Wnt/beta-catenin signalling promotes mesenchymal stem cells to repair injured alveolar epithelium induced by lipopolysaccharide in mice. Stem Cell Res Ther 6(1): 65.
- Meng SS, Guo FM, Zhang XW, Chang W, Peng F, et al. (2018) mTOR/STAT-3 pathway mediates mesenchymal stem cell-secreted hepatocyte growth factor protective effects against lipopolysaccharide-induced vascular endothelial barrier dysfunction and apoptosis. J Cell Biochem 120(3): 3637-3650.
- Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, et al. (2017) Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med 196(10): 1275-1286.
- Wang L, Shi M, Tong L, Wang J, Ji S, et al. (2019) Lung-resident mesenchymal stem cells promote repair of LPS-induced acute lung injury via regulating the balance of regulatory T cells and Th17 cells. Inflammation 42(1): 199-210.
- Yabuki H, Wakao S, Kushida Y, Dezawa M, Okada Y (2018) Human multilineage-differentiating stress-enduring cells exert pleiotropic effects to ameliorate acute lung ischemia-reperfusion injury in a rat model. Cell Transplant 27(6): 979-993.
- Plusa T (2017) Stem/progenitor cells in diseases of the respiratory tract. Polski merkuriusz lekarski: Organ Polskiego Towarzystwa Lekarskiego 42(249): 97-100.
- Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, et al. (2016) Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells 34(8): 2210-2223.
- Lu Z, Chang W, Meng S, Xu X, Xie J, et al. (2019) Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell Res Ther 10: 372.
- Hackstein H, Lippitsch A, Krug P, Schevtschenko I, Kranz S, et al. (2015) Prospectively defined murine mesenchymal stem cells inhibit Klebsiella pneumoniae-induced acute lung injury and improve pneumonia survival. Respir Res 16: 123.
- Consentius C, Akyuz L, Schmidt-Lucke JA, Tschope C, Pinzur L, et al. (2015) Mesenchymal stromal cells prevent allostimulation in vivo and control checkpoints of Th1 Priming: Migration of Human DC to Lymph Nodes and NK Cell Activation. Stem Cells 33(10): 3087-3099.
- Wilke CA, Chadwick MM, Chan PR, Moore BB, Zhou X (2019) Stem cell transplantation impairs dendritic cell trafficking and herpesvirus immunity. JCI Insight 4(18): e130210.
- Yang Y, Chen QH, Liu AR, Xu XP, Han JB, et al. (2015) Synergism of MSC-secreted HGF and VEGF in stabilising endothelial barrier function upon lipopolysaccharide stimulation via the Rac1 pathway. Stem Cell Res Ther 6: 250.
- Kurz SM, Diebold SS, Hieronymus T, Gust TC, Bartunek P, et al. (2002) The impact of c-met/scatter factor receptor on dendritic cell migration. Eur J Immunol 32(7): 1832-1838.
- English K, Barry FP, Mahon BP (2008) Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett 115(1): 50-58.
- Hubel J, Hieronymus T (2015) HGF/Met-Signaling contributes to immune regulation by modulating tolerogenic and motogenic properties of dendritic cells. Biomedicines 3(1): 138-148.
- Baek JH, Birchmeier C, Zenke M, Hieronymus T (2012) The HGF receptor/Met tyrosine kinase is a key regulator of dendritic cell migration in skin immunity. J Immunol 189: 1699-1707.
- Liu J, Zhang X, Chen K, Cheng Y, Liu S, et al. (2019) CCR7 Chemokine Receptor-Inducible lnc-Dpf3 Restrains Dendritic Cell Migration by Inhibiting HIF-1alpha-Mediated Glycolysis. Immunity 50(3): 600-615.
- Liu Q, Chen T, Chen G, Shu X, Sun A, et al. (2007) Triptolide impairs dendritic cell migration by inhibiting CCR7 and COX-2 expression through PI3-K/Akt and NF-kappaB pathways. Mol Immunol 44(10): 2686-2696.
- Filippi I, Morena E, Aldinucci C, Carraro F, Sozzani S, et al. (2014) Short-term hypoxia enhances the migratory capability of dendritic cell through HIF-1alpha and PI3K/Akt pathway. J Cell Physiol 229(12): 2067-2076.
- Xuan NT, Hoang NH, Nhung VP, Duong NT, Ha NH, et al. (2017) Regulation of dendritic cell function by insulin/IGF-1/PI3K/Akt signaling through klotho expression. J Recept Signal Transduct Res 37(3): 297-303.
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, et al. (1992) Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 176(6): 1693-1702.
- Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, et al. (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Method 223(1): 77-92.
- Gibbings SL, Jakubzick CV (2018) Isolation and characterization of mononuclear phagocytes in the mouse lung and lymph nodes. Methods Mol Biol 1809: 33-44.
- Chaudhary P, Babu GS, Sobti RC, Gupta SK (2019) HGF regulate HTR-8/SVneo trophoblastic cells migration/invasion under hypoxic conditions through increased HIF-1α expression via MAPK and PI3K pathways. J Cell Commun Signal 13(4): 503-521.
- Kohler T, Reizis B, Johnson RS, Weighardt H, Forster I (2012) Influence of hypoxia-inducible factor 1alpha on dendritic cell differentiation and migration. Eur J Immunol 42(5): 1226-1236.
- Lu Z, Meng S, Chang W, Fan S, Xie J, et al. (2020) Mesenchymal stem cells activate Notch signaling to induce regulatory dendritic cells in LPS-induced acute lung injury. J Transl Med 18: 241.
- Yuan X, Qin X, Wang D, Zhang Z, Tang X, et al. (2019) Mesenchymal stem cell therapy induces FLT3L and CD1c (+) dendritic cells in systemic lupus erythematosus patients. Nat Commun 10(1): 2498.
- Ji SR, Ma L, Bai CJ, Shi JM, Li HY, et al. (2009) Monomeric C-reactive protein activates endothelial cells via interaction with lipid raft microdomains. FASEB J 23: 1806-1816.
- Chai YS, Chen YQ, Lin SH, Xie K, Wang CJ, et al. (2020) Curcumin regulates the differentiation of naive CD4+T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomed Pharmacother 125: 109946.
- Constabel H, Stankov MV, Hartwig C, Tschernig T, Behrens GM (2009) Impaired lung dendritic cell migration and T cell stimulation induced by immunostimulatory oligonucleotides contribute to reduced allergic airway inflammation. J Immunol 183: 3443-3453.
- Spaggiari GM, Moretta L (2013) Interactions between mesenchymal stem cells and dendritic cells. Adv Biochem Eng Biotechnol 130: 199-208.
- Maguire G (2013) Stem cell therapy without the cells. Commun Integr Biol 6(6): e26631.
- Hu S, Li J, Xu X, Liu A, He H, et al. (2016) The hepatocyte growth factor-expressing character is required for mesenchymal stem cells to protect the lung injured by lipopolysaccharide in vivo. Stem Cell Res Ther 7: 66.
- Singhal E, Sen P (2011) Hepatocyte growth factor-induced c-Src-phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathway inhibits dendritic cell activation by blocking IkappaB kinase activity. Int J Biochem Cell Biol 43: 1134-1146.
- Guak H, Al Habyan S, Ma EH, Aldossary H, Al-Masri M, et al. (2018) Glycolytic metabolism is essential for CCR7 oligomerization and dendritic cell migration. Nat Commun 9: 2463.
- Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, et al. (2004) Visualizing dendritic cell networks in vivo. Nat Immunol 5(12): 1243-1250.
- Legge KL, Braciale TJ (2003) Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity 18(2): 265-277.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Oncology Clinics and Research (ISSN: 2643-055X)
- Dermatology Clinics and Research (ISSN:2380-5609)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- Journal of Spine Diseases










