Review Article
Characterization of Three Different Types of Pineapple Waste Powder
6998
Views & Citations5998
Likes & Shares
Pineapple (Ananas cosmocus) is produced in Malaysia for domestic and export market which consists of 50% waste, thus lead to waste management problem. Hence, this study aimed to determine the effects of different drying temperatures on composition of peel and core of three different types of pineapples (Josapine, Moris and N36) and characterize powders developed from the different parts of the waste. Preliminary test on different drying temperatures (50˚C, 70˚C, 90˚C) and time (7, 9 and 12 h) were conducted to form the waste into powders. Thus, temperature 90˚C for 7 h was selected for further analysis due to higher phenolic content in peel (0.007 ± 0.00 mg/g) and core (0.006 ± 0.0 mg/g) respectively, compared to 50˚C and 70˚C. Physicochemical analysis such as composition of wastes (peel and core), color, water holding capacity (WHC), water solubility index (WSI) and total phenolic content (TPC) were compared between the powders. There was a significant difference in fibre content between N36 (3.50 ± 0.0%) and Morris peel (1.77±0.05%) respectively thus peel powder of N36 pineapple has significantly higher ability to hold water (7.53%) compared to core powders. However, Josapine powders have higher in yellowness (25.95 ± 0.12) than N36 and Morris. The waste powders (peel and core) from these pineapples may be considered as food functional ingredients.
Keywords: Josapine, Morris, N36, Pineapple waste, Total phenolic content and water holding capacity
INTRODUCTION
Pineapple (Ananas cosmosus) is a tropical fruit known as the first commodity crop with high export potential grown in Malaysia. As of 2017, Malaysia ranked 23rd worldwide with pineapple harvested area covering approximately 10,131 Ha and ranked 21st worldwide with total pineapple production of 340, 722 MT [1]. There are nine major pineapple varieties planted in Malaysia, which include Morris, N36, Josapine, Sarawak, Moris Gajah, Gandul, Yankee, Masapine and MD2. Research stated that N36 and Josapine were grown locally for the local fresh fruit market and not grown outside of Malaysia. Pineapple fruit is available throughout the world in three different forms which are fresh fruits, canned fruit and fruit juice.
Rathna kumar et al. [2] reported that half of the whole weight of pineapple was used for consumption and further processing, while another 50% consists of waste. The waste part of the pineapple includes the stem, crown, peel, and core. Production of pineapple is increasing day by day; thus, this creates waste management problems with an increase of pineapple waste.
A previous study conducted in Indonesia by Saraswaty et al. [3], who found that pineapple peel waste contains antioxidant, sugar, a phenolic compound, high fiber and protein content after macerated it in ethanol. They also reported high antioxidant activity in pineapple after extraction using methanol. However, studies detected that methanol is a toxic organic solvent which leads to several health problems. Many have reported on waste (peel and core) composition of pineapple extracted using various concentrations of ethanol due to its ability to extract a high percentage of yield [3].
According to research, pineapple peel powder is a good source of dietary fibre, protein, and minerals capable of accelerating growth and activity of the tested Lactobacilli. Supported by Sah et al. [4] who stated the effect of pineapple waste powder on probiotic growth of yogurt with an increase (0.3-1.4 log cycle) in probiotic populations due to pineapple powder supplementation.
Therefore, this study aims to determine the effects of different drying temperatures on total phenolic compound of peel and core of three different types of pineapples (Josapine, Moris and N36) and characterize powders developed from the different parts of the waste. These findings may expand the application of core and peel as a functional food ingredient.
MATERIALS & METHODS
Sample preparation
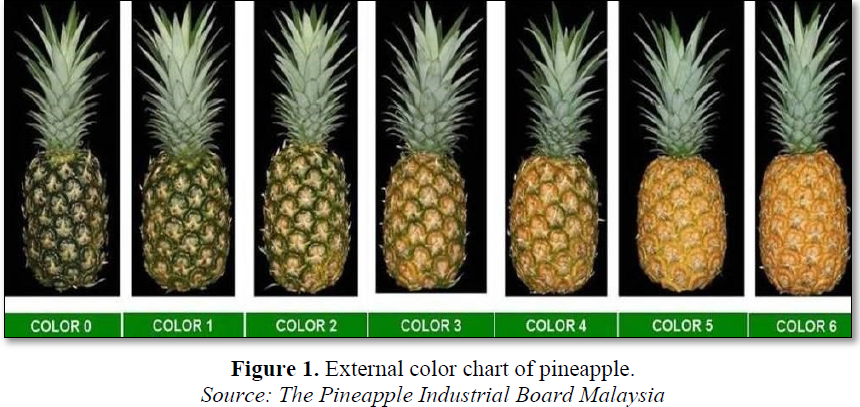
Three types of fresh pineapple fruits (Ananas comosus) were tested in this study, which include Josapine pineapple, Morris pineapple, and N36 pineapple. All these pineapples were purchased from Pasar Besar Meru, Klang which have been harvested from Muar and Pontian, Johor. These fruits were selected based on their uniformity in shape and mature commercial stage at stage 4 as shown in Figure 1. The thick layer of the skin was peeled off from the fruits manually using a sharp knife. The crown of the pineapple was discarded to minimize cross-contamination from foreign matter during processing. Pineapple peels were washed thoroughly under running tap water to get rid of adhered impurities on the surfaces [7].
A preliminary test was conducted to dry all the pineapple wastes (peel and core) using an oven (Binder Fisher Scientific Malaysia Sdn Bhd) at three different temperatures (50˚C, 70˚C and 90˚C) until the texture of the peel and core became hard and the moisture content reaches 12-14%. The dried pineapple peel and core were then ground using dry mill grinder (Panasonic, Malaysia) and sieved through a 40-mesh sieve (MonotaRO, Malaysia) to obtain a powdered form of samples. The powders were stored in a dark bag to prevent oxidation of active compounds due to sunlight and kept in a chiller prior to the analysis [7].

Chemicals and reagents
Petroleum ether, ethyl alcohol, sulphuric acid and sodium hydroxide were purchased from Fisher scientific (M) Sdn Bhd, Malaysia. Folin-Ciocalteu’s phenol and Sodium carbonate Na2CO3 were purchased from EMD Milipore Corporation, Germany.
Proximate analysis
The fresh peel and core pineapple powders from the three types of pineapples were proceeded for proximate analysis to determine ash, moisture content, crude protein, fat and fiber using standard methods of AOAC (2006) 934.01, 942.05, 992.15, 954.02, 978.10, respectively.
Antioxidant activity
Total phenolic content: Folin-Ciacalteau method is used to determine the amount of total phenolic present in the fresh and dried pineapple powders. The sample of 1 g powder was taken and diluted with 50 ml of distilled water. Then, 0.5 ml of the diluted sample was added with 2 ml of Folin reagent which was freshly prepared at 1:10 ratio. After that, 4 ml of 7.5% sodium carbonate was added to the mixture and then shaken for 2 min using a vortex mixer (Labmart, Malaysia) before it kept in the dark for 30 min. The absorbance of the sample was measured using UV-VIS spectrophotometer (Shimadzu, Malaysia) at 765 nm [2]. The total phenolic content of each pineapple peel extract sample was expressed as mg Gallic Acid Equivalent (GAE). All samples were analyzed in triplicates and the average values were calculated [7].
Physical analysis
Water holding capacity (WHC): Water holding capacity (WHC) was determined according to Traynham et al. [8] with some modifications on the amount of sample being tested. Approximately, 0.2 g of pineapple powder was weighed in 100 ml plastic centrifuged bottles. After that, 10 ml of distilled water was added and mixed using a vortex mixer for 2 min. The sample was left for 30 min at room temperature before it was centrifuged at 1200 g (3709 rpm) for 20 min (Kubota, Japan). After the centrifugation process, the supernatant was carefully poured out of the centrifuged bottles. The mass of sample precipitated in the centrifuged bottles was recorded. Water holding capacity (g water/g powder) was calculated using the formula:
where
WFB is weight of bottle after decanting
WDB is weight of dry bottle
WTP is total powder weight
Oil holding capacity (OHC): According to studies, oil holding capacity was calculated with slight modifications on the quantity of powder used. About 0.2 g of pineapple powder was weighed into 100 ml centrifuge bottles. About 10 ml of refined vegetable oil (corn oil) was added into the centrifuged bottle for each sample and it was mixed using vortex mixer for 2 min. Then, the sample was left for 30 min before it was centrifuged at 1200 g (3709 rpm) for 20 min using (Kubota, Japan). After the centrifugation process, the supernatant was carefully poured off from the centrifuged bottle. The mass of the precipitate was recorded. Oil holding capacity (g oil/g dry powder) was calculated using this formula:
Where
WFB is weight of bottle after decanting
WDB is weight of dry bottle
WTP is total powder weight
Powder solubility: The purpose of this solubility test is to study the performance of powder after it was dissolved in water. Powder solubility was tested according to Suzihaque, Hashib and Ibrahim [9] with slight modification by adding 0.2 g of powder sample in a beaker containing 10 ml of distilled water. The mixture was left at room temperature for 5 min. The supernatant was poured onto a pre-weight petri dish and dried in an oven at 105°C for 2 h. The solubility of the powder in percentage was determined by weighing the petri dish and the obtained solids content was divided by the initial sample weight and multiplied by 100 to calculate percentage of solubility [10].
Particle size: The particle size distribution of pineapple peel and core powders a\were determined using laser analyzer. Powders were dispersed by the laser analyzer’s dry powder accessory with air pressure of 4.0 bar and feed rate vibration of 50%. Particle size was evaluated by D [4,3], d (0.9) and d (0.5), which represent the mean diameter and the sizes in microns, at which 90% and 50% of the particles were smaller than the rest of the distribution, respectively. Measurements were performed in triplicate.
Color analysis: According to research, all the powdered samples were spread out about 5 mm diameter on a tray for color analysis by using chroma meter CR-410 (Konica Minolta, Japan). Instrumental color data was provided using CIE system. The L* value indicated darkness (L*=0) to lightness (L*=100), positive a* value indicated red shades while negative values indicated green hues, and positive b* value indicated yellow shades while negative values indicated blue shades. The two chromatic components for both a* value and b* value range from -100 to 100.
Statistical analysis:All results were expressed as the mean ± standard deviation (SD) and statistics were performed using Minitab version 17 statistical package (Minitab Inc., State College, PA, USA). Two-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests were used to identify significant differences at p
RESULTS AND DISCUSSION
Preliminary test analysis
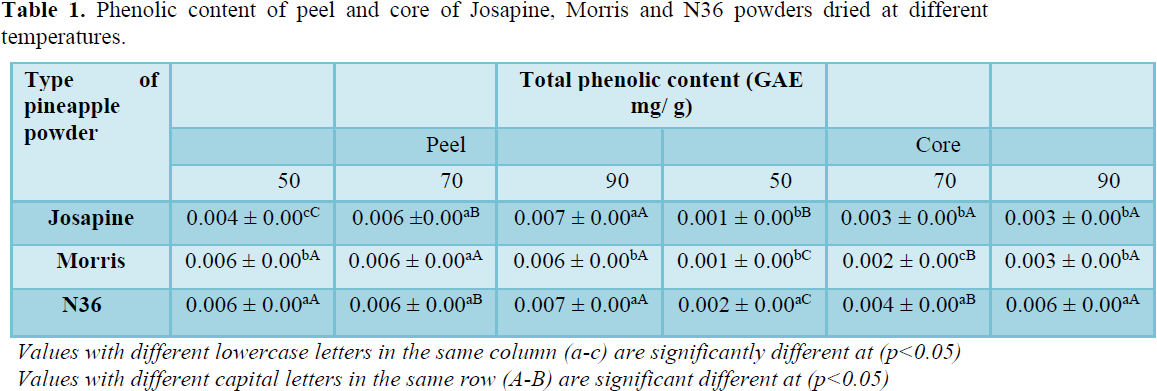
A preliminary test on different drying temperatures (50˚C, 70˚C, and 90˚C) and times (7 h, 9 h and 11 h) was conducted on pineapple wastes (peel and core) to determine the highest total phenolic content (TPC) between the three different types of pineapples. Table 1 shows a significant difference (p<0.05) at 90˚C having the highest total phenolic content (TPC) value in both peel (0.007 mg/g) and core (0.006 mg/g) as compared to temperature 50˚C and 70˚C. Among the three types of pineapples, N36 core (0.006 mg/g) shows a higher TPC value compared to Josapine and Morris pineapple.
This finding indicates that drying at high temperatures will produce high TPC in powders. It is in agreement with previous research which studied the effect of drying at higher temperatures on tomato powder which significantly affected higher content of TPC as compared to low drying temperatures. Thuwapanichayanan et al. [11] also reported that different TPC values might be due to different initial TPC in the species or cultivars or even the geographic origin of the pineapple used. Thus, in this study, drying at temperature 90˚C at a shorter duration (7 h) was selected for further analysis, which contributes to high TPC value in the powders.
Proximate analysis
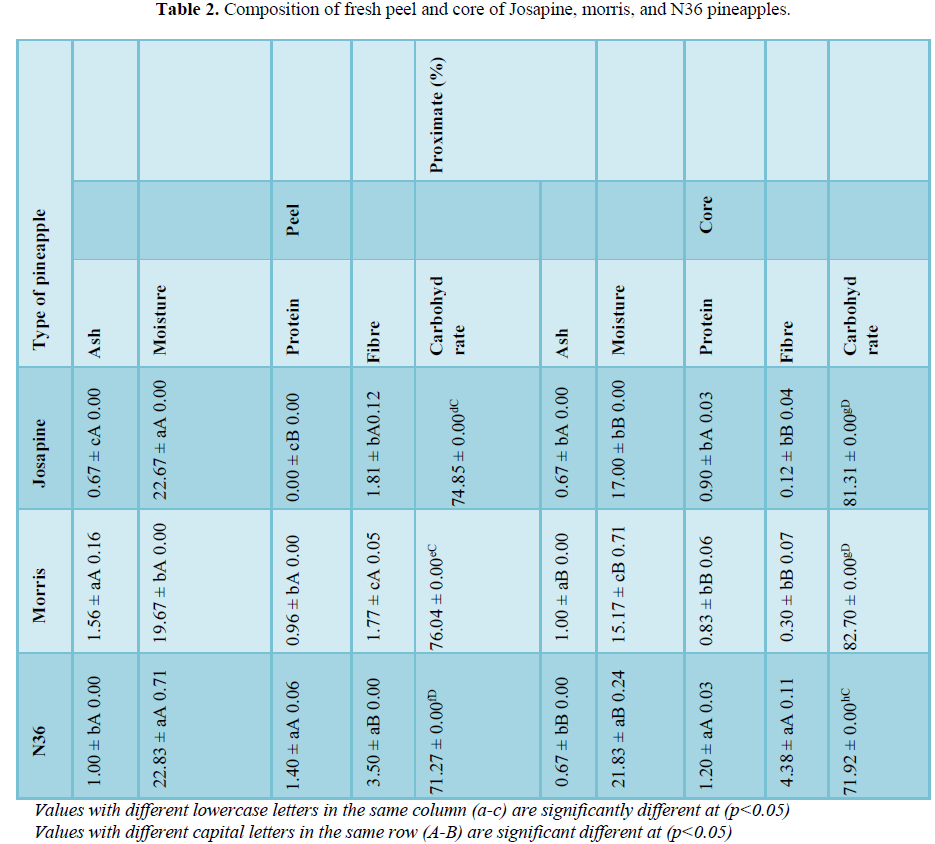
The fresh pineapple was selected at maturity stage four where the colors of the pineapple peel were half green and half yellow. Fresh pineapples were used to determine the ash, moisture, protein, fibre, and carbohydrate content in different parts and types of pineapple. The ash values in Table 2 varied from 0.67 mg/g to 1.56 mg/g for fresh peels and 0.67% to 1.00% for fresh core. Peels presented the largest ash values in Morris (1.56%) with statistically significant differences (p<0.05) among the other two types of pineapple. Other studies found 5.0% of ash in pineapple peel from Brazil higher than Morris peel as stated in Table 2.
The ash composition indicates the presence of mineral in the sample. Variation in mineral content in pineapple could depend on the type of soil where the plants were grown [12].

The ash composition indicates the presence of mineral in the sample. Variation in mineral content in pineapple could depend on the type of soil where the plants were grown [12].
Moisture analysis was analyzed to compare the amount of water present in peel and core in the fruits. From Table 2, N36 peel shows significantly higher moisture content compared to Morris, with the value of 22.83% and 19.67% respectively. This was similar to the fresh core of N36 which has the highest moisture content (21.83%) than the other types of pineapple. High amount of moisture content increases the time needed for the drying process. According to studies, higher moisture content in fruits indicates that the fruits are at the ripening stage. In general, the moisture content of pineapple ranges from 69 to 89.5% but it decreases during storage at room temperature and ripening period.
Pineapple is also known to have low protein content but it contains bromelain (glycoprotein) with protease activity commonly used in the food industry [13]. As presented in Table 2, N36 pineapple has a significantly higher amount of protein content in the peel (1.40%) and core (1.20%) compared to Josapine and Morris pineapple. According to Hassan et al. [12], sulphur containing amino acid methionine and cystine are present in lower amount at early stage, but increase during the ripening stage of the pineapple. Moreover, the protein content in pineapple is said to be related with the water used for irrigation and the fertilizer applied during the fruits was planted [12].
According to Table 2, the fibre content in the peel shows a significant difference (p<0.05) with the highest value found in the N36 peel (3.50%), while the lowest found in the Morris peel (1.77%). For the core sample, N36 (4.38%) shows a significantly higher fibre content as compared to Josapine (0.12%). The result indicates that the fresh peel has high fibre content compared to the fresh core. There is also a significant difference (p<0.05) between Josapine, Morris, and N36 for both peel and core sample which shows that N36 has higher amount of fibre content than the other samples. On the study about pineapple (Ananas comosus L. Merr.) peel fibre, it has been reported that the contents of dietary fibre in pineapple were 1.10% significantly lesser than in pineapples used in this study. This might due to the different varieties of pineapples used in both studies. According to Hassan et. al. [12], different types of pineapple will have a different composition.
Table 2 reveals the carbohydrate content in the peels and cores of the studied pineapples, ranging from 71.27% to 82.70%. Previously, it has been reported that a lesser amount of carbohydrate present in watermelon (32.16 g/100 g), pawpaw (37.49 g/100 g) and banana (43.30 g/100 g) as compared to in pineapples. This might be due to the differences in varieties of cultivars.
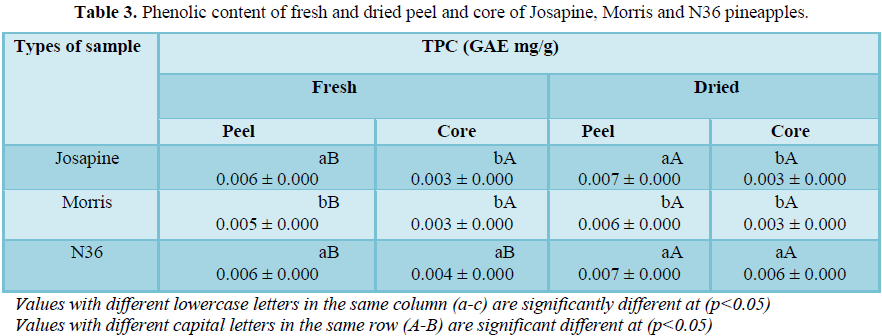
Total phenolic content of fresh and dried pineapple Table 3 shows the highest total phenolic content was in the dried peel (0.007 mg/g) as compared to the fresh peel (0.006 mg/g) with a significant difference (p<0.05). There is also a significant difference (p<0.05) between N36 dried core and fresh core with the value of 0.006 mg/g and 0.004 mg/g, respectively. Thus, this finding indicates that dried peel and core have a higher amount of TPC compared to the fresh peel and core. According to research, heat treatments help in increasing TPC value in a product, which the heat may provide energy to break the linkage between phenolics and the insoluble polyesters, thus potentially increase the polyphenol bio accessibility. Therefore, this might be the reason of the dried pineapple has a higher TPC value compared to fresh pineapple.
Particle size
Table 4 shows a significant difference (p<0.05) between the peel and core powders. Among the three types of pineapple powders, N36 for both peel and core show the largest particle size of 212.46 µm and 554.08 µm, respectively. This might be due to the high fibre content in the N36 peel and core powders compared to other samples. According to Muhamad et al. [14], reported that low fibre content in pineapples will contribute to the finer of powder particles.

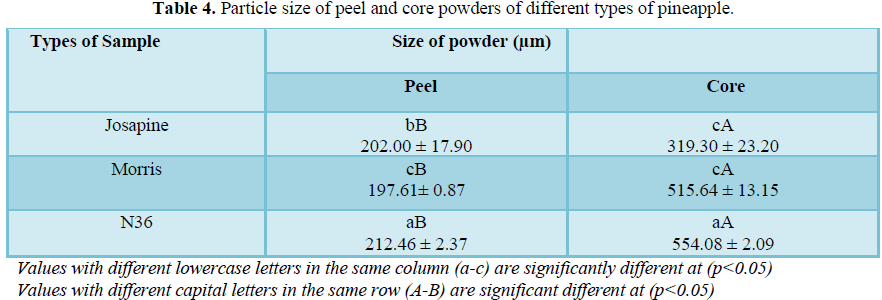
This can relate due to the low fibre content in Josapine and Morris pineapples peel and core.
Water holding capacity (WHC) and oil holding capacity (OHC)
From the results shown in Table 5, peel powders had a significantly (p<0.05) higher percentage of WHC as compared to core powders. Among the three types of pineapple powders, N36 shows higher percentage of WHC for both peel and core sample with the value of 7.53% and 6.34%, respectively. Based on a previous study conducted by Felli et al. [15], soy flour has the ability to hold water at 6.75%. This shows that the performance of WHC in pineapple peel powder is better than soy flour in binding water.
Drying at high temperature during processing may slightly affect the WHC in pineapple peel powder. Supported by another study on soy powders, they reported that heating at high temperature can help to unfold and denatured the protein structure and expose the side chains that can bind water and helps in increasing the WHC performance. In this study, it shows that the core powder has a low ability to absorb water due to the low protein content.
According to Table 5, N36 peel powders show a significantly higher ability to hold oil (4.7%) than Josapine peel (3.61%). The OHC of pineapple peel was about four times higher than wheat flour (1.12%). Previous research stated that the oil absorption ability of food material depends on the type and content of hydrophobic fraction present in the matrix structure. The presence of hydrophobic amino acid in the structure of a powder sample may be responsible for its capacity to absorb the oil. However, the ability of the N36 to hold the oil is low compared to dried durian seed gums (114.9 to 132.8 g oil/100 g gum). This might be due to less amount of protein content and zero amount of fat in the pineapple peel and core powders. Another study stated that the presence of fibre in pineapple peel and core also influenced the ability of WHC and OHC in the powders.
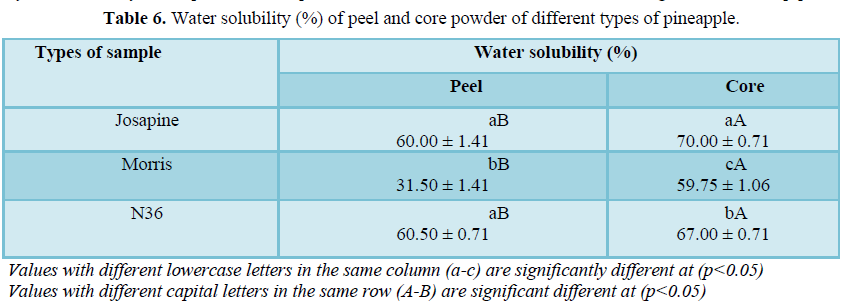
Water solubility
From Table 6, the solubility of N36 peel was significantly higher compared to Morris peel powder with the values of 60.5% and 31.5%, respectively. There was also a significant difference (p<0.05) between Josapine core (70%) and Morris core (59.75%). It indicates that the core powders have a higher ability to solubilize in water since they have high percentage of water solubility. All the pineapple powders have moisture content between 10-12% as mentioned in the method. Thus, this might be related to Samborska [16], who reported that low moisture content of powders will increase the water solubility index.

From Table 6, the solubility of N36 peel was significantly higher compared to Morris peel powder with the values of 60.5% and 31.5%, respectively. There was also a significant difference (p<0.05) between Josapine core (70%) and Morris core (59.75%). It indicates that the core powders have a higher ability to solubilize in water since they have high percentage of water solubility. All the pineapple powders have moisture content between 10-12% as mentioned in the method. Thus, this might be related to Samborska [16], who reported that low moisture content of powders will increase the water solubility index.

Moreover, it has been reported that the solubility of powder depends on the moisture content, in which high moisture content (15%-38%) will decrease the performance of solubility. The solubility of the powdered sample is essential as the rehydration process will occur when the dried pineapple powder comes into contact with water. A good rehydration process will wet the dried powders quickly and it will dissolve without floating in the solution [9].

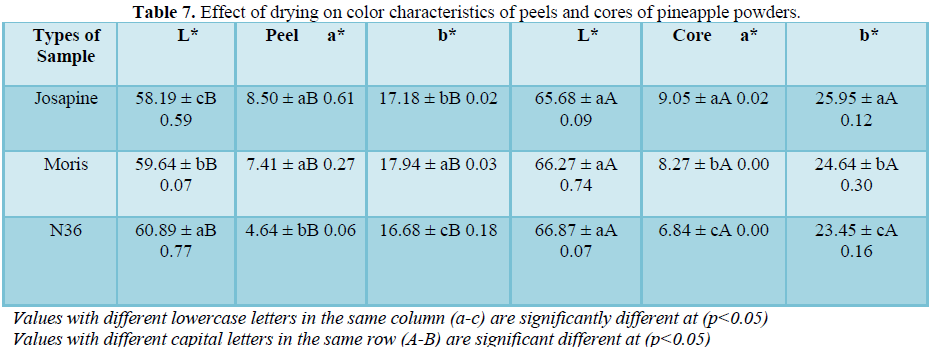
Color
According to Table 7, N36 peel powders has a significantly (p<0.05) lighter color (L=60.89) compared to Josapine peel powders (L=58.19). However, there are significant difference (p<0.05) between N36 core powders (L=66.87) and N36 peel powders (L=60.89) which N36 core powders show lighter color compare to N36 peel powders. The result was supported by Anim (2012), who as a pineapple trader, stated that N36 pineapple has its own characteristics which the color of the flesh and core are naturally pale yellow and more to white color even though it was ripened and reach to the maturity stage.
According to Table 7, N36 peel powders has a significantly (p<0.05) lighter color (L=60.89) compared to Josapine peel powders (L=58.19). However, there are significant difference (p<0.05) between N36 core powders (L=66.87) and N36 peel powders (L=60.89) which N36 core powders show lighter color compare to N36 peel powders. The result was supported by Anim (2012), who as a pineapple trader, stated that N36 pineapple has its own characteristics which the color of the flesh and core are naturally pale yellow and more to white color even though it was ripened and reach to the maturity stage.
In addition, all powders show positive a* value with a significant difference (p<0.05) between Josapine peel (a=8.50) and N36 peel (a=4.64), which indicates that Josapine peel powders have more redness color as compared to N36 peel powders. Similarly, Josapine core powders (a=9.05) has higher redness than N36 core (a=6.84). This finding indicates that the drying temperature at 90˚C gives affected the color of the powders after it was dried. The changes in color parameters during drying depended on the temperature of drying air in which high air-drying temperature will lead to more visible changes in color.
Positive b* value indicates the yellowness of the powders. From Table 7, Josapine core (b=25.95) shows significantly (p<0.05) a higher b* value than N36 core (b=23.45). It has been reported that the yellowness of the product strongly depends on drying temperature. N36 peel and core powders indicates that the powder has lighter color compare to Josapine and Morris powders thus, N36 powders may be applied as food additives without affecting the original color of the food.
CONCLUSION
In this study, N36 pineapple waste (peel and core) has proven that it contains significantly high in protein and fibre compared to Josapine and Morris pineapple. There is a significant difference (p<0.05) between N36 core and N36
peel on fibre content which shows higher amount in the core (4.38%) compared to the peel (3.50%).

TPC was found significantly higher in N36 dried core (0.006 GAE mg/g) than the fresh core. N36 peel also shows the higher ability to hold water (7.53%) and soluble in water (60.50%) among the three types of pineapple. In order to help the country in reducing waste, N36 pineapple powders dried at 90°C may be considered as a functional food ingredient as it consists of higher fibre content, better WHC and solubility with paler color than Josapine and Morris. The powder may have the potential to be applied as a food additive in beverage products as it does not affect the original color of the food [17-25].
REFERENCES
-
Malaysia Pineapple Industrial Board (2017) The pineapple statistical manual of 2018. Available online at: http://www.mpib.gov.my/en/publication/
-
Rathnakumar K, Anal AK, Lakshmi K (2017) Optimization of ultrasonic assisted extraction of bioactive components from different parts of pineapple waste. Int J Agric Environ Biotechnol.
-
Saraswaty V, Risdian C, Primadona I, Andriyani R, Andayani DGS, et al. (2017) Pineapple peel wastes as a potential source of antioxidant compounds. IOP Conference Series: Earth and Environmental Science, 60, 012013.
-
Sah BNP, Vasiljevic T, McKechnie S, Donkor ON (2016) Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT-Food Sci Technol 65: 978-986.
-
Maran PJ, Manikandan S, Nivetha VC, Dinesh R (2017) Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centred response surface design. Arab J Chem 10: S1145-S1157.
-
Naviglio D, Conti S, Ferrara L, Santini A (2010) Determination of moisture in powder and lyophilised saffron (Crocus sativus L.) by Karl Fischer Method~!2009-08-29~!2009-12-08~!2010-02-11~! Open Food Sci J 4(1): 1-6.
-
Maran et al. (2017). Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centered response surface design. Arab J Chem.
-
Traynham TL, Myers DJ, Carriquiry AL, Johnson LA (2007) Evaluation of water-holding capacity for wheat–soy flour blends. J Am Oil Chemists’ Soc 84(2): 151-155.
-
Suzihaque MUH, Hashib SA & Ibrahim UK (2015) Effect of inlet temperature on pineapple powder and banana milk powder. Procedia - Soc Behav Sci 195: 2829-2838.
-
Alfaro L, Chotiko A, Chouljenko A, Janes M, King JM, et al. (2018). Development of water-soluble chitosan powder and its antimicrobial effect against inoculated Listeria innocua NRRL B-33016 on shrimp. Food Control 85: 453-458.
-
Thuwapanichayanan R, Phowong C, Jaisut D, Štencl J (2014) Effects of pre-treatments and drying temperatures on drying characteristics, antioxidant properties and color of ginger slice. Acta Univ. Agric. et Silvic. Mendelianae Brun 62(5): 1125-1134.
-
Hassan A, Wills R, Atan R, Othman Z, Fatt LP, et al. (n.d.). Blackheart disorder in fresh pineapple. (1): 7.
-
Rowan AD (2013). Handbook of proteolytic enzymes, pp: 1874-1875.
-
Muhamad II, Katan NS, Shaharuddin S, Zaidel DNA (2015) Effects of preparation methods on the properties of pineapple fibres.
-
Felli R, Yang TA, Wan Abdullah WN & Shahjalal (2018). Effects of incorporation of jackfruit rind powder on chemical and functional properties of bread. Trop Life Sci Res 29(1): 113-126.
-
Samborska K (2013) Physicochemical properties of spray dried honey preparations.
-
Burgain J, Petit J, Scher J, Rasch R, Bhandari B, et al. (2017) Surface chemistry and microscopy of food powders. Prog Surface Sci 92(4): 409-429.
-
Salim S (2016) Transforming the Malaysia pineapple industry. 5th International Plantation Industry Conference and Exhibition.
-
Kaneshiro WS, Burger M, Vine BG, de Silva AS, Alvarez AM (2008) Characterization of Erwinia chrysanthemi from a bacterial heart rot of pineapple outbreak in Hawaii. Plant Disease 92(10): 1444-1450.
-
Mohammad S, Ghazali KH, Zan NC (2011) Article of classification of fresh N36 pineapple crop using image processing tehnique.
-
Murrieta-Pazos I, Gaiani C, Galet L, Calvet R, Cuq B, et al. (2012) Food powders: Surface and form characterization revisited. J Food Eng 112(1-2): 1-21.
-
Rawat S (2015) Food spoilage: Microorganisms and their prevention.
-
Rosdan M (2012) Physicochemical properties of josapine pineapple (Ananas comosus), 10.
-
Shamsudin R, Daud WRW, Takriff MS, Hassan O (2007) Physicochemical properties of the Josapine variety of pineapple fruits. Int J Food Eng 3(5).
-
Thalip AA, Tong PS, Ng C (2015) The MD2 “Super Sweet” Pineapple (Ananas comosus). J Agric Sci.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)



