4501
Views & Citations3501
Likes & Shares
Since late 2019 a pandemic infection associated with a coronavirus first identified in Wuhan, China, has ravaged global societies. Subsequently some consensus seems to be reached that recovery from socioeconomic and medical morass which had developed would involve the rapid development and implementation of the socioeconomic universal vaccination program. Without precedent, this took place in the absence of consensus on the origin and epidemiology of infection; without detailed knowledge and investigation into the nature of natural host resistance to the pathogen; by “speed-tracking” novel vaccine designs for clinical use, without refined knowledge of possible short-term and longer-term implications of vaccine administration; and perhaps most reprehensible, by essentially mandating vaccine uptake by imposing draconian restrictions on non-vaccinated individuals on a world-wide basis. The medical/bioethical/sociological and philosophical literature has been inundated with contradictory interpretations of both the justification for, and evidence of, the usefulness of these approaches. In the following review we have highlighted what we believe to have been the major fallacies in many of the arguments raised in support of the current dogmas, and identify some key points which suggest a way forward to a more rational approach to tackling the next pandemic when, rather than if, it arrives.
Keywords: SARS-CoV-2, Vaccination, Mucosal immunity, Innate immunity, Virus originINTRODUCTION
Current approaches to vaccination assume without proof that:
- Following immunization most individuals are at similar risk of disease
- Relevant host resistant mechanisms (innate and/or acquired immunity) have been identified, and can be targeted appropriately to boost resistance
- After vaccination, individuals within a population react immunologically in the same way in terms of protective antibodies and/or cell-mediated reactivity (one size fits all) with equivalent and minimal side effects
- Vaccination dose and frequency of administration is invariant in the population
These assumptions have been applied to widespread delivery of vaccines for a number of infectious diseases, with effective control for many of those. However, a clear weakness of this approach is that it discounts the growing evidence for individual variability in risk, in immune responsiveness, and in response to different doses of vaccine, and the growing evidence that altering the route of delivery (induction of systemic immunity versus local intra-nasal mucosal immunity) can introduce further important variables for clinical efficacy. These issues have come to the fore while tailoring individual approaches to cancer therapy, but are now becoming more concerning as we come to grips with novel emerging infections, as has been highlighted during the recent SARS-COV-2 pandemic.
We discuss below in more detail how innate immunity is likely an important component of viral resistance, and that viral responses to the innate immune system can help explain mutagenesis of SARS-CoV2 virus in the host. We also suggest that the inattention to mucosal immunity as a major component of respiratory virus infection, with instead a focus on induction of systemic immunity for SARS-CoV2 through conventional intramuscular injection, is a major error, and may have led to a gross misrepresentation of current vaccine efficacy and utility.
ORIGIN AND EPIDEMIOLOGY OF SARS-COV-2 (AND OTHER EMERGENT) INFECTIONS
For the past 30 months, the world has been ravaged by a pandemic caused by SARS-CoV-2, identified initially in late 2019 in Wuhan, China. By mid-2020 there had arisen global consensus that the way forward from the hysteria and draconian measures which were implemented (societal “lock-downs” in the face of the risk of overwhelming and/or collapse of medical care) was through rapid development and implementation of a universal vaccination program. However, unlike past precedents, this took place in the absence of consensus on the origin and epidemiology of infection; without detailed knowledge and investigation into the nature of the mechanism of host resistance to the pathogen; and by “speed-tracking” novel vaccine designs to clinical use, with minimal to no large-scale clinical trials, and in the absence of any detailed knowledge of possible short-term and longer-term implications of this vaccine’s administration.
We have suggested in many publications [1-4] that there is a compelling argument to be made that the origin of the current SARS-CoV-2 outbreak, and possibly of other emergent novel infections, is attributable to an “in-fall” of infectious particles from the stratosphere [5]. We can highlight many of the arguments supporting this notion with specific attention to a series of so-called COVID-19 mystery community transmissions which occurred in a defined arc across the inner Western and outer Northern suburbs of Melbourne, Victoria in May-June 2020 [6] and in May-June 2021 [7]. These could not be traced to any direct infected contacts nor could they be directly genomically linked to any known infection clusters (e.g., among infected international travelers in hotel quarantine or in aged care and nursing homes). As a consequence of the government response to this perceived emergency, large numbers of PCR COVID-19 tests on oronasal swabs were conducted (> 30, 000 per day at peak) with all positive cases quarantined at home. Contact tracing was conducted by teams of experienced tracers, yet despite a total clamp on individual mobility, new mystery outbreaks continued to occur in the 2020 and 2021 epidemics in Victoria. Detailed analysis in 2020 showed that more than 25-30% of all tracked Covid-19 variants were genomically-unlinked “mystery infections” without a known infection contact [6] as shown in Figures 1 and 2.
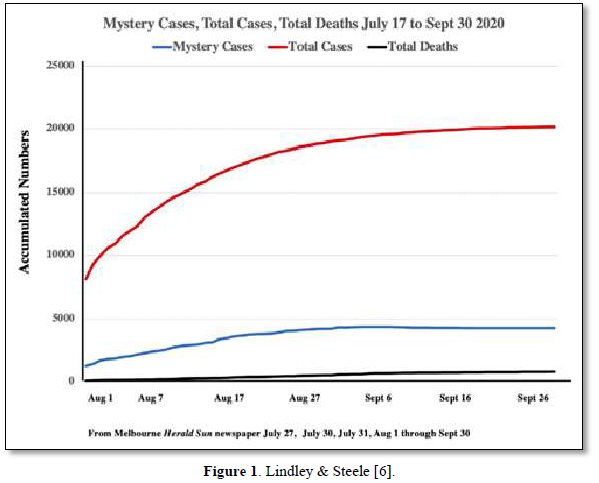
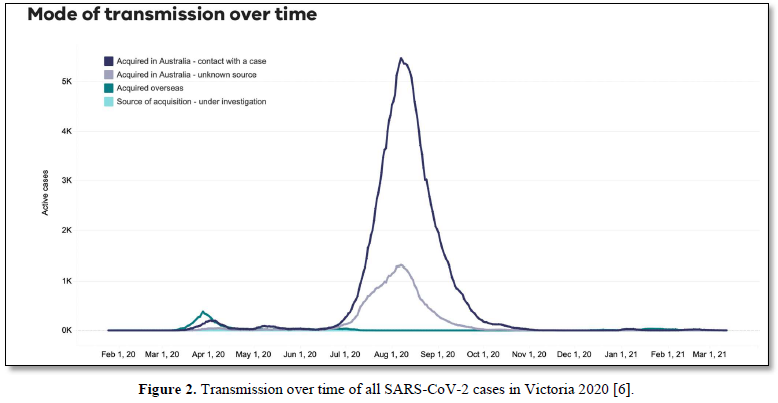
In the smaller 2021 epidemics many of the viral variants of concern (PANGO classification) were clearly mature human-passaged virions, many of which were also identified in the large Indian April-May 2021 epidemic. The public domain data in Victoria support the hypothesis that a heterogeneous set of these 2021 “Indian” variants delivered into a tropospheric aerosol plume [7], were transported by prevailing tropospheric global wind systems via the Indian Ocean and Southern Ocean (Roaring Forties West to East on the 40° S Latitude line) to Victoria, Australia. Indeed, as we have and others have argued before, there is precedent for such global wind transportations in the history of past Influenza virus pandemics in the last 100 years and the present observations relating to COVID-19 events in Australia are likely but one of many such incidents [8,9]. These confirmed unlinked “mystery case” infections in Victoria, Australia in 2020 and 2021 are interpreted as a clear signature of viral in-fall from the troposphere leading to a virus contaminated environment. This leads to the ignition of respiratory tract COVID-19 infections in unsuspecting victims who introduce the infection by touching their nose and mouth with their contaminated fingers. As we have established from public domain data, the major viral amplifications occur in immune defenseless elderly subjects with co-morbidities who spread the viral particles via aerosols to contaminate their own closed environment, with trillions of virions facilitating further spread across multiple aged care facilities [6].
The discussion that follows provides a more detailed summary consensus view of the current knowledge regarding mammalian host responses to infections, and in turn contrasts that evidence with the approach used in vaccination against SARS-CoV-2.
INNATE IMMUNITY TO PATHOGENS
Mammalian immunity in general, including for SARS-CoV-2, has both an innate and adaptive arm. Innate immunity acts rapidly to control viral replication in infected healthy subjects through type I and type III interferon inducible anti-viral immunity, primarily deaminases which attack DNA or RNA of invading viruses by extensively mutating their genomes with C-to-U (T) and A-to-I(G) mutations, crippling its replicative efficiency [10,11]. Elderly patients lacking this rapid innate response are at very high risk for severe outcomes following SARS-CoV-2 infection, including increased morbidity and mortality [12]. Type I and III interferon inducible genes include APOBEC and ADAR, which as described in Figure 1 and elsewhere [5,6] can also play a role in “haplotype switching” of SARS-CoV-2-expressed genes, leading in turn to the diversification of the virus genetic pattern seen in some subjects, but not in those with impaired innate immunity. Figure 3 shows the causal links between deaminase mutagenic activity, SARS-Cov-2 infection, and the role of the host innate and adaptive immune response, and the subsequent possible accumulation of collateral cell damage [13].
Innate immunity can be “trained” to provide improved immunity on reinfection with the same, and possible even other, pathogens [14], helping explain why infant mortality, and even adult mortality, is less in Bacillus Calmette-Guerin (BCG) vaccinated cohorts (BCG admixed with adjuvants is an excellent inducer of innate immune responses) than in non-vaccinated cohorts from the same population [15]. Even live-attenuated vaccines for tuberculosis, measles, and polio can “train” the innate immune system, likely involving histone modifications and epigenetic reprogramming of monocytes to develop an inflammatory phenotype, and improved broad resistance to other infectious diseases, of which SARS-CoV-2-2 infection may be an example [15,16]. Comparisons of innate immune responses to Influenza and SARS-CoV-2 in nasal washes from infected adults suggested there was some difference in innate responses following SARS-CoV-2 infection, with decreased IFN-associated transcripts compared with influenza-infected individuals [17], Importantly, comparison of subjects post natural infection vs SARS-CoV-2 vaccination (SARS-CoV-2 BNT162b2 mRNA) showed that only in naturally infected patients, and not vaccinated individuals, was exposure associated with heightened clinically significant mucosal immunity [18].
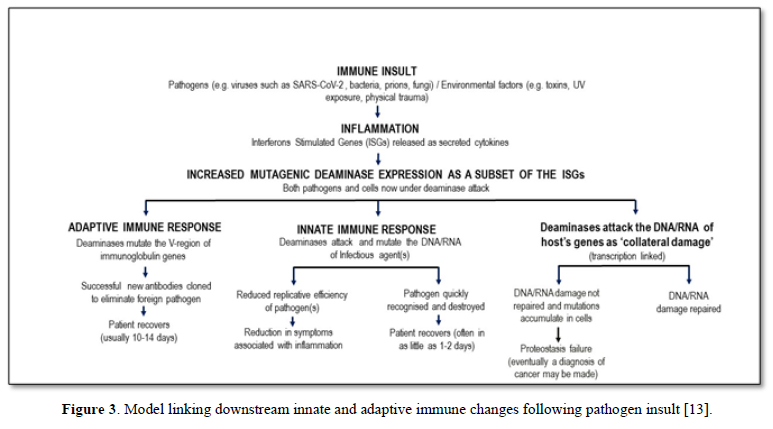
Indeed, the value of any (vaccine) strategy deliberately to boost innate immunity was never at the forefront of any early attempts to control this pandemic. It may be that the adjuvant used in vaccines to date does indeed augment innate immunity, and this may (not any antigen-specific moiety in the vaccine) even be responsible for any observed vaccine protection-this too has never been investigated. Instead all effort has been made to develop vaccines which are then tested, using serum IgG as a marker, for their efficacy in inducing a response to the injected material-this is simply a test of the ability of the host to respond immunologically, not of the clinical utility of the vaccine, nor of the value of serum IgG to be a surrogate marker for such utility (Figure 3).
ADAPTIVE (ACQUIRED) T AND B LYMPHOCYTE MEDIATED IMMUNITY
Unlike the innate immune response, acquired immunity takes some 10-14 days post pathogen exposure to become active, but in general shows much greater diversity for pathogen recognition and is primarily responsible for immunologic memory. Deliberate controlled priming by vaccination exposure to pathogen moieties had been claimed to generate great successes in global infectious disease control [19,20]. Not surprisingly then considerable effort was directed to this aim for SARS-CoV-2, focusing on immunity to the receptor-binding domain (RBD) of the spike (S) protein of SARS-CoV-2 which controls viral entry into cells. It has become very apparent that there is considerable heterogeneity in epitope recognition within different individuals/populations, and this has likely contributed to variable efficacy in vaccine utility [13]. However, what has remained unexplored is the relative importance of mucosal vs systemic immunity in natural or vaccine-induced protection, with most studies focusing on the (more easily measured/quantitated) systemic IgG response. It may indeed be that serum IgG levels can serve as a surrogate marker of activation, within the respiratory tract, of B/T cells activated for IgA production, but that has never been tested. We have discussed this issue in depth elsewhere [13] and highlight more issues in the following.
MUCOSAL IMMUNITY AND RESISTANCE TO/RECOVERY FROM SARS-COV-2
It has been known for many years, that the best form of protective immunity for pathogens invading by the nasal or oral route are local secretory IgA responses [21]. Recent analyses on SARS-CoV-2 reinfections and transmissions in vaccinated individuals, and studies assessing immunization against influenza and SARS-CoV-2 are consistent with this concept [22,23].
Measurement of humoral responses to SARS-CoV-2 and analysis of specific neutralizing antibodies in the serum, saliva, and bronchoalveolar fluid of 159 patients following natural infection with SARS-CoV-showed that early viral specific humoral responses were dominated by IgA antibodies with peaks during the third week post-infection, with IgA contributing to virus neutralization to a greater extent than IgG or IgM antibodies [24]. Anti-viral IgA serum concentrations decreased after 1 month but neutralizing IgA remained detectable in saliva for up to 10 weeks. An independent study also concluded that while serum neutralization and effector functions correlated with systemic SARS-CoV-2-specific IgG responses, mucosal neutralization was associated with nasal SARS-CoV-2- IgA, along with less severe disease [25]. Animal (mice) studies have shown that unlike a systemic (im) vaccination protocol, only an intranasal dose of adenovirus vaccine induced high levels of neutralizing antibodies, enhanced both systemic and mucosal IgA and T cell responses, and prevented SARS-CoV-2 infection in both the upper and lower respiratory tracts [26]. The validity of mucosal immunization for protection was confirmed in an independent vaccine study in macaques [27]. Multiple other studies have reached similar conclusions regarding the importance of induction of mucosal immunity for protection against pathogens targeting the respiratory system [28-30], results consistent with evidence for an increased susceptibility to SARS-CoV-2 in IgA deficient subjects [31].
RISK OF SARS-COV-2 VACCINES
In the early period following introduction of novel SARS-CoV-2 vaccines, it gradually became apparent that there was a significant unanticipated adverse effect (venous thromboembolism, VITT) described in a subpopulation of subjects [32], leading eventually to reluctance in many countries to continue use of this particular vaccine. Other groups have focused on the theoretical risk associated with other novel vaccines (especially mRNA vaccines), arguing that their “rush into service” has ignored potential concerns with their use, particularly the concern regarding induction of autoimmune reactivity [33-35]. Indeed, a comparison of immunogenic epitopes in SARS-CoV-2-S proteins, and other SARS-CoV-2 proteins with human protein concluded that only one immunogenic epitope in SARS-CoV-2 had no homology to human proteins, and that many of the overlaps with human proteins could theoretically help explain some of the symptoms associated with the pathogenesis of SARS-CoV-2 [36]. A summary of reported adverse events can be found at the CDC website [37], though it should be noted that given the mandated implementation of government vaccination policies world-wide, all adverse events are not necessarily captured simply by medical events [38].
CONCLUSIONS
There is evidence that we are now approaching an entrenchment phase in the response to the SARS-CoV-2 pandemic, with evolution of less virulent viral variants, better protection of vulnerable population cohorts (especially the elderly), and adherence to better public health measures all combining to improve the overall outlook. At all levels, politically, sociologically, ethically, scientifically and medically, there have been instances of major mismanagement and misunderstanding, coupled with gross errors of judgement, which have clearly cost lives [38]. As discussed above there is still concern that we have failed to recognize the importance of implementation of basic science knowledge, both new research and understanding old observations, which even now would likely improve the future course of the disease. It is clear too that we need to remain vigilant, having implemented so many previously untried and untested therapies, for the appearance of new signs and symptoms in treated patients which are early indications of adverse events. As stressed before, we would argue also that critical evaluation of evidence for a “viral infall” from the stratosphere as a source of this (and previous/future epidemics) may highlight ways we can begin to develop “early warning systems” [39].
- Hoyle F, Wickramasinghe NC (1993) Our Place in the Cosmos: The Unfinished Revolution. J.M. Dent Ltd, Lond
- Steele EJ, Al-Mufti S, Augustyn KA, Chandrajith R, Coghlan JP, et al. (2018) Cause of Cambrian Explosion - Terrestrial or Cosmic? Prog Biophys Mol Biol 136: 3-23.
- Wickramasinghe NC, Steele EJ, Gorczynski RM, Temple R, Tokoro G, et al. (2020) Growing Evidence against Global Infection-Driven by Person-to-Person Transfer of COVID-19. Virol Curr Res 4: 1.
- Wickramasinghe NC, Steele EJ, Wallis DH, Wainwright M, Tokoro G, et al. (2021) Footprints of Past Pandemics in the Human Genome. Virol Curr Res 5: 4.
- Steele EJ, Gorczynski RM, Lindley RA, Carnegie PR, Rebhran H, et al. (2022) Overview SARS-CoV-2 Pandemic as January-February 2022: Likely Cometary Origin, Global Spread, Prospects for Future Vaccine Efficacy. Infect Dis Ther 3(1): 1-16.
- Lindley RA, Steele EJ (2021) Analysis of SARS-CoV-2 haplotypes and genomic sequences during 2020 in Victoria, Australia, in the context of putative deficits in innate immune deaminase anti-viral responses. Scand J Immunol 94: e13100.
- Steele EJ, Gorczynski RM, Carnegie P, Tokoro G, Wallis DH, et al. (2021) COVID-19 Sudden Outbreak of Mystery Case Transmissions in Victoria, Australia, May-June 2021: Strong Evidence of Tropospheric Transport of Human Passaged Infective Virions from the Indian Epidemic. Infect Dis Ther 2(1): 1-28.
- Hoyle F, Wickramasinghe NC (1979) Diseases from Space. J.M. Dent Ltd, London.
- Hammond GW, Raddatz RL, Gelskey DE (1989) Impact of atmospheric dispersion and transport of viral aerosols on the epidemiology of Influenza. Rev Infect Dis 11: 494-497.
- Samuel CE (2011) Adenosine deaminases acting on RNA (ADARs) are both antiviral and proviral. Virology 411: 180-193.
- Steele EJ, Lindley RA (2020) Analysis of APOBEC and ADAR deaminase-driven Riboswitch Haplotypes in SARS-CoV-2 RNA strain variants and the implications for vaccine design. Res Rep 4: e1-e146.
- Lucas C, Wong P, Klein J, Castro TBR, Silva J, et al. (2020) Longitudinal analyses reveal immunological misfiring in severe SARS-CoV-2. Nature 584: 463-469.
- Gorczynski RM, Lindley RA, Steele EJ, Wickramasinghe CNC (2021) Nature of Acquired Immune Responses, Epitope Specificity and Resultant Protection from SARS-CoV-2. J Pers Med 11: 1253.
- Netea MG (2013) Training innate immunity: The changing concept of immunological memory in innate host defense. Eur J Clin Investig 43: 881-884.
- Parmar K, Siddiqui A, Nugent K (2021) Bacillus Calmette-Guerin Vaccine and Nonspecific Immunity. Am J Med Sci 361: 683-689.
- Chumakov K, Avidan MS, Benn CS, Bertozzi SM, Blatt L, et al. (2021) Old vaccines for new infections: Exploiting innate immunity to control COVID-19 and prevent future pandemics. Proc Natl Acad Sci USA 118(21): e2101718118.
- Gao KM, Derr AG, Guo Z, Nundel K, Marshak-Rothstein A, et al. (2021) Human nasal wash RNA-seq reveals distinct cell-specific innate immune responses between influenza and SARS-CoV-2. JCI Insight 6(22): e152288.
- Ivanova EN, Devlin JC, Buus TB, Koide A, Cornelius A, et al. (2021) Discrete immune response signature to SARS-CoV-2 mRNA vaccination versus infection. medRxiv 2021: 255677.
- Wilyman J (2015) A critical analysis of the Australian government’s rationale for its vaccination policy, Doctor of Philosophy thesis, School of Humanities and Social Inquiry, University of Wollongong, 2015. Available online at: https://ro.uow.edu.au/theses/4541
- Steele EJ (2022) Wilyman report on vaccines: How do we handle the next pandemic, small, large or predicted. Submitted, Open Research, Infectious Disease and Therapeutics.
- Wilkie BN (1982) Respiratory tract immune response to microbial pathogens. J Am Vet Med Assoc 181: 1074-1079.
- Bleier BS, Ramanathan M, Lane AP (2021) COVID-19 Vaccines May Not Prevent Nasal SARS-CoV-2 Infection and Asymptomatic Transmission. Otolaryngol Head Neck Surg 164: 305-307.
- Fröberg J, Diavatopoulos DA (2021) Mucosal immunity to severe acute respiratory syndrome coronavirus 2 infection. Curr Opin Infect Dis 34: 181-186.
- Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, et al. (2021) IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med 13(577): eabd2223.
- Butler SE, Crowley AR, Natarajan H, Xu S, Weiner JA, et al. (2021) Distinct Features and Functions of Systemic and Mucosal Humoral Immunity Among SARS-CoV-2 Convalescent Individuals. Front Immunol 11: 618685.
- Hassan AO, Kafai NM, Dmitriev IP, Fox JM, Smith BK, et al. (2020) A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 183: 169-184.
- Sui Y, Li J, Zhang R, Prabhu SK, Andersen H, et al. (2021) Protection against SARS-CoV-2 infection by a mucosal vaccine in rhesus macaques. JCI Insight 6(10): e148494.
- Xiao Y, Lidsky PV, Shirogane Y, Aviner R, Wu CT, et al. (2021) A defective viral genome strategy elicits broad protective immunity against respiratory viruses. Cell 184: 6037-6051.
- Oh JE, Song E, Moriyama M, Wong P, Zhang S, et al. (2021) Intranasal priming induces local lung-resident B cell populations that secrete protective mucosal antiviral IgA. Science Immunology 6: eabj5129.
- Afkhami S, D’Agostino MR, Zhang A, Stacey HD, Marzok A, et al. (2022) Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell 185: 896-915.
- Quinti I, Mortari EP, Salinas AF, Milito C, Carsetti R (2021) IgA Antibodies and IgA Deficiency in SARS-CoV-2 Infection. Front Cell Infect Microbiol 11: 655896.
- Cines DB, Bussel JB (2021) SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N Engl J Med 384: 2254-2256.
- Lyons-Weiler J (2020) Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. J Transl Autoimmun 3: 100051.
- Dotan A, Muller S, Kanduc D, David P, Halpert G, et al. (2021) The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev 20: 102792.
- Bozkurt B, Kamat I, Hotez PJ (2021) Myocarditis With COVID-19 mRNA Vaccines. Circulation 144: 471-484.
- Lu L, Xiong W, Mu J, Zhang Q, Zhang H, et al. (2021) The potential neurological effect of the COVID-19 vaccines: A review. Acta Neurol Scand 144: 3-1
- CDC Government (2019) Selected Adverse Events Reported after COVID-19 Vaccination. Available online at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
- Bardosh K, de Figueiredo A, Gur-Arie R, Jamrozik E, Doidge J, et al. (2022) The unintended consequences of COVID-19 vaccine policy: Why mandates, passports and restrictions may cause more harm than good. BMJ Global Health 7: e008684.
- Qu J, Wickramasinghe NC (2020) The world should establish an early warning system for new viral infectious diseases by space-weather monitoring. MedComm 1(3): 423-426.





