3563
Views & Citations2563
Likes & Shares
Antibiotics are the most commonly used biocides in nano-fibers to provide antibacterial characteristics. Nano-fibers have been used to encapsulate antibiotics such as tetracycline hydrochloride, ciprofloxacin, levofloxacin, and Moxifloxacin [7]. Flouro-quinolones are a promising class of antibiotics used to treat external ocular diseases topically. Ofloxacin (OFL) is a flouro-quinolone antibiotic that is effective against Gram-positive and Gram-negative bacteria [8]. Electro-spinning is a unique, simple, efficient and versatile method to prepare polymeric fibers with diameter in the range of nanometer to submicron size using electrically charged jet of polymer solutions or melts [9-13]. High surface area that alters drug [13] pore size within the nano scale [14] simple drug entrapment, [15] three-dimensional structure [15]and short diffusion passage length [16] are some of the functional properties of polymeric nano-fibers produced by electro-spinning. Electro-spun fibers are ideal candidates as drug delivery systems for ophthalmic [17] periodontal [11] transdermal [18] topical (as a wound dressing) [19] and colon targeted delivery [20] due to their unique features. Electro-spun fibers can help minimize the drug's lowest required dosage and undesired side effects, resulting in less systemic absorption [21]. According to one of the patented studies, the fibers can be put on the conjunctiva or sclera of the eye and will progressively erode away, releasing the therapeutic medicament for ocular administration [22].
Collagen is a natural extracellular matrix (ECM) component of many tissues, such as skin, bone, tendon, ligament, and other connective tissues. Among the isotypes of collagen, type I is the principal structural and functional protein and is composed of two α chains and one β chain. The underlying chains that form these natural polymers are arranged into a repeating pattern that forms a coiled structure. The specific complement of subunits present within the fibril defines the material properties of the natural polymer [23]. The mechanical strength of electro-spun collagen nano-fibers can be enhanced with the addition of PLGA materials [24]. Polylactide–polyglycolide (PLGA) is in the class of synthesized biodegradable and biocompatible copolymers, from which resorbable sutures, resorbable surgical clips, and controlled release implants are made. PLGA also falls within the class of copolymers that have been used for implantable and injectable controlled-release, drug delivery systems [25]. These copolymers, with a history of safe use, have been approved for human use. After being introduced into the body, PLGA material induces only a minimal inflammatory response and biodegrades through the hydrolysis of its ester linkages to yield biocompatible lactic and glycolic acids [26].
Antibiotic-loaded scaffolds [27] made out of biodegradable polymeric membranes have advantages in several ways. First, biodegradable membranes provide bactericidal concentrations of antibiotics for the prolonged time needed to completely treat the particular infection. Second, variable biodegradability from weeks to months may allow many types of infections to be treated. Third, the biodegradable membranes dissolve, thus there is no need for removal; and lastly, because the biodegradable membranes dissolve slowly, the soft tissue or bone defect will slowly fill with tissue, so there is no need for reconstruction.
In the present study, a sandwich-structured nanofibrous matrix was produced via electro-spinning to develop biodegradable and biomimetic drug-eluting dressings. The blended solutions were electro-spun into sandwich structured membranes, with PLGA/collagen for the surface layers and PLGA/OFL for the core layer.
MATERIALS AND METHODS
Materials
The poly (D, L)- lactic-co-glycolic acid (PLGA) used was commercially available material and had a ratio of 50:50 and an intrinsic viscosity of 0.4, collagen type I, drug commercial grade ofloxacin and 1,1,1,3,3,3-hexafluoro-2- propanol (HFIP) were purchased from Sigma–Aldrich (Saint Louis, MO, USA).
Electrospinning
The electro-spinning setup utilized in this study consisted of a syringe and needle (the internal diameter 0.42 mm), a ground electrode, an aluminum sheet, and a high voltage supply (Liu et al., 2010). The needle was connected to the high voltage supply, which could generate positive DC voltages and current up to 35 kV and 4.16 mA/125W, respectively, PLGA/collagen (280 mg/140 mg, w/w) and PLGA/ofloxacin (240 mg/35 mg w/w) were dissolved in 1 ml of HFIP each. For the electro-spinning of sandwich-structured nano-fibers, a predetermined volume of PLGA/collagen solution was delivered and electro-spun by a syringe pump with a volumetric flow rate of 3.6 ml/h, followed by the electro-spinning of PLGA/ofloxacin solution with a volumetric flow rate of 1.2 ml/h as the core layer, and finally by the spinning of another PLGA/collagen layer. The distance between the needle tip and the ground electrode was 9 cm, and the positive voltage applied to polymer solutions was 17 kV. 1 ml of PLGA/collagen solution, 4 ml of PLGA/OFL solution, and 1 ml of PLGA/collagen solution produced a membrane of 0.107 mm in thickness shown in Table 1, the experiment was carried out at room temperature. After electro-spinning, the specimen was placed in a vacuum oven at 40◦C for 72 h for the solvents to evaporate.
CHARACTERIZATION OF NANO-FIBERS
Fourier Transform-Infrared Analysis
The FT-IR is used to characterize and to know the chemical interactions between ofloxacin, PLGA and other Excipients. The spectra of ofloxacin, PLGA, collagen was recorded using KBr pellet method in an FT-IR spectrophotometer (JASCO 4100 type A) with the range of 4000cm-1 to 400cm-1.
Surface Analysis
The surface morphology of the Bio composite scaffold was studied using Scanning Electron Microscopy (SEM).
Scanning Electron Microscopy
The morphology of the fibers was evaluated using scanning electron microscope (SEM). A Vega 3 Tescan was used to characterize the fibrous morphology of the electro-spun scaffolds. The samples imaged at an accelerating voltage of 20 kV. The fiber diameter distribution and angular deviation of the fibers from the direction of collector rotation in scaffolds were obtained by analyzing the SEM micrographs by image-analysis software (Image-pro plus, Media Cybernetics Co.).
X-Ray Diffraction Analysis
X-ray diffraction analysis (XRD) was performed with a PAN analytical Xpert Pro X-Ray Diffractometer using Ni filtered Cu Kα radiation. The sample for evaluation was taken on the glass slide and placed on the X- Ray Diffractometry. The scanning rate was continued over a 2Ɵ range of 10 to 90°.
In-Vitro Release Studies
Ofloxacin loaded fibers were cut in 1cm2 (1x1cm) pieces. The 1cm2 fibers specimens were weighed by analytical balance in order to obtain theoretical drug content, regarding polymer to-drug ratio. Then 1cm2 fiber specimens were immersed in release medium, 15 mL of buffer saline (pH 7.4) in glass flacons. The glass flacons were placed in a thermo-stated horizontal shaker and the drug release studies were performed at 50 rpm at 35±0.5o C. Samples of 1.0mL were withdrawn and replaced with the equal volume of fresh medium at predetermined time intervals. The amount of OFL released was analyzed by a UV spectrophotometer at the wavelength of 287 nm.
IN VITRO ANTIBACTERIAL ACTIVITY
Preparation of Inoculum
Stock cultures were maintained at 4°c on slant of nutrient agar. Active cultures for experiments were prepared by transferring a loop full of cells from the stock cultures to test tubes of nutrient broth for bacteria that were incubated at 24hrs at 37ºC. The assay was performed by agar well diffusion method.
Antibacterial Activity
Antibacterial activity of sample was determined by well diffusion method on Muller Hinton agar (MHA) medium. For optimized formulation 1 cm2 (1x1cm) fiber mats were cut and weighed. Average OFL content was calculated considering polymer: drug ratio. Before each in vitro antibacterial test, the bacterial strain was first grown overnight on Muller-Hinton Agar medium (MHA) at 37°C. After overnight culture at 37°C bacterial suspensions were diluted 1/10 and inoculated onto an MHA plate. Then 1cm2 fiber samples were placed in contact with an agar plate inoculated with bacteria and inhibition diameters, corresponding to the bacteria-free zone, were measured (in mm) after 24 h incubation at 35°C. All microbiological studies were performed in an aseptic area in a laminar flow hood.
IN-VITRO CYTOTOXICITY STUDY
Determination of Mitochondrial Synthesis by Mtt Assay
- The cell culture was centrifuged, and the cell count was adjusted to 1.0x105cells/ml using DMEM medium containing 10% FBS.
- To each well of a 96 well flat bottom micro titer plate, 100µl of the diluted cell suspension (approximately-10,000 cells/well) was added.
- After 24 h, when the cell population was found adequate, the cells were centrifuged, and the pellets were suspended with 100µl of different test sample concentrations prepared in maintenance media. The plates were then incubated at 37°C for 48 h in 5%CO2 atmosphere, and microscopic examination was carried out and observations recorded every 24 h.
- After 48 h, 20 µl of MTT (2mg/ml) in MEM-PR (MEM without phenol red) was added.
- The plates were gently shaken and incubated for 2 h at 37°C in 5% CO2 atmosphere.
- The 100µl of DMSO was added and the plates were gently shaken to solubilize the formed formazan.
- The absorbance was measured using a micro plate reader at a wavelength of 540nm.
In Vitro Proliferation Assay
Exponentially growing HaCaT cells were washed and seeded at 17000 cells/well (in 200 μl of growth medium) in 96 well micro plates. After 24 h incubation, a partial monolayer was formed then the media was removed and 200 μl of the medium containing sample were added (30, 15, 7.5, and 3.75µg/ml) and re-incubated for 48 h. Then 100 μl of the medium were aspirated and 15 μl of the MTT solution was added to the remaining medium (100 μl) in each well. After 4 h of contact with the MTT solution, blue crystals were formed. 100 μl of the stop solution was added and incubated further for 1h. Reduced MTT was assayed at 550 nm using a microplate reader.
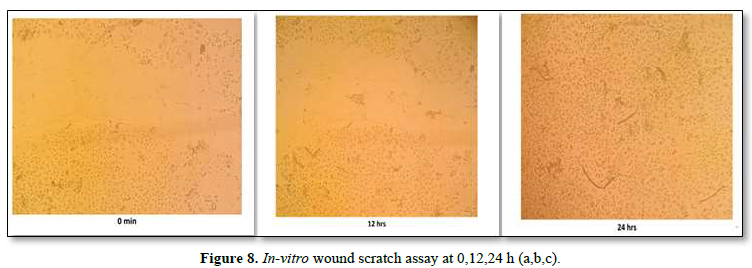
In-Vitro Wound Scratch Assay
The study was conducted using a wound scratch assay, which is a technique for studying cell migration. The wound scratch assay was performed on HaCaT cell lines. The cells were spaced by scraping directly onto the cell site to form an incision across the center of the cell plate. The lesions were then photographed at different time intervals (0 min, 12 h and 24 h), and cell growth in the wound was observed.
RESULTS AND DISCUSSION
Fourier Transform-Infrared Analysis
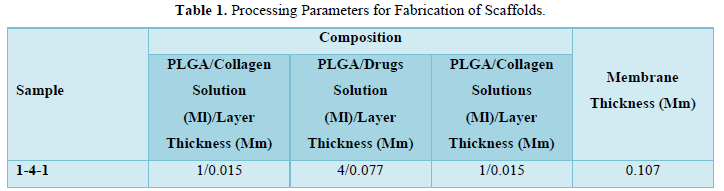
The presence of functional groups was confirmed by using FT-IR (Instrument-SCO 4100 type A). The readings were obtained between 400cm-1 to 4000cm-1 using KBR pellet technique. FT-IR was carried out for drug and polymers. The results of the analysis showed various stretching, bending and rocking vibrations based on the groups present. The strong absorption peaks at 1759, 1182, and 1132 cm-1 indicated the stretching vibration of the C=O bond, the C-O-C bond, and the C-O single bond, respectively in PLGA. The spectra of collagen exhibit N-H Bending, C=O; C=O, Stretching at 1650 and 1900cm-1 respectively. The FTIR spectrum of ofloxacin shows that the absorption peaks were mainly located at 2750 (O-H & N-H Stretching, C-H Stretching), 1500(C-H Bending, O-H Bending) and 1050cm-1 (C-H Bending, O-H Bending). The results exhibited that there is no interaction between active ingredient and other Excipients (Figure 1).
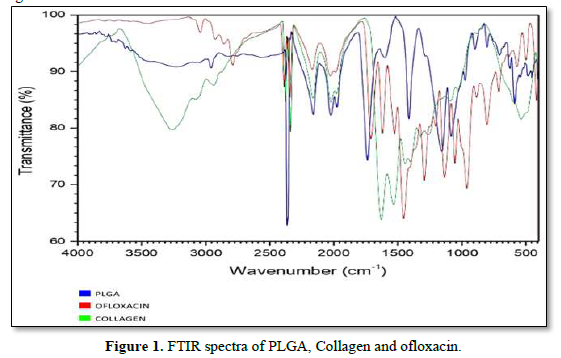
Surface Analysis-Scanning Electron Microscopy
Scanning electron microscopy was also carried out to identify the surface morphology of the scaffold. The scaffold was examined by SEM where SEM is a part of a dual beam instrument Vega 3 Tescan. The images shown in Figure were taken with an acceleration voltage of 20KV, with varying magnification of 25000X and 50000X.The fabrication of drug-eluting nanofibrous matrices via electro-spinning is highly desirable because the core PLGA/drugs nanofibrous membrane can provide sustainable release of pharmaceuticals [28-30] while the surface PLGA/collagen nano-fibers acts as a scaffold for cell growth and proliferations [31,23] Collagen is a principal structural component of the ECM matrix. In this study, continuous PLGA and collagen nano-fibers were obtained by electro-spinning. Figure 2 shows the SEM micrographs of PLGA/drugs nano-fibers electro-spun under different magnification. The diameters of the spun nano-fibers PLGA/drugs ranged from 52 nm to 66 nm. The images exhibited that the scaffold was found to have very porous structure with smooth surface morphology.
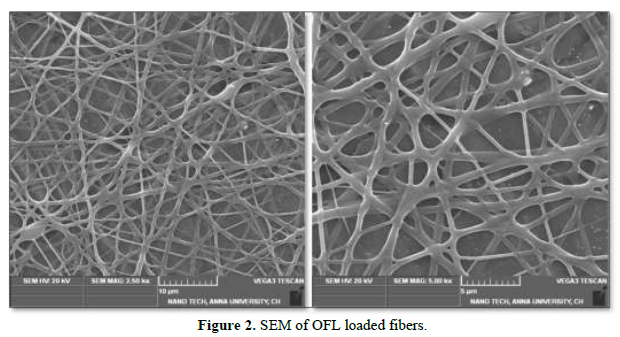
X-Ray Diffraction Analysis
XRD patterns of the ofloxacin incorporated scaffold are shown in Figure 3. In XRD analysis, the peaks were obtained at 2Ɵ level at positions 20.21, 21.24, 23.70, 27.22, 30.95, 31.37, 34.90, 43.14, 46.06, 57.80, 68.14, 79.71. Hence, the formulated scaffold was found to exhibit crystalline structure of scaffold.
In-Vitro Release Studies
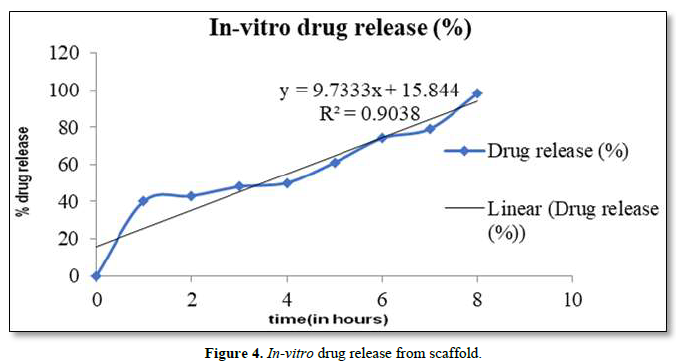
In vitro drug release studies were evaluated. Drug release profiles of the OFL from nano-fibers were shown in Figure 4. Experiments were performed triplicate and error bars indicates standard deviation (± S.D). Sahoo et al. (2010) implied that drug release from polymeric matrices was usually affected by water penetration in the matrix, hydration, and swelling, diffusion of the dissolved drug and the erosion of gelatinous layer. The electro-spun fibers usually show an initial burst release because most of the drugs are mostly accumulate on the fiber surface during the electro-spinning process. It is believed that at higher concentration of OFL, the solved drug in the polymer solution has more tendency to migrate to surface or near the surface of fibers during the electro-spinning process. When the drug amount is increased, drug molecules may aggregate more on the fiber surface due to the high ionic strength of the fiber. Since there are limited physical interactions between the drug and polymer matrix which would led to an even larger initial burst of drug as seen in fibers. Therefore, the diffusion of OFL to the medium became higher leading to a faster drug release rate.
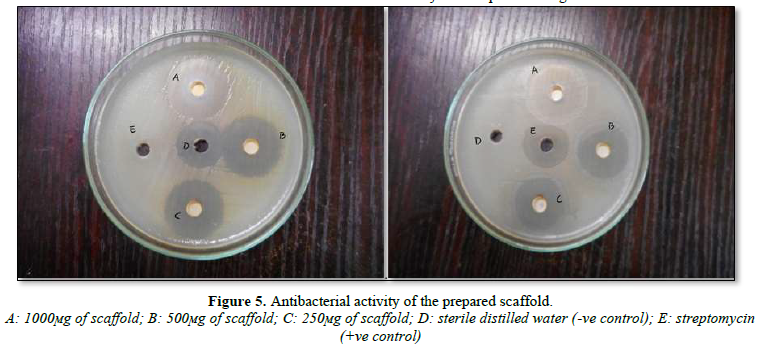
In-Vitro Antibacterial Activity
The formulation exhibited good antibacterial activity. Well defined zones of inhibition of the growth of bacterial cells were recorded for drug loaded fibers. The 1 cm2 (1x1cm) fiber mats containing OFL 44±2 mm inhibition diameter for S. aureus and 46±2 mm inhibition diameter for E. coli (Figure 5). The results of this study clearly demonstrated that drug loaded nanofiber mats freely released OFL and thus inhibited the growth and multiplication of the tested bacteria. In addition, released OFL from electro spun scaffold was shown to retain its biological functions, and the process of electro spinning had no adverse effect on the activity of incorporated drug.
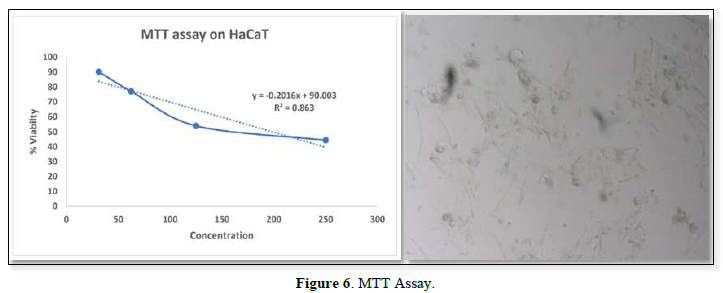
CELL LINE STUDIES
In-Vitro Cytotoxicity Study by Determination of Mitochondrial Synthesis by Mtt Assay
Cell viability assay was performed using HaCaT cells, the cytotoxic study result showed that 30.250 µg/ml, as maximum concentration. From the result it was inferred that beyond the concentration 30.250 µg/ml it exhibited cytotoxic effect (Figure 6).
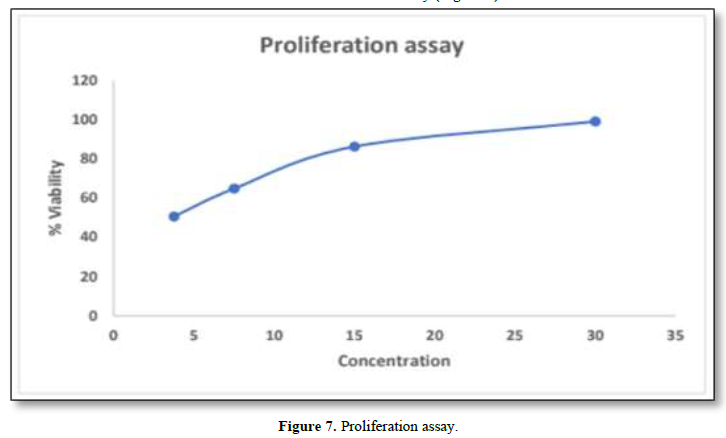
In-Vitro Proliferation Assay
In-Vitro proliferation assay was performed by using HaCaT cells. The maximum concentration obtained from MTT assay was found to be 30µg/ml. The results showed that greater viability was found to be 99.107%. From this it was inferred that decrease in the concentration, decreases viability (Figure 7).
In-Vitro Wound Scratch Assay
The wound scratch assay was performed on HaCaT cell lines. The cells were spaced by scraping directly onto the cell site to form an incision across the center of the cell plate. The lesions were then photographed at different time intervals (0 min, 12 h and 24 h) shown in the Figure 8a,b,c. From the results it was inferred that the proliferation and migration of either keratinocytes or fibroblasts was seen during 24th h, further it was confirmed that the formulated scaffold has the potential to repair the injured tissues. So, it could be an effective source for the treatment of corneal wound healing.
CONCLUSION
In the current work, a sandwich-structured matrix produced by the electro-spinning process was introduced for the application of corneal damage. The in vitro release of ofloxacin from electro-spuns and which structured polylactide-polyglycolide (PLGA)/collagen nanofibrous membranes was investigated. Ofloxacin possess high activity as a wound healing accelerator. The compatible properties of PLGA/collagen polymer with that of the skin helps to formulate the drug into them and act as an effective carrier. A nanofibrous scaffold was prepared using two polymers as mentioned earlier with the advanced technique called electro-spinning along with the drug incorporation in polymeric solution. The prepared scaffolds were studied for its characteristic properties such as, Scanning electron microscopy, FT-IR, X-ray diffraction, in vitro release studies and in vitro antimicrobial studies. Owing to the sufficient porosity, improved antibacterial activity and extended drug release, the drug loaded scaffold would be a promising biomaterial for corneal tissue engineering applications. From this research, it was concluded that the drug loaded scaffold is a viable alternative to existing conventional dosage forms which lead to improved bioactivity and a promising biomaterial for corneal tissue engineering applications in case of administration affords resulting in better patient compliance and cost-effective therapy in the field of biomedical application. Results indicated that nanofibrous sandwiched scaffolds containing ofloxacin were well sustained for the sustained release of ofloxacin and are promising as the carrier for the drug delivery in corneal tissue engineering due to their compliance with the corneal epithelial cells. By adopting the electro-spinning technique, we will be able to manufacture biodegradable biomimetic nanofibrous extracellular membranes for long-term drug delivery of various pharmaceuticals.
- Pratoomsoot C, Tanioka H, Hori K, Kawasaki S, Kinoshita S, et al. (2008) A Thermo reversible hydrogel as a biosynthetic bandage for corneal wound repair. Biomaterials 29(3): 272-281.
- Whitcher JP, Srinivasan M, Upadhyay MP (2001) Corneal blindness: A global perspective. Bull World Health Organ 79: 214-221.
- World Health Organization (2023) Prevention of blindness and visual impairment: Priority eye diseases. Available online at: http://www.who.int/blindness/causes/priority/en/index9.html
- Cornea transplantation fact sheet. Available online at: http://www.organdonation.nhs.uk/newsroom/fact_sheets/cornea_transplantation_fact_sheet.asp
- World Health Organization (2012) Visual impairment and blindness. Fact sheet no. 282. Available online at: http://www.who.int/mediacentre/factsheets/fs282/en/index.html
- Zhang, Z, Niu G, Choi JS, Giegengack M, Atala A, et al. (2015) Bioengineered Multilayered Human Corneas from Discarded Human Corneal Tissue. Biomed Material 10: 035012.
- Hu X, Liu S, Zhou G, Huang Y, Xie Z, et al. (2014) Electrospinning of polymeric nano-fibers for drug delivery applications. J Control Release 185: 12-21.
- Ustundag-Okur N, Gokce EH, Bozbiyik DI, Egrilmez Sa, Ozer O, et al. (2014) Preparation and in vitro-in vivo evaluation of ofloxacin loaded ophthalmic nanostructured lipid carriers modified with chitosan oligosaccharide lactate for the treatment of bacterial keratitis. Eur J Pharm Sci 63: 204-215.
- Xu X, Yang L, Xu Xi, Wang Xi, Chen Xu, et al. (2005) Ultrafine medicated fibers electro spun from W/O emulsions. J Control Release 108(1): 33-42.
- Lee KH, Kim HY, Khil MS, Ra YM, Lee DR (2003) Characterization of nano-structured poly(ε-caprolactone) nonwoven mats. Polymer 44: 1287-1294.
- Zamani M, Morshed M, Varshosaz J, Jannesari M (2010) Controlled release of metronidazole benzoate from poly ε-caprolactone electro spun nano-fibers for periodontal diseases. Eur J Pharm Sci 75: 179-185.
- Zeng J, Chen X, Xu X, Liang Q, Bian Xi, et al (2003) Ultrafine fibers electro spun from biodegradable polymers. J Applied Polym Sci 89: 1085-1092.
- Meechaisue C, Dubin R, Supaphol P, Hoven VP, Kohn J (2006) Electro spun mat of tyrosine-derived polycarbonate fibers for potential use as tissue scaffolding material. J Biomater Sci Polym Ed 17(9): 1039-1056.
- Uyar T, Besenbacher F (2008) Electrospinning of uniform polystyrene fibers: The effect of solvent conductivity. Polymer 49: 5336-5343.
- Natu MV, Sousa HCde, Gil MH (2010) Effects of drug solubility, state, and loading on controlled release in bicomponent electro spun fibers. Int J Pharm 94: 50-58.
- Ignatova MG, Manolova NE, Toshkova RA, Rashkov IB, Gardeva EG, et al. (2010) Electro spun nanofibrous mats containing quaternized Chitosan and Polylactide with in vitro antitumor activity against HeLa cells. Biomacromolecules 11(6): 1633-1645.
- Wang TJ, Wang IJ, Lu JN, Young TH (2012) Novel chitosan-polycaprolactone blends as potential scaffold and carrier for corneal endothelial transplantation. Mol Vis 18: 255-264.
- Madhaiyan K, Sridhar R, Sundarrajan S, Venugopal JR, Ramakrishna S (2013) Vitamin B12 loaded polycaprolactone nano-fibers: A novel transdermal route for the water-soluble energy supplement delivery. Int J Pharm 444: 70-76.
- Jannesari M, Varshosaz J, Morshed M, Zamani M (2011) Composite poly (vinyl alcohol)/poly (vinyl acetate) electro spun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int J Nanomedicine 6: 993-1003.
- Shen X, Yu D, Zhu L, White CB, White K, et al. (2011) Electro spun diclofenac sodium loaded Eudragit L 100-55 nano-fibers for colon-targeted drug delivery. Int J Pharm 408: 200-207.
- Zamani M, Prabhakaran MP, Ramakrishna S (2013) Advances in drug delivery via electro spun and electro sprayed nanomaterials. Int J Nanomed 8: 2997-3017.
- Bango Jr JJ, Bowman L, Wnek G, Tauber S, Fuerst R (2009) U.S. Patent 2009/0269391.
- Matthews JA, Wnek GE, Simpson DG, Bowlin GL (2002) Electrospinning of collagen nano-fibers. Biomacromolecules 3: 232-238.
- Yang Y, Zhu X, Cui W, Li X, Jin Y, et al. (2009) Electro spun composites mats of poly [(d, l-lactide)-co-glycolide] and collagen with high porosity as potential scaffolds for skin tissue engineering. Macromol Mater Eng 294: 611-619.
- Kumbar SG, Nukavarapu SP, James R, Nair LS, Cato T, et al. (2008) Electro spun poly (lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 29: 4100-4107.
- Ali SA, Doherty PJ, Williams DF (1993) Mechanisms of polymer degradation in implantable devices 2. Poly (dl-lactic acid). J Biomed Materials Res 27: 1409-1418.
- Zilberman M, Elsner JJ (2008) Antibiotic-eluting medical devices for various applications. In J Control Release 130: 202-215.
- Meng ZX, Xu XX, Zheng W, Zhou HM, Li L, et al. (2011a) Preparation and characterization of electro spun PLGA/gelatin nano-fibers as a potential drug delivery system. Colloids Surf B Biointerfaces 84: 97-102.
- Meng ZX, Zheng W, Li L, Zheng YF (2011b) Fabrication, characterization, and in vitro drug release behavior of electro spun PLGA/chitosan nanofiber scaffold. Mater Chem Phys 125: 606-611.
- Kontogiannopoulos KN, Assimopoulou AN, Tsivintzelis I, Panayiotou C, Papageorgiou VP (2011) Electrospun fiber mats containing shikonin and derivatives with potential biomedical applications. Int J Pharm 409: 216-228.
- Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK (2002) Electro spun nanofibrous structure: A novel scaffold for tissue engineering. J Biomed Mater Res 60: 613-621.










