4377
Views & Citations3377
Likes & Shares
MATERIAL AND METHODS
Preformulation study is the first step in the rational development of dosage form of a drug substance. It can be defined as an investigation of physical and chemical properties a drug substance alone and when combined with excipients. The overall objective of preformulation testing is to generate information useful to the formulator in developing stable & bioavailable dosage forms which can be mass produced. Obviously, the type of information needed depend on the dosage form to be developed. Even after developing a formulation and method of manufacture on these principles, it is still necessary to confirm stability and bioavailability, but there is a smaller probability that the formulation will fail. If two or three formulations are developed in parallel, there is even greater probability that one will be significantly minimize the risks of failure and increase the likelihood of producing a high quality. The study contains physical evaluation, solubility, melting point, determination of pH, loss on drying, flow properties, bulk properties, tapped density, compressibility index, hausner ratio and determination of λ max of drug simvastatin etc.
Preparation of simvastatin buccal tablet
Direct compression was taken after to manufacture the buccal tablets of Simvastatin. Six different formulations (SF1 - SF6) were set up by direct compression. Every one of the polymers chose, drug and excipients were gone through strainer no. 40 preceding utilizing into plan. The sum and proportion of drug and polymers were weighed according to given in Table 1 and all the definition were utilized for encourage assessments parameters. Excipients like Sodium bicarbonate, citrus extract anhydrous, Magnesium Stearate were selected for the examination. Carbopol as buccal mucoadhesive polymers. Steps associated with the manufacture of tablets, first the medication; polymer and different excipients selected were gone through 40 mesh sieves. Required amount of medication, polymer and excipients were weighed legitimately and moved into polyethylene pack and the mix was blended for no less than 15 min. The mix acquired was then lubricated by including 1% magnesium stearate and again blended for another 5min.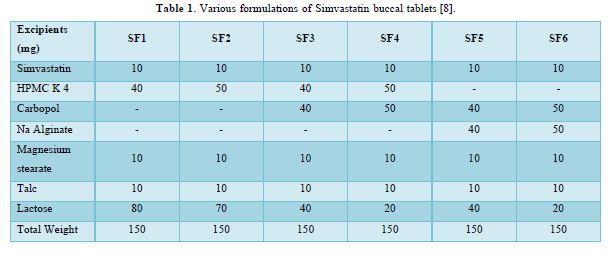
Characterization of tablets: All the tablets were evaluated for following various parameters which includes following parameters
General Appearance: Withdrawn 10 tablets from various batches were randomly selected and organoleptic properties such as color, odor, taste, shape, were evaluated. Appearance was judged visually. Very good (+++), good (++), fair (+) poor (-), very poor (- -).
Thickness and diameter: Thickness and diameter of tablets were determined using Vernier caliper. Five tablets from each batch were used, and an average value was calculated.
Uniformity of weight: Twenty tablets were randomly selected from each batch individually weighed, the average weight and standard deviation of 20 tablets was calculated.
Hardness: For each formulation the hardness of five tablets was resolved utilizing the Monsanto hardness tester (Cadmach).
Friability: The friability of sample of 10 tablets was estimated utilizing a Friability tester (Electro Lab). Ten tablets were weighed, rotated at 25 rpm for 4 min. Tablets were reweighed after removal of fines (dedusted) and the percentage of weight loss was calculated.
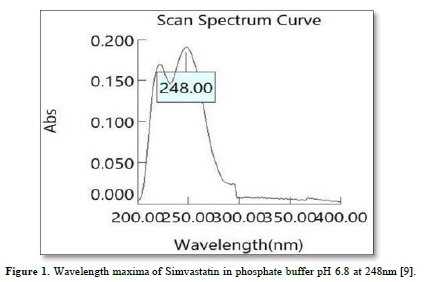
Drug content: Twenty tablets were taken and amount of drug present in each tablet was determined. The tablets were crushed in a mortar and the powder equivalent to 10mg of drug was transferred to 10ml standard flask. The powder was dissolved in 5 ml of phosphate buffer pH 6.8 and made up to volume with of phosphate buffer pH 6.8. The sample was mixed thoroughly and filtered through a 0.45μ membrane filter. The filtered solution was diluted suitably and for drug content by UV spectrophotometer at λ max of 248nm using of phosphate buffer pH 6.8 as blank.
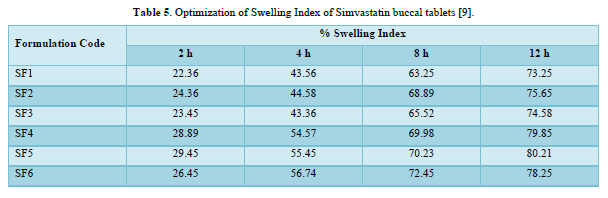
Swelling Index: Swelling study of individual polymers and combinations was carried out using eight-stage USP type 1 (basket) Dissolution Test Apparatus (Lab India, DS 8000) at 50 rpm, and phosphate buffer pH 6.8 was used as medium, and the temperature was maintained at 37 ± 0.5°C. Weight of individual tablet was taken prior to the swelling study (W1). The tablet was kept in a basket. The weight of tablet was taken at time interval of 2, 4, 8, 12 h (W2). Percent hydration (swelling index) was calculated using the following formula: Swelling index = (W2 - W1) × 100/W2
Where W1 is the initial weight of tablet and W2 is the weight of hydrated tablet
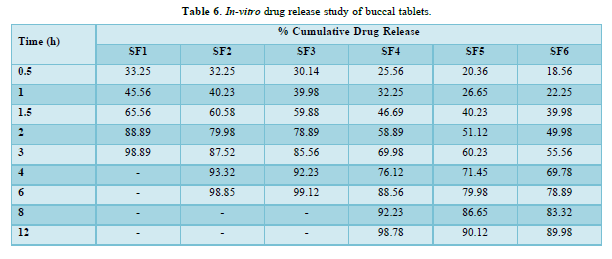
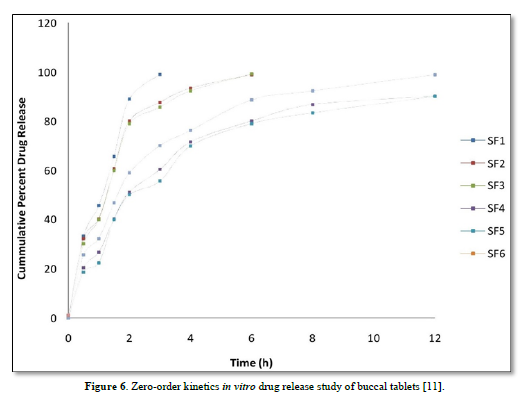
In-vitro dissolution rate studies: In vitro drug release of the sample was done using USP-type II dissolution apparatus (Paddle type). The dissolution medium, 900 ml phosphate buffer pH 6.8 was set into the dissolution flask maintaining the temperature of 37±0.5°C and rpm of 75. One simvastatin tablet was set in every container of dissolution apparatus. The mechanical assembly was permitted to keep running for 10 h. Sample measuring 5 ml were pulled back after each 1 h up to 2 h using 10ml pipette. The new disintegration medium (37°C) was supplanted each time with a similar amount of the sample and takes the absorbance at 248.0 nm using spectroscopy. The quantitative analysis of the qualities got in dissolution/release tests is simpler when mathematical formulas that express the dissolution comes about as an element of a portion of the measurement frames attributes are utilized. The pharmaceutical dosage frames following this profile release a similar measure of medication by unit of time and it is the ideal method of medication release keeping in mind the end goal to accomplish a pharmacological prolonged action [8].
RESULT AND DISCUSSION
The sensory characters of prepared simvastatin drug were white to off-white powder, Odorless and tasteless characteristics. The solubility of Simvastatin drug in distilled water is sparingly soluble, 0.1 N Hydrochloric acid is slightly soluble, ethanol and methanol are freely soluble, and phosphate buffer pH 6.8 is soluble. The Melting point of Simvastatin is 136-138°C and average pH of solution of drug is 7.37.
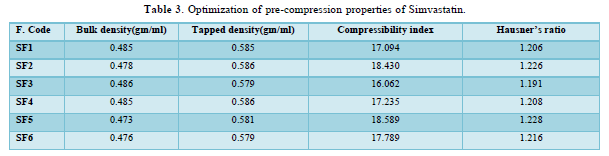
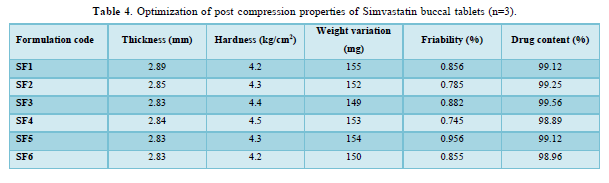
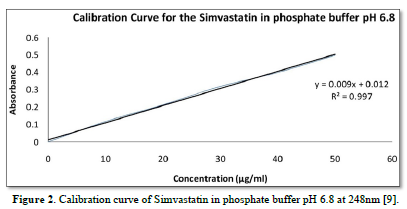
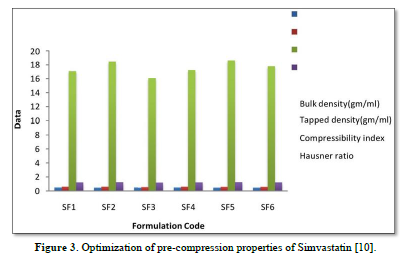
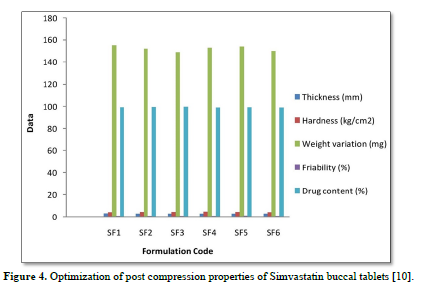
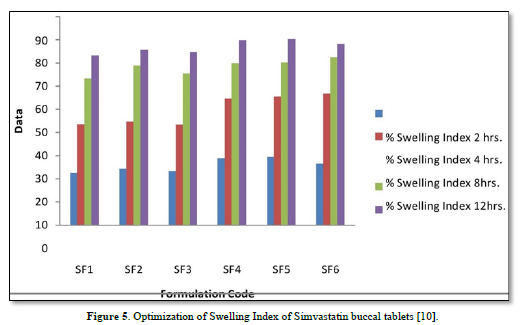
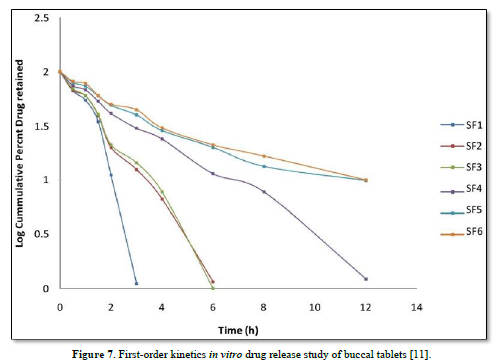
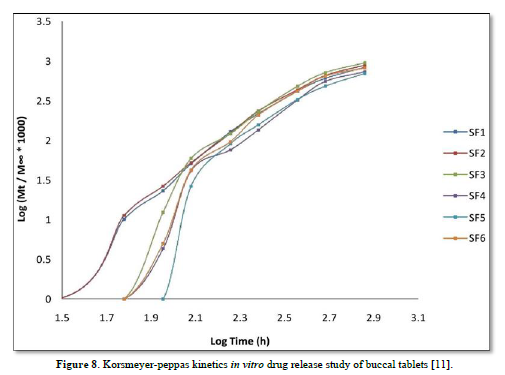
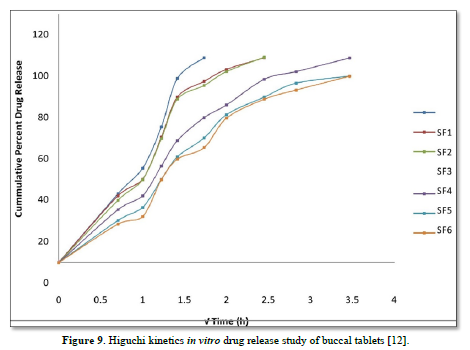
The average percent of loss of drying is 1.1%. The flow properties of drug were estimated in terms of Carr’s index, Hausners ration and angle of repose and it was 6.722 % CI, 1.072 HR and 23.12° Angle of repose. The linear regression analysis was done on Absorbance data points. The results are as follow for standard curve was determined and it was Slope 0.009, intercept 0.012 and correlation coefficient (r2) is 0.997. The prepared formulations were optimized with various parameters and pre-compression properties of simvastatin mucoadhesive granules were good flow in nature (Tables 2 & 3). The optimization of post compression properties of simvastatin buccal mucoadhesive tablets (n=3). The Thickness (mm) varied from 2.83 - 2.89 mm, Hardness were varied from 4.2 - 4.5 kg/cm2, Weight variation were varied from 149 - 155 mg, Friability were varied from 0.745 -0956 % and drug content were varied from 98.89 -99.56 % (Table 4). The swelling index of simvastatin buccal tablets after 12 h was varied from 73.25 - 80.21 % (Table 5). The invitro drug release study was carried out and when the regression coefficient values of were compared, it was observed that ‘r’ values of first order was maximum i.e. 0.965 hence indicating drug release from formulations was found to follow first order kinetic (Figures 1-9).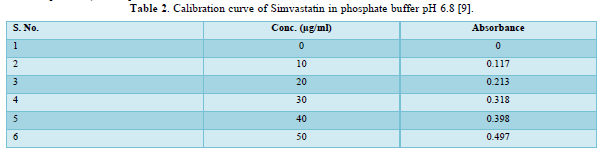
SUMMARY AND CONCLUSION
From the present study the following conclusions were made. Buccal tablets of Simvastatin using HPMC K4, Carbopol 934 and Na Alginate prepared by direct compression method were found to be good without chipping, capping and sticking. The drug content was uniform in all the formulations of tablets prepared. Low values of standard deviations indicate uniform distribution of drugs within the matrices. The drug polymer ration influenced the release of drug from the formulations. An increase in polymer decreased the drug release. Formulation SF4 with drug polymer (HPMC K4, carbopol and Na Alginate) has shown promising results as per USP test II requirements. Among these formulations SF4 is acceptable for further pharmacodynamic and pharmacokinetic evaluation.
- Koirala S, Nepal P, Ghimire G, Basnet R, Rawat I, et al. (2021) Formulation and evaluation of mucoadhesive buccal tablets of Heliyon 7(3): e06439.
- Kaelbe D H and Moacanin J 1977) A surface energy analysis of Polym 18: 475-481.
- Rathbone MJ, Tucker IG (1993) Mechanisms, barriers and pathways of oral mucosal drug permeation. Adv Drug Deliv Rev 13: 1-22.
- Khanvilkar K, Donovan MD, Flanagan DR (2001) Drug transfer through mucus. Adv Drug Deliv Rev 48(2-3): 173-193.
- Duchene D, Touchard F, Peppas NA (1998) Pharmaceutical and medical aspects of Bioadhesive system for drug administration. Drug Dev Ind Pharm 14: 283-381.
- Mathiowitz E, Chickering DE, Jacob JS (2001) US No. 6: 197-346.
- Russo E, Selmin F, Baldassari S, Gennari CGM, Caviglioli G, et al. (2016) A focus on mucoadhesive polymers and their application in buccal dosage forms. J Drug Deliv Sci Technol 32 B: 113-125.
- Mishra S, Kumar G, Kothiya P (2012) Formulation and Evaluation of Buccal Patches of Simvastatin by Using Different Polymers. Pharm Innov 1(7): 87-92.
- Eyad SMAN, Shawabkeh RA, Azzam A (2006) High-performance liquid chromatographic determination of simvastatin in medical drugs. J Anal Chem 61: 63-66.
- Pethe A, Salunkhe SP (2014) Formulation and evaluation of mucoadhesive buccal tablet of simvastatin. Int J Pharm Bio Sci 5(3): 268-278.
- Bruschi ML (2015) Woodhead Publishing (Cambridge: Elsevier)- 64.
- Dash S, Narasimha Murthy P, Nath L, Chowdhury P (2010) Kinetic modeling on drug release from controlled drug delivery system. Acta Poloniae Pharmaceutica Drug Res 67(3): 217-223.











