4298
Views & Citations3298
Likes & Shares
USES
- Oculocutaneous Albinism
OCA which is characterized by diminished melanin pigmentation in the skin and eyes, as well as vision loss, is brought on by a mutation in the TYR gene. Post-natal visual development is influenced by retinal pigment epithelium. Nitisinone is FDA approved and did not result in an increase in iris melanin content but may increase hair and skin pigmentation in patients with OCA-1 [4].
- Hereditary Tyrosinemia 1
HT is an autosomal recessive inborn error of Tyr metabolism brought on by a lack of fumaryl acetoacetate hydrolase which leads to increased maleylacetoacetate precursors which get converted to succinyl acetoacetate. The highly reactive electrophilic toxic metabolites bind to sulfhydryl groups. It clinically presents as photophobia, palmoplantar keratoderma, acute or chronic liver disease, renal dysfunction associated with Fanconi syndrome, hypophosphatemic rickets, polyneuropathy and abdominal pain resembling acute porphyria. Elevated methionine metabolites produce a boiled cabbage odor [5,6]. Nitisone is known to prevent acute life-threatening complications when started in the newborn period started with a dose of 1-2mg/kg/day. Reducing the dose of the drug by determining the level of plasma and urine succinylacetone levels [7,8].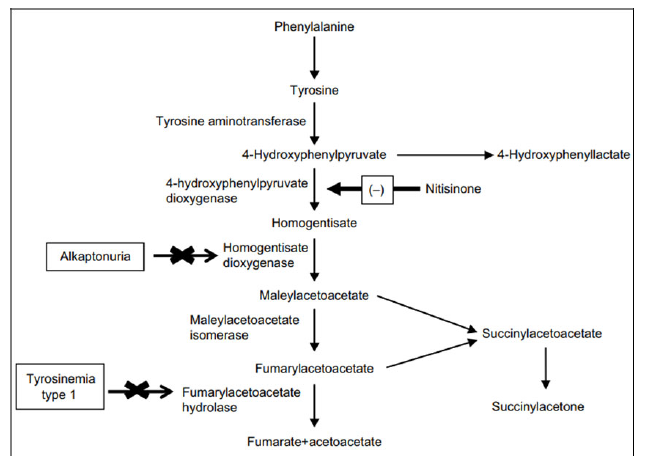
- Alkaptonuria
Alkaptonuria (AKU; OMIM 203500) is a rare autosomal recessive disease caused by a deficiency of homogentisate 1, 2-dioxygenase (HGD) that converts intermediate HGA to MAA in the Tyr metabolism pathway [3]. Characteristic dark urine is seen in this disease due to the polymerization of HGA to Benzoquinone acetic acid and deposition and adhering to connective tissue like cartilage forms a pathological pigmentation called ochronosis [9].
- African trypanosomiasis
Tsetse fly transmits this fatal disease and no vaccine available to prevent the disease, so control of transmission depends on elimination of tsetse fly population. Neurotoxic pesticides are gold standard for disease control with negative impact on environment. Nitisinone targets tyrosine pathway and RNA interference of either of enzymes in tyrosine degradation pathway was lethal to tsetse fly [10].
Other non-dermatological uses are ocular neuroblastoma [11,12].
PHARMACOKINETICS
The drug level peaks after 1- 4 h in plasma after ingestion with 95% protein binding. Half-life of the drug is approximately 54 h. Modification of nitisinone in liver and renal dysfunction is yet to be studied [11].
The drug is available as capsule (2mg, 5mg, 10mg, and 20 mg), tablet (2mg, 5mg, and 10mg) and oral suspension (4mg/ml) availability in India.
It is administered 1 h prior of 2 h after a meal [11].
SIDE EFFECTS
Nitisinone has minimal adverse events with ocular symptoms like irritation, corneal erosions, photophobia if not on tyrosine restricted diet in HT. It is not known whether nitisinone has direct effects for CNS symptoms such as hypokinesis, seizure, and headache. Transient thrombocytopenia, leucopenia, and minimal GI symptoms such as diarrhea, and enanthem have been reported. Rare dermatological side effects such as alopecia, exfoliative dermatitis, xeroderma, pruritus have been reported [12].
The pregnancy category is not determined all pregnancies were uneventful and there was no evidence of NTBC-induced harm to the developing fetus. It is not known whether nitisinone is present in breast milk while lactating. No contraindications have been labeled till now [12].
CONCLUSION
We want to conclude that even though nitisone is a rarely used drug get familiarize by the drug and its availability. It is the drug that inhibits the tyrosine degradation pathway with minimal side effects. The drug is yet to bed explored further.
CONFLICTS OF INTEREST
None.
- Michaeley WJ, Kratz GW (1986) Certain 2-(2-substituted benzyl)-1, 3-cyclohexanediones. European Patent Application 0135191.
- Prisbylla MP, Onisko BC, Shribbs JM, Mutter LC, Adams D, et al. (1993) The novel mechanism of action of the herbicidal triketones. In: Proceedings of the Brighton Crop Protection Conference-Weeds, London, UK 2: 731-738.
- Lock EA, Ellis MK, Gaskin P, Robinson M, Auton TR, et al. (1998) From toxicological problem to therapeutic use: the discovery of the mode of action of 2-(2-nitro4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), its toxicology and development as a drug. J Inherit Metab Dis 21(5): 498-506.
- Adams DR, Menezes S, Jauregui R, Valivullah ZM, Power B, et al. (2019) One-year pilot study on the effects of nitisinone on melanin in patients with OCA-1B. JCI insight 4(2): e124387.
- Angileri F, Bergeron A, Morrow G, Lettre F, Gray G, et al. (2015) Geographical and ethnic distribution of mutations of the fumarylacetoacetate hydrolase gene in hereditary tyrosinemia type 1. JIMD Rep 19: 43-58.
- Van Spronsen FJ, Thomasse Y, Smit GP, Leonard JV, Clayton PT, et al. (1994) Hereditary tyrosinemia type I: A new clinical classification with difference in prognosis on dietary treatment. Hepatology 20(5): 1187-1191.
- Russo PA, Mitchell GA, Tanguay RM (2001) Tyrosinemia: A review. Pediatr Dev Pathol 4(3): 212-221.
- Mitchell G, Russo PA, Dubois J, Alverez F (2007) Tyrosinemia. In: Suchy FJ, Sokol RJ, Balistreri WF, editors. Liver Disease in Children. 3rd Ed. New York: Cambridge University Press; pp: 694-713.
- Peker E, Yonden Z, Sogut S (2008) From darkening urine to early diagnosis of alkaptonuria. Indian J Dermatol Venereol Leprol 74(6): 700.
- Sterkel M, Haines LR, Casas-Sánchez A, Adung'a VO, Vionette-Amaral RJ, et al. (2021) Repurposing the orphan drug nitisinone to control the transmission of African trypanosomiasis. PLoS Biol 19(1): e3000796.
- Olsson B, Cox TF, Psarelli EE, Szamosi J, Hughes AT, et al. (2015) Relationship Between Serum Concentrations of Nitisinone and Its Effect on Homogentisic Acid and Tyrosine in Patients with Alkaptonuria. JIMD Rep 24: 21-27.
- Aktuglu-Zeybek AC, Zubarioglu T (2017) Nitisinone: A review. Orphan Drugs Res Rev 7: 25-35.



