6292
Views & Citations5292
Likes & Shares
Soil samples were procured from a nearby area of a medical center in Mumbai, India. After performing suitable dilutions of samples in saline, they were plated on Luria Bertani agar plates for isolation. The colony morphology and Gram nature of the well-isolated colonies were determined. The bacterial culture was further confirmed by biochemical tests and molecular characterization.
Biochemical tests
Methicillin resistance of the Staphylococcus aureus strains was detected using the oxacillin agar test. As a highly sensitive and efficient test, the samples were isolated on a Mannitol salt agar (MSA) consisting of 6 µg/mL of oxacillin [9]. The samples were isolated on these plates and incubated at 37°C for 24 h [10].
16S rRNA sequencing and Identification of gene
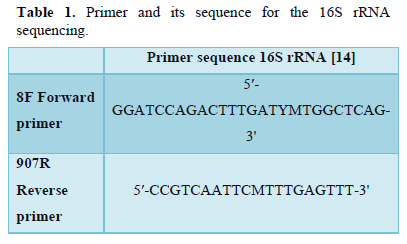
The CTAB method was used for the isolation of genomic DNA [11]. The 16S rRNA gene was amplified from extracted DNA using universal primers 8F and 907R (Table 1). The reaction mixture volume was 25µL consisting of 18µL nuclease-free water, 2.5µL Taq Buffer,1.25µL each primer, 1µL of Template DNA, 0.5 µL dNTPs, 0.5 µL Taq DNA polymerase. Cycling conditions included 30 amplification cycles, Pre denaturation for 5 min at 95°C, 1 min of Denaturation at 95°C, 1 min of annealing at 55°C, extension for 1 min at 72°C lastly termination at 72°C for 5 min [12]. Agarose Gel Electrophoresis was used to observe the amplified products. Followed by identification of gene using PCR. The methicillin-resistant mecA gene is highly conserved in Staphylococcal species; therefore, they are used for the detection of MRSA. PCR amplification using the primers for these genes is a standard for identifying MRSA [13]. The PCR amplification for MRSA was performed according to the method described by Geha et al with slight modification. The primers that were used for mecA gene amplification are given in Table 2. 200 µM dNTPs, 2.5 mM (each) primers, 1.25 U of Taq DNA polymerase, 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 µM MgCl2, and 0.5% Tween were added and reaction mixture of 25 µL was prepared. The amplification steps included 33 cycles, Pre-Denaturation of 5 min at 94°C, 45 seconds of Denaturation at 94°C, 45 seconds of Annealing at 50°C, 1 min of Primer extension at 72°C and 5 min of final extension at 72°C. The gene sequences were submitted to GenBank. The isolates' accession numbers are listed in Table 3.


Sample collection and Extraction of Essential Oil
Chrysopogon zizanioides was acquired at a regional market in Mumbai, Maharashtra. After that, it was brought to the research facility. The roots were cleaned with distilled water to remove any accumulated dirt before oil extraction. Roots were air dried for one week in the shade. The essential oil was extracted by hydrodistillation in a modified Clevenger apparatus for 2 h from 200 g of sample in 2 L of distilled water and stored at 4°C [16]. The extracted oil was further analyzed for its composition and antimicrobial properties against isolated test pathogen.
Chemical Characterization using GC-MS
The GC-MS technique was used to investigate the volatile components of the essential oil (Shimadzu GCMS-QP2010) [17]. The evaluation was carried out on the Rtx-5MS column (5 percent diphenyl and 95% dimethylpolysiloxane chemistry, 30 m length, 0.25 m diameter, and 0.25 mm film thickness) [18]. Helium was employed as a carrier gas with a flow rate of 14 mL/min to assess 1 µL of the essential oil in a split ratio of 1:10. To examine the CzEO components, they were subjected to regulated experimental conditions, Oven temperature, which was programmed to differ in a gradient fashion from 70°C with the isothermal environment for 1 min, with a 6 °C/min increase to 260 °C with a 10.00 min isothermal condition. The essential oil components were identified by matching their mass spectra to those in the Wiley and NIST libraries [19].
Analysis of antibacterial activity by Minimum Inhibitory Concentration (MIC)
Using the resazurin microplate assay, the minimum inhibitory concentration (MIC) of Chrysopogon zizanioides essential oil against the test culture (MRSA) was measured. The method was performed as outlined by Jha [4]. The bacterial suspension was calibrated with a saline solution (0.8%) to 105 CFU/mL. To determine the minimal inhibitory concentration (MIC), several essential oil concentrations were formulated in 2 percent DMSO [20]. The microbial growth was assessed after incubation. After adding 5µL of 2 mg/mL resazurin to each well and incubating at 37°C for 30 min, the MIC was determined. The change in color of resazurin in the microplate wells was used to detect microbial growth [21]. In this assay, the antibiotic Kanamycin was utilized as a positive control.
Kill Time Analysis
Time-kill curves that monitor bacterial growth and fatality across a wide range of antimicrobial levels have been widely used to determine antimicrobial efficacy over time [22]. The time-kill curve for CzEO against MRSA was assessed. Bacterial cells were incubated in fresh Nutrient broth at 37°C for 24 h. Following the measurement of the O.D. of the 24 h grown cultures, dilutions of the culture were prepared with an inoculum of approximately 106 CFU/mL before the addition of the Chrysopogon zizanioides essential oil [23]. A growth control was maintained by adding essential oil to the nutrient broth. Dilutions were agitated in an incubator set to 37°C. At 5, 10, 15, 20, 25, 30, 45, and 60-min intervals, 100 μL of the sample was retained and rinsed with 900 μL of sterile phosphate buffer (pH 7.0) [24]. After centrifugation at 1000 rpm for 10 min, the samples were re-suspended in a sterile phosphate buffer. Aliquots of each culture were recovered and plated on the Nutrient agar by the spread plate method. After 24 h at 37°C, the number of colonies on the plates was recorded [25]. A bactericidal effect was defined as a decline in the number of colonies from the initial amount over a specific period.
Antibacterial Kinetics Assay
Essential oils have been found to have strong antiseptic, antibacterial, antiviral, antioxidant, anti-parasitic, anti-fungal, and insecticidal properties [26]. The bactericidal effects of the essential oil were analyzed using the growth curve assay [27]. The essential oil was incubated with 24-h-old culture of Methicillin-resistant Staphylococcus aureus at a dilution of 106 CFU/mL at 1X MIC and 2X MIC in Luria Bertani broth. Additionally, control was maintained without the essential oil. Samples of the cultures were taken at appropriate intervals (0, 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, and 36 h). The numbers of colonies were calculated by plating 100µL of the sample on Luria Bertani agar plates and incubating them at 37ºC for 24 h.
Cell membrane integrity:
Experiment involving leakage of DNA and RNA through the bacterial membrane
Essential oils (EOs) and their components play a vital role in antimicrobial activity. Due to their hydrophobic nature, EOs migrate rapidly across the lipids of bacteria's cell membranes, disrupting cell wall structures and making them more permeable, resulting in ion and other cellular material leakage and, eventually, cell death [28]. The experiment was carried out as follows; MRSA was incubated for 24 h in a Luria Bertani medium at 37°C. Logarithmic growth phase bacterial cells were treated with Chrysopogon zizanioides essential oil at 1 × MIC and 2 × MIC levels. The culture was incubated at 37°C and hourly samples were taken. Subsequently, cells were separated by centrifugation (10,000 g) at 4°C for 15 min. The supernatant’s optical density at 260 nm was evaluated using Nanodrop 2000 UV–vis spectrophotometer by measuring the amounts of Nucleic Acid (ng/μL) released from the cytoplasm.
Experiment involving leakage of proteins through the bacterial membrane
The characterization of proteins that release into the supernatant is one of the crucial indicators for the assessing permeability of the cell membrane [29]. The Bradford method was used to determine the protein concentrations in supernatants [30]. The test pathogen was treated with the essential oil at 1× MIC and 2× MIC concentrations, and hourly samples were taken. Cells were separated by centrifugation (10,000 g) at 4°C for 15 min. To determine the concentrations of proteins released from the cytoplasm, the supernatant was used to measure the optical density at 595 nm using Nanodrop 2000 UV-vis spectrophotometer.
RESULT AND DISCUSSION
Bacterial Strain Characterization
Soil samples taken from the vicinity of the medical center were diluted in saline, plated on the LB plates, and incubated for 24 h at 37°C. Methicillin resistance of Staphylococcus aureus was tested using the oxacillin agar test. After incubation, the colonies that grew on the respective plates were labeled as MRSA. For the molecular identification of MRSA, genomic DNA isolation was carried out. Sequencing of the 16S rRNA was performed. The 16S rRNA genes of all isolated bacteria were amplified. The amplicons were then purified and sequenced using a column. To compare the obtained nucleotide sequences to known sequences in the National Center for Biotechnology Information (NCBI) database, the Basic Local Alignment Search Tool (BLAST) was utilized. Later, PCR amplification was carried out. PCR was used for identification and confirmation since it has many advantages over other methods like picking the true positives from the positive results obtained from the other methods [31]. PCR detection was carried out using primers for the target gene, mecA for MRSA. The amplified product was found to be 310 bp in the case of MRSA. As a result, it was established that the strain identified from the soil sample was MRSA. Post extraction of essential oil it was analyzed for its chemical composition and antibacterial activity.
GC-MS Analysis of the Composition of the Essential Oil
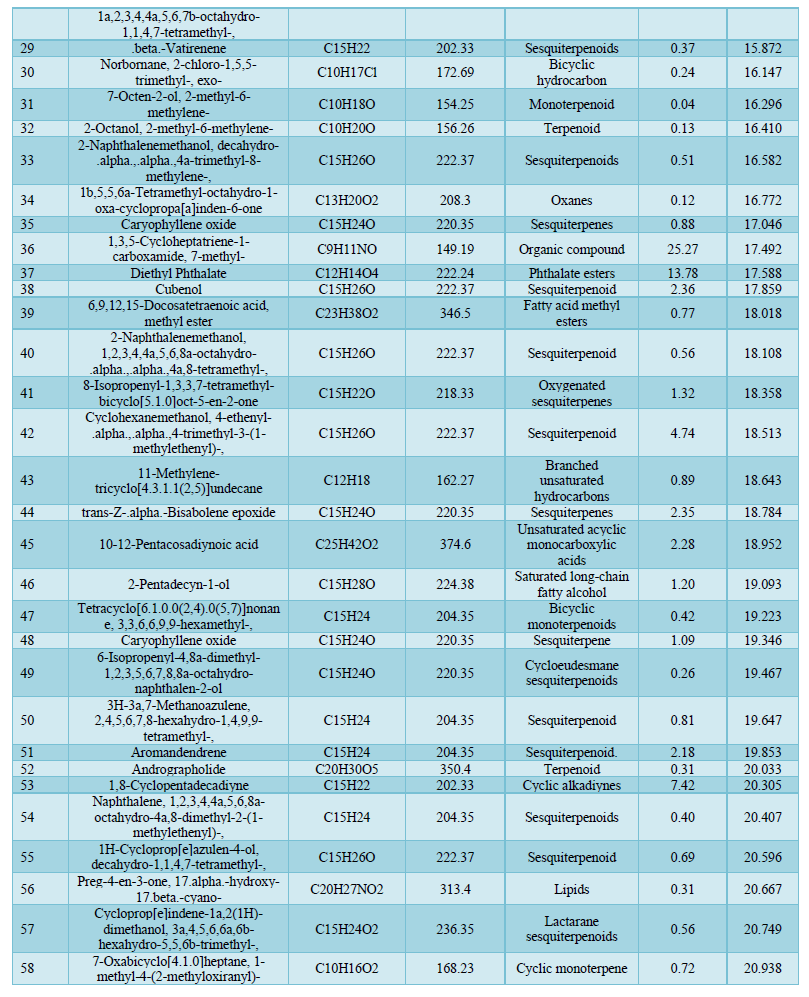
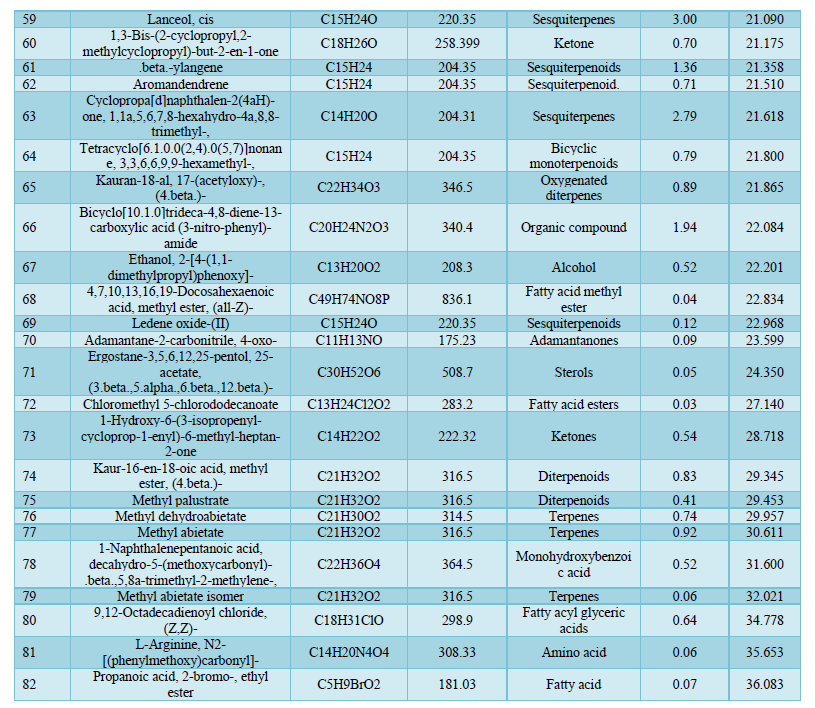
Analysis by Gas Chromatography-Mass Spectrometry system revealed a total of 82 components of the Chrysopogon zizanioides essential oil. The most common classes present in the essential oil are sesquiterpene (31.73%), which includes cyclic and oxygenated sesquiterpenes, the organic compound (29.27%), fatty acid ester (19.7 %), terpenes (3.59%), which includes cyclic and diterpenoids, and monoterpene (2.14%), which includes cyclic and oxygenated monoterpenes. The principal components of the essential oil identified were 1,3,5-Cycloheptatriene-1-carboxamide,7-methyl-(25.27%); Diethyl Phthalate (13.78%); 1,8-Cyclopentadecadiyne (7.42%) and Cyclohexanemethanol, 4-ethenyl-.alpha.,.alpha.,4-trimethyl-3-(1-methylethenyl)-(4.74%) having retention time of 17.492, 17.588, 20.305 and 18.513 respectively. The rest of the oil corresponds to other identified compounds such as Copaene, Aromandendrene, and others. Due to the numerous components present, the essential oil offers a variety of therapeutic and industrial purposes. 1,3,5-cycloheptatriene-1-carboxamide,7-methyl-, the most abundant compound in the essential oil belongs to the family of carboxamides with known antibacterial activity against Methicillin-resistant Staphylococcus aureus [32]. Cinnamaldehyde (0.50%) is an organic compound well known for its anti-tyrosinase activity [33]. Cinnamaldehyde is widely used as an antimicrobial against oral bacteria and effectively eliminates Gram-positive and Gram-negative bacterial biofilms by inhibiting cell wall synthesis [34,35]. Diethyl phthalate present in the essential oil is used as a potent antimicrobial agent against Aspergillus species and it has several industrial uses [36]. Aromandendrene being a sesquiterpene bears a reactive exocyclic methylene group, and a cyclopropane ring grants it the ability to alkylate proteins and disrupt the conformation of proteins making it effective against Methicillin-resistant Staphylococcus aureus [37]. Thus, this essential oil has the potential to be employed in the pharmaceutical industry owing to its many capabilities, can also be used as a cosmetical product, and has the potential to be used as a substitute for several commercially available antibiotics (Table 4).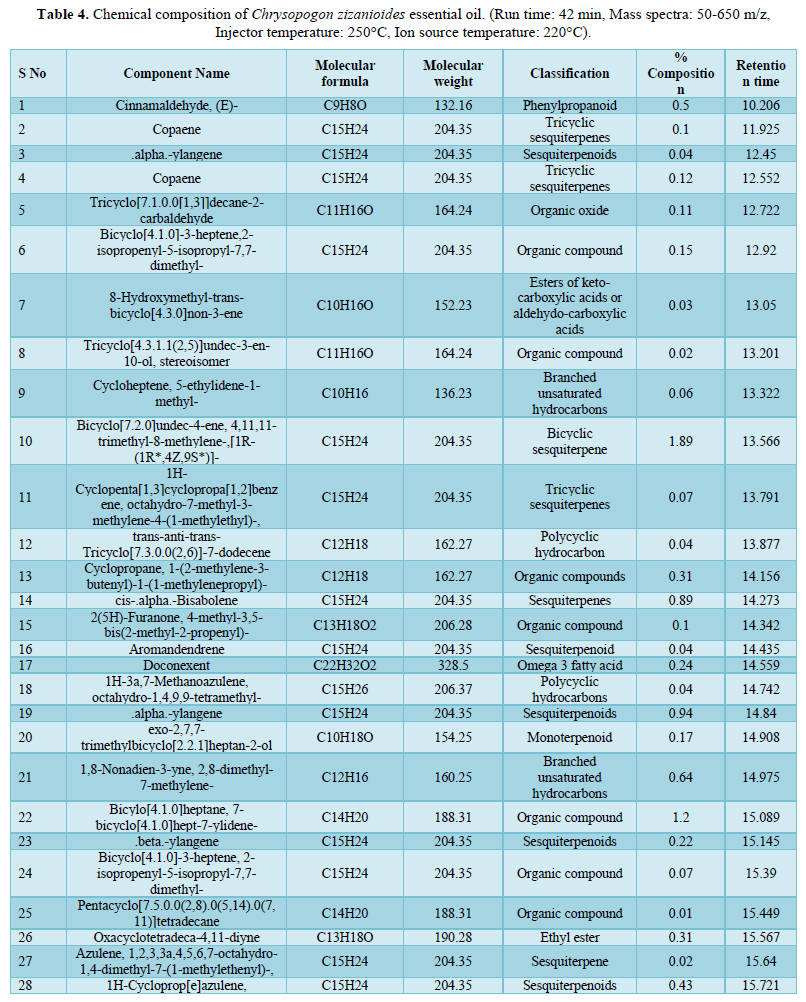
Antibacterial activity by MIC
MICs are predominantly used to assess the in vitro efficacy of novel metabolites [38]. CzEO showed strong activity against Gram-positive MRSA the MIC values of the test organism were 15.625μg/mL. These antibacterial attributes of the essential oil can be attributed to the presence of the principal bioactive constituents, especially Cinnamaldehyde and Diethyl phthalate [39]. The primary mechanism of action of these components includes gene transcription and protein production restriction. Cinnamaldehyde has been shown to significantly inhibit antibiotic-resistant biofilms [40]. The investigated essential oil and its main active components could be potential candidates to be used as natural alternatives for further applications.
Kill Time Analysis
Essential oils are effective in suppressing the growth of drug-resistant bacterial strains [41]. Time-kill assays were used to investigate the cell viability (kill-time) of Chrysopogon zizanioides essential oil, and the results were expressed as a logarithm of colony-forming units. 24-h-old cultures of Methicillin-resistant Staphylococcus aureus which were diluted to 106 CFU/mL were used. As reported in the Figure 1 below the survival rate of treated MRSA after 5-10 min decreased from 8.2 log CFU/mL to 7.5 log CFU/mL over the first hour after treatment with Chrysopogon zizanioides essential oil. According to existing studies, Chrysopogon zizanioides essential oil has a quick decapitating effect on MRSA’s growth, with a bactericidal effect after 1 h of incubation [42]. As previously evidenced by GC-MS analysis, compounds such as Cinnamaldehyde [43], 1,3,5-Cycloheptatriene-1-carboxamide, 7-methyl, Diethyl Phthalate,1,8-Cyclopentadecadiyne [44-46] are commonly associated with antimicrobial properties. According to a previous report, Chrysopogon zizanioides essential oil contains components that have antimicrobial activity against a wide range of microorganisms, most likely due to their ability to complex with extracellular and soluble proteins as well as the bacterial cell wall; more lipophilic active compounds may disrupt microbial membranes [47]. Thus, combining various components can have synergistic or additive effects; despite being present in low concentrations, these minor components may enhance the effect of oil or have other targets in microbial species [48].
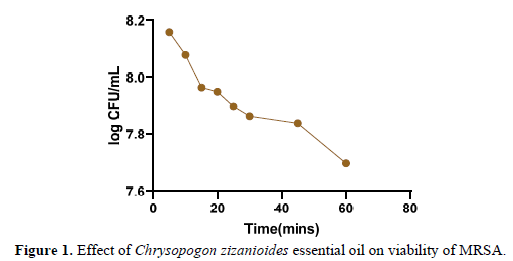
Antibacterial Kinetics Assay
The bactericidal effects of Chrysopogon zizanioides essential oil were analyzed using the growth curve assay [27]. The growth curves of MRSA affected by the essential oil are presented in Figure 2 below. It can be observed in the graphs that the essential oil showed significant inhibition of the growth of the test isolate at 1X MIC and 2X MIC concentrations. The inhibition of the test cultures at 2X MIC was greater than 1X MIC. A steady death phase was observed for both the concentrations of the essential oils. This assay provides evidence that the essential oil of Chrysopogon zizanioides inhibits MRSA at all phases of bacterial growth. CzEO serves as a promising antibacterial agent.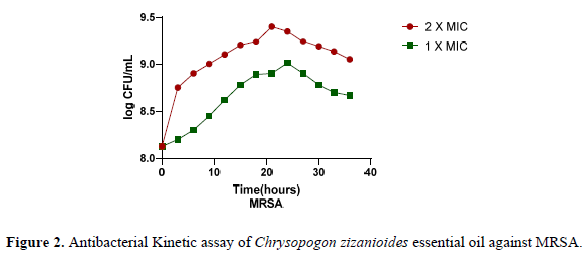
Cell membrane integrity
Experiment involving leakage of DNA and RNA through the bacterial membrane:
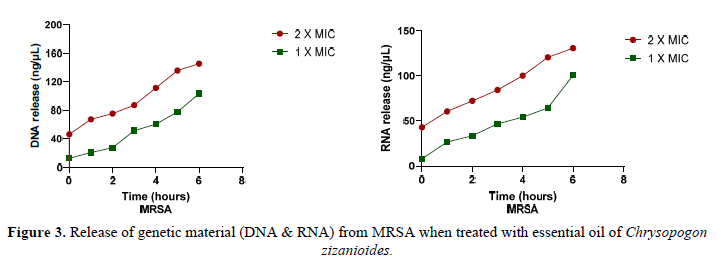
Leakage of DNA and RNA content was evaluated to confirm the impact of Chrysopogon zizanioides essential oil on the permeability of bacterial cell membranes [49]. Figure 3 illustrates the effect of Methicillin-resistant Staphylococcus aureus treatment with Chrysopogon zizanioides essential oil at increasing time intervals. When the Essential oil was added to the bacterial isolate, DNA and RNA were released throughout the six hours of incubation. At 2X MIC, the concentration of DNA and RNA released in the supernatant was higher than the concentration of both nucleic acids released at 1X MIC. According to previous research, the various components of CzEO have highly hydrophobic properties that allow them to be distributed in the lipids of cell membranes and mitochondrion. Cinnamaldehyde-(E) further affects the structural integrity and function of cell membranes, thus causing easier penetration in membranes and resulting in cell content leakage [50].
Experiment involving leakage of proteins through the bacterial membrane:
The hydrophobicity of essential oils allows them to partition into the lipids of bacteria's cellular membranes, interrupting the structure and making it more porous [51]. Proteins are essential components of microbial species. The impact of Chrysopogon zizanioides essential oil on protein leakage from MRSA was investigated. The results shown in the graph above indicate that the essential oil of Chrysopogon zizanioides induces protein leakage from the cells into the supernatant of pathogen by damaging the bacterial cell membrane. The protein release concentration was measured using Bradford’s method beginning at one hour and expanding for six hours treatment period. The concentration of protein released in the supernatant at 2X MIC was higher than the concentration of protein released at 1X MIC for the isolate (Figure 4). Results demonstrated that the compounds Caryophyllene oxide [52]; Aromandendrene when present in high concentrations, caused cellular protein leakage by disrupting cellular integrity. Although a certain amount of bacterial cell leakage can be tolerated without exacerbating cell death, increased loss of cell contents or critical output of molecules and ions can induce cell death.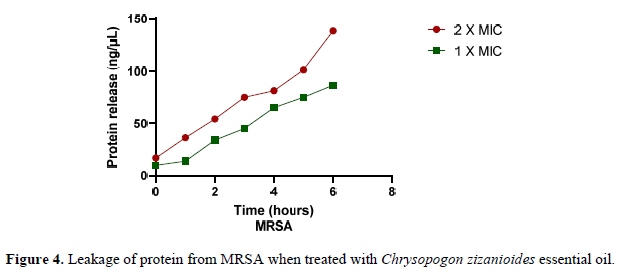
CONCLUSION
Treatment of Methicillin-resistant Staphylococcus aureus (MRSA) is becoming increasingly tedious. MRSA is potentially deadly bacteria with complex pathogenic mechanisms and are associated with high rates of mortality. On investigation of the distinct characteristics of the Essential oil, the findings were successful in demonstrating the therapeutic potential. GC-MS analysis reveals various components such as 1,3,5-Cycloheptatriene-1-carboxamide,7-methyl-, Caryophyllene oxide, Aromandendrene, Diethyl Phthalate, 1,8-Cyclopentadecadiyne and Cinnamaldehyde which have a promising antimicrobial attribute. In the growth curve, essential oil showed appreciable results by inhibiting growth. The leakage of Nucleic acids and Proteins after the introduction of the essential oil further exhibits the effect of essential oil on membrane permeability. This confirmation provides a budding demand for consequent exploration of Essential oils and their mechanism of action. In addition to reducing the lowest effective dose of the medications, and reducing their potential adverse effects and treatment costs, EOs can be utilized as an alternative therapeutic application.
ETHICS DECLARATIONS
Ethics approval and consent to participate: Not applicable.
Data Availability Statement: All data generated or analyzed during this study are included in this published article.
Author Contributions: All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical statement: No animals were harmed during this study.
- Dandachi I, Chaddad A, Hanna J, Matta J, Daoud Z (2019) Understanding the epidemiology of multi-drug resistant gram-negative bacilli in the middle east using a one health approach. Front Microbiol 10: 1941.
- Prestinaci F, Pezzotti P, Pantosti A (2015) Antimicrobial resistance: A global multifaceted phenomenon. Pathog Glob Health 109(7): 309-318.
- Furuno JP, Perencevich EN, Johnson JA, Wright MO, McGregor JC, et al. (2005) Methicillin-resistant Staphylococcus aureus and Vancomycin-resistant Enterococci Co-colonization. Emerg Infect Dis 11(10): 1539-1544.
- Jha V, Shaikh MA, Devkar S, Patel R, Walunj S, et al. (2022) Exploration of Chemical Composition and Unveiling the Phytopharmaceutical Potentials of Essential Oil from Fossilized Resin of Pinus succinefera. Int Res J Pure Appl Chem 23(3): 19-34.
- Iseppi R, Mariani M, Condò C, Sabia C, Messi P (2021) Essential oils: A natural weapon against antibiotic-resistant bacteria responsible for nosocomial infections. Antibiotics 10(4): 417.
- Clarke S (2008) Families of compounds that occur in essential oils in Essential Chemistry for Aromatherapy, Elsevier pp: 41-77.
- Jafri H, Ansari FA, Ahmad I (2019) Prospects of Essential Oils in Controlling Pathogenic Biofilm. in New Look to Phytomedicine: Advancements in Herbal Products as Novel Drug Leads pp: 203-236.
- Duarte A, Ferreira S, Silva F, Domingues FC (2012) Synergistic activity of coriander oil and conventional antibiotics against Acinetobacter baumannii. Phytomedicine 19(3-4): 236-238.
- Anand KB, Agrawal P, Kumar S, Kapila K (2009) Comparison of cefoxitin disc diffusion test, oxacillin screen agar, and PCR for mecA gene for detection of MRSA. Indian J Med Microbiol 27(1): 27-29.
- Ateba CN, Lekoma KP, Kawadza DT (2013) Detection of vanA and vanB genes in vancomycin-resistant enterococci (VRE) from groundwater using multiplex PCR analysis. J Water Health 11(4): 684-691.
- Jha V, Sarang C, Sawant D, Nalawade K, Dhamapurkar V, et al. (2022) Exploration of Probiotic Potential of Lactic Acid Bacteria Isolated from Different Food Sources. Am J BioScience 10(3): 118-130.
- Kusumaningrum HD, Handayani L, Novrianti R (2016) Partial sequencing of 16S rRNA gene of selected staphylococcus aureus isolates and its antibiotic resistance. Media Peternakan Fakultas Peternakan Institut Pertanian Bogor 39(2): 67-74.
- Hososaka Y, Hanaki H, Endo H, Suzuki Y, Nagasawa Z, et al. (2007) Characterization of oxacillin-susceptible mecA-positive Staphylococcus aureus: A new type of MRSA. J Infect Chemother 13(2): 79-86.
- Ben-Dov E, Shapiro OH, Siboni N, Kushmaro A (2006) Advantage of using inosine at the 3′ termini of 16S rRNA gene universal primers for the study of microbial diversity. Appl Environ Microbiol 72(11): 6902-6906.
- Geha DJ, Uhl JR, Gustaferro CA, Persing DH (1994) Multiplex PCR for Identification of Methicillin-Resistant Staphylococci in the Clinical Laboratory. J Clin Microbiol 32(7): 1768-1772.
- Jha V, Risbud A, Kasbe S, Thube S, Preman G, et al. (2022) GC-MS Analysis and Investigation of Bioactive Potential of Essential Oil from Citrus aurantium var. amara. Int J Pharm Chem 8(3): 29-39.
- Raja MB, Rajamani K, Suresh J, Joel AJ, Uma D (2018) Chemical composition of Vetiver root oil obtained by using GCMS analysis. J Pharmacogn Phytochem 7(6): 1709-1713.
- Chakraborty A, Sankaran V, Ramar M, Chellappan DR (2015) Chemical analysis of leaf essential oil of Cinnamomum verum from Palni hills, Tamil Nadu. J Chem Pharm Sci 8(3): 476-479.
- NIST: National Institute of Standards and Technologies, Mass Spectra Libraries. Available online: http://www.sisweb.com/software/nist-gc-library.htm
- Alam A, Rehman NU, Ansari MN, Palla AH (2021) Effects of essential oils of elettaria cardamomum grown in india and guatemala on gram-negative bacteria and gastrointestinal disorders. Molecules 26(9): 2546.
- David A, Wang F, Sun X, Li H, Lin J, et al. (2019) Chemical Composition, Antioxidant, and Antimicrobial Activities of Vetiveria zizanioides (L.) Nash Essential Oil Extracted by Carbon Dioxide Expanded Ethanol. Molecules 24(10): 1897.
- Foerster S, Unemo M, Hathaway LJ, Low N, Althaus CL (2016) Time-kill curve analysis and pharmacodynamic modelling for in vitro evaluation of antimicrobials against Neisseria gonorrhoeae. BMC Microbiol 16: 216.
- Siriporn O, Waranee P, Chadarat A, Srikanjana K (2011) Killing kinetics and bactericidal mechanism of action of Alpinia galanga on food borne bacteria. Afr J Microbiol Res 5(18): 2847-854.
- Mayaud L, Carricajo A, Zhiri A, Aubert G (2008) Comparison of bacteriostatic and bactericidal activity of 13 essential oils against strains with varying sensitivity to antibiotics. Lett Appl Microbiol 47(3): 167-173.
- Rall V, Fernandes A, Fernandes J (2011) Antimicrobial activity of propolis and essential oils and synergism between these natural products. J Venom Anim Toxins Incl Trop Dis 17(2): 159-167.
- Chouhan S, Sharma K, Guleria S (2017) Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Medicines (Basel) 4(3): 58.
- Bouyahya A, Abrini J, Dakka N, Bakri Y (2019) Essential oils of Origanum compactum increase membrane permeability, disturbs cell membrane integrity, and suppresses quorum-sensing phenotype in bacteria. J Pharm Anal 9(5): 301-311.
- Bhavaniramya S, Vishnupriya S, Al-Aboody MS, Vijayakumar R, Baskaran D (2019) Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci Technol 2(2): 49-55.
- Tang C, Chen J, Zhang L, Zhang R, Zhang S, et al. (2020) Exploring the antibacterial mechanism of essential oils by membrane permeability, apoptosis and biofilm formation combination with proteomics analysis against methicillin-resistant staphylococcus aureus. Int J Med Microbiol 310(5): 151435.
- Bradford MM (1976) A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem 72: 248-254.
- Pillai MM, Latha R, Sarkar G (2012) Detection of Methicillin Resistance in Staphylococcus Aureus by Polymerase Chain Reaction and Conventional Methods: A Comparative Study. J Lab Physicians 4(2): 083-088.
- Semelková L, Janďourek O, Konečná K, Paterová P, Navrátilová L, et al. (2017) 3-Substituted N-Benzylpyrazine-2-carboxamide Derivatives: Synthesis, Antimycobacterial and Antibacterial Evaluation. Molecules 22(3): 495.
- Rao PV, Gan SH (2014) Cinnamon: A multifaceted medicinal plant. Evidence-based Complementary and Alternative Medicine. Preprint available online at: https://doi.org/10.1155/2014/642942
- He Z, Huang Z, Jiang W, Zhou W (2019) Antimicrobial Activity of Cinnamaldehyde on Streptococcus mutans Front Microbiol 10: 2241.
- Gill AO, Holley RA (2004) Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl Environ Microbiol 70(10): 5750-5755.
- Jaynthy C, Janu NP (2014) Antimicrobial activity of diethyl phthalate: An in-silico Asian J Pharm Clin Res 7(4): 141-142.
- Mulyaningsih S, Sporer F, Zimmermann S, Reichling J, Wink M (2010) Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 17(13): 1061-1066.
- Loomba P, Taneja J, Mishra B (2010) Methicillin and vancomycin resistant aureus in hospitalized patients. J Glob Infect Dis 2(3): 275-283.
- Rao RR, Suseela MR (2022) Vetiveria Zizanioides (Linn.) Nash A Multipurpose Eco-Friendly Grass Of India. pp: 439-442. Available online at: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.565.5245&rep=rep1&type=pdf
- Wang S, Kang OH, Kwon DY (2021) Trans-cinnamaldehyde exhibits synergy with conventional antibiotic against methicillin-resistant staphylococcus aureus. Int J Mol Sci 22(5): 1-9.
- Tariq S, Wani S, Rasool W, Shafi K, Bhat MA, et al. (2019) A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb Pathog 134: 103580.
- Li ZH, Cai M, Liu YS, Sun PL, Luo SL (2019) Antibacterial Activity and Mechanisms of Essential Oil from Citrus medica L. Var. Sarcodactylis. Molecules 24(8): 1577.
- Wei QY, Xiong JJ, Jiang H, Zhang C, Wen Y (2011) The antimicrobial activities of the cinnamaldehyde adducts with amino acids. Int J Food Microbiol 150(2-3): 164-170.
- Howyzeh MS, Noori SAS, Shariati JV (2018) Essential oil profiling of Ajowan (Trachyspermum ammi) industrial medicinal plant. Ind Crops Prod 119: 255-259.
- Kumar NN, Ramakrishnaiah H, Krishna V, Deepalakshmi AP (2015) GC-MS Analysis and Antimicrobial Activity of Seed Oil of Broussonetia Papyrifera (L) Vent Pichia Signal Sequences View project Dichrostachys Cinerea View project. Int J Pharm Sci Res 13(8): 3954-3960.
- Singh SK (2011) Antimicrobial activity of alcoholic and aqueous extracts of Vetiveria zizanioides. J Pharm Res 4(5): 1343-1344.
- Bassolé IHN, Juliani HR (2012) Essential oils in combination and their antimicrobial properties. Molecules 17(4): 3989-4006.
- Wu Y, Bai J, Zhong K, Huang Y, Gao H (2017) A dual antibacterial mechanism involved in membrane disruption and DNA binding of 2R,3R-dihydromyricetin from pine needles of Cedrus deodara against Staphylococcus aureus. Food Chem 218: 463-470.
- Sikkemat J, Bontt JA de, Poolmann B (1994) Interactions of Cyclic Hydrocarbons with Biological Membranes. J Biol 269(11): 8022-8088.
- Magiatis P, Skaltsounis AL, Chinou I, Haroutounian SA (2002) Chemical Composition and in-vitro Antimicrobial Activity of the Essential Oils of Three Greek Achillea Species. Z. Naturforsch 57(3-4): 287-290.
- Zouhir A, Jridi T, Nefzi A, ben Hamida J, Sebei K (2016) Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) by antimicrobial peptides (AMPs) and plant essential oils. Pharm Biol 54(12): 1-15.
- Denyer S, Hugo WB (1990) Mechanisms of action of chemical biocides, Society of Applied Bacteriology Technical Series no. 27.






