Review Article
Pharmaceutical Analysis - An Introduction
6694
Views & Citations5694
Likes & Shares
Introduction: With the current scenarios around the world including medical emergencies the need to establish and know about pharmaceutical industry and laboratory techniques are a must. It is rapidly growing industry with the average growth is more than 5% according to one analysis.
Area Covered: This paper deals with the basic pharmaceutical analysis types and commonly used methods. Also, in brief the industries available in India.
Keywords: Pharmaceutical analysis, Analytical methods, Chromatography, Spectroscopy, Electrochemical methods
PHARMACEUTICAL ANALYSIS
Definition- Pharmaceutical analysis is defined as “A branch of Practical chemistry that involves a series of process for identification, determination, quantification and purification of a substance, separation of the components of a solution or mixture, or determination of structure of chemical compounds”. Substance here could be a single compound or a mixture of compounds and methods could be Manual, Chemical and Instrumental Method.
TYPES OF CHEMICAL OR PHARMACEUTICAL ANALYSIS
Mainly of two types:
- Qualitative (Identification)
- Quantitative (Estimation)
- Qualitative (Identification)
As the name suggests it is done to establish the quality or composition of natural/synthetic substances. Or whether the substance or compound is present in the sample or not. An unknown substance is taken for analysis and the presence or absence of any compound is detected in it by using any of the above-mentioned methods. The methods will detect whether the compound of interest is present or not but how much the quantity of compound in question is present will be specified or detected by Quantitative methods.
For e.g.: Detection of evolved gas, formation of precipitates, limit tests, color change reaction. Melting point and boiling point tests etc.
- Quantitative (Estimation)
Done to quantify any compound or substance in the sample. Through Quantitative methods one can detect the presence of the compound in number or percentage.
Quantitative techniques are based on:
- The quantities performance of suitable chemical reaction and either measuring the amount of reagent added to complete the reaction or measuring the amount of reaction product obtained.
- The characteristic movement of a substance through a defined medium under controlled conditions.
- Electrical measurement
- Measurement of some spectroscopic properties of the compound
COMMONLY USED METHODS FOR PHARMACEUTICAL ANALYSIS [1-5] (DETAIL GIVEN IN FIGURE 1)
These are divided into two parts:
- Instrumental methods
- Non-Instrumental
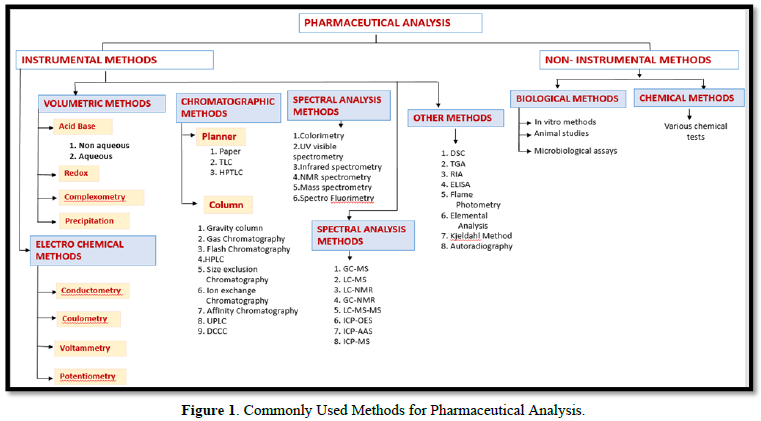
NMR: Nuclear Magnetic Resonance; TLC: Thin Layered Chromatography; HPTLC: High Performance Thin Layered Chromatography; HPLC: High Performance Liquid Chromatography; UPLC: Ultra Performance Liquid Chromatography; DCCC: Droplet Counter Current Chromatography; GCMS: Gas Chromatography with Mass Spectroscopy; LCMS: Liquid Chromatography with Mass Spectroscopy; LC-NMR: Liquid Chromatography with NMR, GC-NMR: Gas Chromatography with NMR; LC-MS-MR: Liquid Chromatography with Two Mass Spectrometer; ICP-OES: Inductively Coupled Plasma Optical Emission Spectrometry; ICP-AAS: Inductively Coupled Plasma Atomic Absorption Spectroscopy; ICP-MS: Inductively Coupled Plasma Mass Spectrometer; DSC: Differential Scanning Calorimetry; TGA: Thermo Gravimetric Analysis; RIA: Radioimmunoassay; ELISA: Enzyme-Linked Immunosorbent Assay
Few of the Important Methods Used for Pharmaceutical Analysis:
- Titration:
Various types of Titration methods observed are [6-7]:
- Aqueous acid base titration: An aqueous acid –base titration is the determination kf the concentration of an acid or base by exactly neutralizing the acid or base with an acid or base of known concentration. This allows for quantitative analysis of the concentration of an unknown acid-base solution. It can also be used to find purity of chemicals.
- Nonaqueous acid-base titration: It is the titration of substances dissolved in solvents other than water. It provides a solvent in which organic compounds are soluble. Most commonly used procedure in this titration of organic bases with perchloric acid in anhydrous acetic acid.
- Redox titration: It is based on a redox reaction between analyte and titrant. Concentration of certain chemicals in pharmaceutical compounds can be determined through redox titration.
- Complexometric titration: It is a form of volumetric analysis in which the formation of a colored complex is used to indicate the end point of a titration.
- Potentiometric titration: It is similar to direct redox reaction technique and useful means of characterizing an acid. No indicator is used here and the potential is measured across the analyte, typically an electrolyte solution.
- Amperometric titration: Refers to a class of titration in which the equivalence point is determined through measurement of the elective current produced by the titration reaction.
- Aquametry: Aquametry in analytical chemistry refer to analytical processes to measure the water present in materials.
- Nephelometry: It is used in immunology to determine the levels of several blood plasma proteins.
- Polarimetry: It is a sensitive, non-destructive technique for measuring the optical activity exhibited by inorganic and organic compounds.
- Refractometry: It is a method of measuring substance’s refractive index in order to assess their composition or purity.
Overall Titration had been used widely such as in determination of medicines such as Captopril, albendazole and gabapentin etc. Advantages associated with these methods are saving time and labor, high precision and no reference standards are needed for final result.
- Spectroscopy methods [8]
Spectroscopy is the science which deals with the interaction between a matter and an electromagnetic radiation.
Ultraviolet and visible spectroscopy is a type of absorption spectroscopy that uses the ultraviolet and visible parts of the electromagnetic spectrum.
There are many types of spectroscopy methods, commonly used are:
- Ultraviolet-visible spectroscopy or ultra-violet visible spectrophotometry in ultraviolet of infrared spectral region. In these the electromagnetic spectrum, sample atoms and molecules undergo electronic transitions by absorbing radiation energy from light source.
- Nuclear magnetic resonance spectroscopy (NMR Spectroscopy)- In NMR, nuclei in a magnetic field absorb and re-emit electromagnetic radiation. This energy is at a specific resonance frequency which depends on the strength of the magnetic field and the magnetic properties of the isotope of the atoms.
- Mass Spectroscopy- In this, molecules are bombarded with an electric beam of electrons. Molecules are then ionized and fragmented. Each kind of ion has a particular ratio of mass i.e. (m/e Ratio). For most ions the charge is one and thus m/e ratio is simply the molecular mass of the ion.
- Atomic absorption Spectroscopy (AAS)- It is a electroanalytical procedure for the quantitative determination of chemical elements using the absorption of optical radiation (Light) by free atoms in the gaseous state.
- Chromatography [9]
Chromatography is a technique used for separating and /or identifying the components in a mixture. It is powerful method in industry.
Some common types of chromatography used are:
- Paper chromatography
- Gas chromatography
- Liquid Chromatography
- High performance liquid Chromatography
- Gel filtration chromatography
- Fluorometry
It is an analytical method for detecting fluorescent compound using a beam of ultraviolet light that excites the compounds and causes them to emit visible light.
- Biological and microbiological methods:
Biological methods are generally used when the potency of a drug or its derivative cannot be properly determined by any physical and chemical methods. These are measured by bio-assays. Microbiological methods are used to observe potency of antibiotics or anti-microbial agents. On the other hand, Anti-microbiological methods are used for inhibition of growth of bacteria of the sample is compared with that of the standard antibiotics.
VALIDATION PARAMETERS
[10-11]
[10-11]
Various validation parameters used are:
- Accuracy
- Precision
- Repeatability
- Reproducibility
- Intermediate precision
- Selectivity (Specificity)
- Linearity and Range
- Sensitivity
- Limit of detection (LOD)
- Limit of quantitation (LOQ)
- Ruggedness
- Robustness
Scope of pharmaceutical analysis [12]
- Examination of raw material
- Examination of Various drug samples
- Various qualitative and quantities analysis of compound.
- For diagnosis of diseases by various chemical methods,
- Determination of Radioactive compound.
- Determination of naturally occurring phytochemical compounds.
- Determination of impurities in different samples of water.
- Determination of active ingredient or additional impurities.
- Concentration of drug in plasma or biological fluids.
CLASSIFICATION OF PHARMACEUTICAL ANALYSIS LABORATORY PRESENT IN INDIA
They are classified into following five categories:
- Governmental Regulatory Agencies-Established by Central or State govt. There work is to continuously monitor and analysis and set standards for all drugs that are manufactured, sold and consumed in India. For e.g., Indian pharmacopoeia commission (IPC) and Central Drug Standard Control Organization (CDSCO).
- Manufacturers of Drugs- Both private and government manufactures who have the capacity and manpower to produce and analysis drugs.
- Manufacturers of Raw material of Drugs-Various industries, again private and government who manufactures Raw materials. In turn they have established chemical laboratories to carry of pharmaceutical analysis.
- Non - commercial research centers- They have central instrumentation laboratories (CILs) and can take part in chemical analysis individually.
- Consulting Laboratories- Many private and Governmental consulting agencies are available in India. They can be hired to perform pharmaceutical analysis.
CONCLUSION
Pharmaceutical Industry is ever developing industry with rapidly evolving innovative techniques. Overall, the types of basic pharmaceutical industry are described in this paper with brief introduction to each. Also, the validation parameters commonly involved and scope of pharmaceuticals techniques are mentioned.
-
Siddiqui MR, AlOthman ZA, Rahman N (2017) Analytical techniques in pharmaceutical analysis: A review, Arab J Chem 10(1): P S1409-S1421.
-
Choudhary A (2017) Pharmaceutical guidelines. Accessed on July 16, 2021. Available online at: https://www.pharmaguideline.com/2017/01/different-types-of-titrations.html
-
Valcarcer M (2000) Principles of Analytical Chemistry. 1st Springer-Verlag, Berlin Heidelberg.
-
Swartz ME, Krull IS (2006) Analytical Method Development and Validation. 2nd Springer.
-
Seiler JP (2005) Good Laboratory Practices. 2nd Springer-Verlag, Berlin Heidelberg.
-
Liang J, Zhu J, Gong L, Liu X, Wang B (2018) Potentiometric titration for the high precision determination of active components in six types of chemical disinfectants. PLoS One 13(9): e0203558.
-
David P (2019) Acid-Base Titration, Undergraduate Journal of Mathematical Modelling: One + Two: 10 (1).
-
Worsfold P, Townshend A, Poole C (2005) Encyclopedia of Analytical Science. 2nd Elsevier pp: 391-399.
-
Coskun O (2016) Separation techniques: Chromatography. North Clin Istanb 3(2): 156-160.
-
Rahman N, Anwar N, Kashif M (2005) Application of pi-acceptors to the spectrophotometric determination of lisinopril in commercial dosage forms Farmaco 60 (7): 605-611.
-
Rajput D, Rajat V, Goyal A (2013) Validation of Analytical Methods for Pharmaceutical Analysis. Int J Pharm Erudition 3(1): 31-40.
- Ahmed S, Islam Md, Ullah B, Biswas SK, Azad Md AS, et al. (2020) A review article on Pharmaceutical Analysis of Pharmaceutical industry according to Pharmacopeia. Orient J Chem 36(1): 112-114.



