Research Article
Enhanced Corneal Permeation of Pilocarpine using Micro emulsion Technology
4334
Views & Citations3334
Likes & Shares
Pilocarpine has been widely used as a topical meiotic for controlling elevated intraocular pressure associated with glaucoma. Pilocarpine belongs to Class-2 of BCS classification system. Because of its low solubility and high permeability, it exhibits shorter half-life. Hence, the persons who are advised for Pilocarpine medications are recommended for repeated dose administrations However; this drug has a very low bioavailability and a limited active period due to both poor corneal penetration and extensive precorneal loss. It has partition coefficient 0.95 which means the hydrophilic behavior, but the cornea is lipophilic, therefore large quantities of Pilocarpine have to be administered frequently into the eyes of patients in order to achieve effective therapeutic results. In this study, we were formulated and characterize microemulsion (ME) carriers as an ocular delivery system of Pilocarpine for treatment of glaucoma. Micro emulsion formulations were prepared by mixing of appropriate amount of surfactants including Tween 80 and Labra sol, co-surfactant such as propylene glycol (PG) and oil phase including isopropyl myristate - Transcotul P (10:1 ratio). The prepared MEs were evaluated regarding their particle size, stability, scanning electron microscopy (SEM), release and permeation of the drug through rabbit cornea. The results showed that the mean droplet sizes of ME formulations were in the range of 3.22-110nm. Drug release profile showed that 12.2% of the drug released in the first 8 hours of experiment.
Keyword: Micro emulsion, Pilocarpine, Ocular delivery, Rabbit cornea
INTRODUCTION
Ocular absorption of topically applied ophthalmic drugs via the cornea is in constant competition with systemic absorption via the conjunctival and nasal mucosae [1]. Drug permeation through corneal epithelial cells may be affected by several species-specific influx and efflux transporters [2].
Since 10% or less of a topical dose is usually absorbed into the eye, theoretically 90% or more would be available for absorption into the bloodstream, potentially eliciting systemic side effects. Rapid clearance and low bioavailability are among the other drawbacks of the current drug deliveries [3]. Because the eye is a closed system with limited volume to administer, finding a noninvasive way for ophthalmic drug delivery using the least volume should be aimed [4]. Topical administration of drugs is the most popular and simplest treatment in ophthalmology [5]. Systemic rout is an alternative delivery approach, yet not an ideal one as other limitation including first-pass hepatic metabolism, blood–ophthalmic barriers, side effects, and low eye vascularization arise [6]. Relatively little has been known about the influence of formulation composition on the systemic absorption of ocular applied drugs.
Pilocarpine has been widely used as a topical meiotic for controlling elevated intraocular pressure associated with glaucoma. However, this drug has a very low bioavailability and a limited active period due to both poor corneal penetration and extensive precorneal loss. Therefore, large quantities of Pilocarpine have to be administered frequently into the eyes of patients in order to achieve effective therapeutic results. The phenomenon results in undesirable side effects, however including meiosis, myopia and poor patient compliance. The excess Pilocarpine, which cannot be absorbed by eye tissue, is eliminated through the lower eyelid and partially penetrates the lacrimo-nasal canal causing mucosa irritation [2]. The absorption of Pilocarpine into the systemic circulation, during the draining process from the precorneal, has also been observed [7]. Due to impermeability and natural anatomical and physiological barrier characteristics, finding alternate formulation approaches has become a concern for pharmaceutical experts [8]. The permeation of therapeutic molecules through the eye membrane is mainly influenced by physicochemical properties of drug and permeation transport routes [9]. Ideally, a drug carrier should improve the drug corneal contact time and retard the precorneal drug loss, while the controlled delivery system releases Pilocarpine at a constant rate so that the unnecessary high drug concentrations can be avoided. Therefore, high therapeutic efficacy with reduced side effects can be achieved [7].
Micro emulsions (MEs) are systems well known for their excellent long-term stability and ease of preparation. They are defined as systems containing water, oil and emulsifier constituting a single optically isotropic and thermodynamically stable liquid solution. ME structure can be oil-in-water (o/w), water-in-oil (w/o), or bicontinuous, i.e., effectively continuous in both water and oil. Beside the basic advantages of MEs, including physical stability and ease of preparation, these systems may offer additional benefits for periodontal uses [10]. MEs are inexpensive, and their production is relatively easy. In addition, they are thermodynamically stable however; their microstructure is continuously changing in the bicontinuous region [1].
MEs are favorable especially as ophthalmological water-continuous vehicle because they are transparent and thermodynamically stable [11]. MEs are also used in contact lenses as drug delivery systems [12]. Some of the advantages of MEs include improvement of drug solubilization, increased stability, reduced dosing frequency, high encapsulation efficiency, biocompatibility, and ease of preparation and administration [13,14].
In this study, the potential use of ME as a drug carrier for a long-acting delivery system for Pilocarpine is investigated. Also, the in vitro effects of the prepared delivery systems were investigated in rabbit eyes.
MATERIALS AND METHODS
Materials
Pilocarpine was purchased from sinadaru Company (Iran), Labrasol and Transcutul P were kindly gifted from Gattefosse Company (France). Isopropyl myristate, Tween 80 and PG were obtained from Merck (Germany).
Animals
Male New Zealand white rabbits weighing 2.5-3.0 kg were used, which was approved by the Animal Ethical Committee, Ahvaz Jundishapur University of Medical Sciences (permit no: N-95).
Methods
Solubility of Pilocarpine
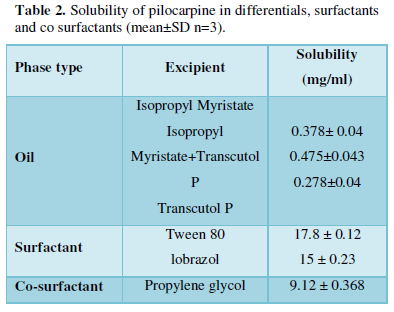
Solubility of Pilocarpine was determined in different oils (oleic acid, isopropyl myristate, Transcutol P), surfactants (Labrasol, Tween 80) and co-surfactant (Propylene glycol) by dissolving an excess amount of Pilocarpine in 3ml of oil, and other components using a stirrer at 37 ± 0.50C for 72 h. The equilibrated samples were then centrifuged at 10000rpm for 30 min to remove the undissolved drug. The solubility of Pilocarpine was determined by analyzing the filtrate spectrophotometrically using a nano-specterophotometer (Biochrom WPA Bioware) after dilution with phosphate buffer pH=7.4 at 213 nm [15].
Pseudo-ternary phase diagram construction
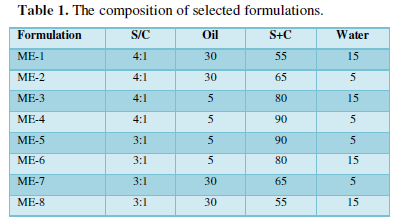
To investigate concentration range of components for the existing boundary of MEs, pseudo-ternary phase diagrams were constructed using the water titration method. Two phase diagrams were prepared with the 3:1 and 4:1 weight ratio of (Labrasol+Tween 80) / Propylene glycol respectively. Oil phase (Isopropyl miry state: Transcotul P) and the surfactant mixture were mixed at the specific percentage. These mixtures were diluted drop wise with double distilled water, under moderate agitation. When a clear liquid was observed, the samples were identified as MEs [16]. Full factorial design was used concerning with 3 variables at 2 levels for formulations. The major independent variables included surfactant/cosurfactant ratio (S/C), percentage of oil (% oil) and water percentage (%w). Eight different formulations with low and high values of oil (5% and 30%), water (5% and 15%), and S/C mixture ratio (3:1and 4:1) were prepared for formulating the MEs.
Preparation of pilocarpine MEs
There are three main considerations to choose the best ME formulation, including selection of oil phase, surfactant and co-surfactant [17]. Various MEs were selected from the pseudo ternary phase diagram with 3:1, and 4:1 weight ratio of Labra sol /Tween 80/Propylene glycol. Pilocarpine (0.5%) was added to oil phase, after adding S/C (surfactant/cosurfactant) mixture, an appropriate amount of double distilled water was added to the mixture drop by drop and the MEs containing Pilocarpine were obtained by stirring the mixtures at ambient temperature. The components of formulations are shown in Table 1.
Characterization of MEs
Droplet size measurement
The average droplet sizes of MEs were measured at 250C with a Scatter Scope 1 Quidix apparatus.
Viscosity determination
A Brookfield rheometer was used for measuring the viscosities of MEs at 25°C.
PH determination
The pH of the ME samples was determined with a Mettler (Mettler Toledo, Wan, Kowloon, Hong Kong) pH meter at 25° C without dilution [16].

Stability experiments
The stability experiments were carried out using a centrifuge stress test and temperature stability. MEs were stored in different temperatures (4°C, 25°C, 37°C) according to the ICH protocols for three months and then assessed by monitoring time- and temperature-dependent variations of the physicochemical properties, such as phase separation, precipitation, droplet size. In addition, the ME formulations were centrifuged at 10000 rpm for 30 minutes at 25°C. After centrifugation, the instability of the ME samples was visually evaluated using the degree of phase separation [15].
Differential scanning calorimetry (DSC)
A Mettler-Toledo DSC was made use of to carry out the thermal analysis of MEs. For this purpose, a small amount of specimen was weighed in aluminum foam, and then sealed so that they did not exchange any material with the outside environment. DSC was performed in cooling manner. In cooling method, the samples were exposed to a temperature of +30° C to -50° C and a scan speed of 5°C/min. The rate of decrease in temperature was 5°C/min in this method [18].
Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) was used to characterize microstructure of emulsions. SEM images of samples were taken by LED 1455VP, Germany [18].
Release study
Franz diffusion cells (contact area of 3.4618 cm2) with a cellulose membrane were utilized to determine the release rate of Pilocarpine from different ME formulations. The cellulose membrane was first hydrated in distilled water at 25°C for 24 h and then was clamped between the donor and receptor compartments of the cells. Each diffusion cell was filled with 25 ml of phosphate buffer (pH = 7.4). The receptor phase was constantly stirred by externally driven magnetic bars at 600 rpm throughout the experiment. Pilocarpine ME (3g) was accurately weighted and placed in donor compartment. At 0.5, 1, 2, 3, 4, 5, 6, 7 and 8hour time intervals, 2 ml sample was removed from buffer for spectrophotometric determination and replaced immediately with an equal volume of fresh receptor solution. Samples were analyzed by UV visible spectrophotometer (BioWaveII, WPA) at 213 nm. The results were plotted as cumulative released drug percent versus time.
The corneal Permeability studies
The corneal permeability experiments were performed using fabricated Franz diffusion cells with a contact area of approximately 0.348 cm2. The excised rabbit corneas were kept between the donor and receptor chambers so that they could face the receptor section without suffering any damage. The receiving phase consisted of 10 mL of simulated tear fluid (pH = 7.4), which was thermo stated at 37±0.5°C and magnetically stirred at 200 rpm throughout the experiment. Then, 0.5 g Pilocarpine ME samples (containing 0.5% of the drug) were accurately weighed and placed on the surfaces of corneas. The corneal permeability studies were used under non-occlusive conditions to allow air to permeate the corneal tissues. At predetermined interval times (0.5, 1, 2, 3, 4, 5, and 6 h), a 1 mL sample was withdrawn from the receptor medium for UV spectrophotometric analysis at 231 nm. The extracted samples were immediately replaced with an equivalent volume of fresh simulated tear fluid until to keep sink condition in the receptor chamber during corneal permeability studies. The free drug MEs were used as blanks. The solution of Pilocarpine was used as positive control in this work. Finally, the results were plotted in the form of cumulative permeated drug percent versus time.
Calculation of permeation parameters
The permeation parameters of the corneas were determined using obtained corneal permeation data, including flux (cornea permeation rate at steady state, Jss), corneal permeability coefficient (P), lag time (Tlag), and apparent diffusivity coefficient (Dapp). Flux was determined from the linear section of the slope of the permeability curve. The permeability coefficient (P, cm/s) was presented based on the equation P = Jss/CV, where Jss and Cv define the permeation rate at steady state and initial Pilocarpine concentration in the donor chamber, respectively. Meanwhile, the apparent diffusivity coefficient was determined using the equation Dapp = h2/6 Tlag. The lag time parameter was calculated by extrapolating the line of steady-state onto the time axis. The enhancement ratio (ER) was determined to explain the relative improvement of the permeation parameters in Pilocarpine ME samples concerning the solution of Pilocarpine (1% drug) parameters. ER was measured by dividing the amount of permeation in the ME formulation by the amount of permeation in the solution of Pilocarpine.
RESULTS AND DISCUSSION
The maximum solubility of Pilocarpine was found in isopropyl myristate: Transcutul P (10:1) (0.378±0.04). In addition, the highest drug solubility of Pilocarpine in surfactants were found in Labrasol (15 ± 0.23), and Tween 80 (17.8 ± 0.12). Based on the solubility studies of Pilocarpine in oil, surfactant and co-surfactant and the preformulation studies it was found IPM-Transcutul P, Labrasol, Tween 80 and propylene glycol were the most appropriate combination for preparation of the ME (Table 2). In order to develop ME formulations the optimum oil was selected by determining the concentration of Pilocarpine that would be dissolved. For the determination of the ME zone based on the different S/C ratios two phase, diagrams were drawn (Figure 1).
To enhance drug solubility, stability and permeation properties, the mutual use of ME system and cyclodextrins has been suggested previously [19].
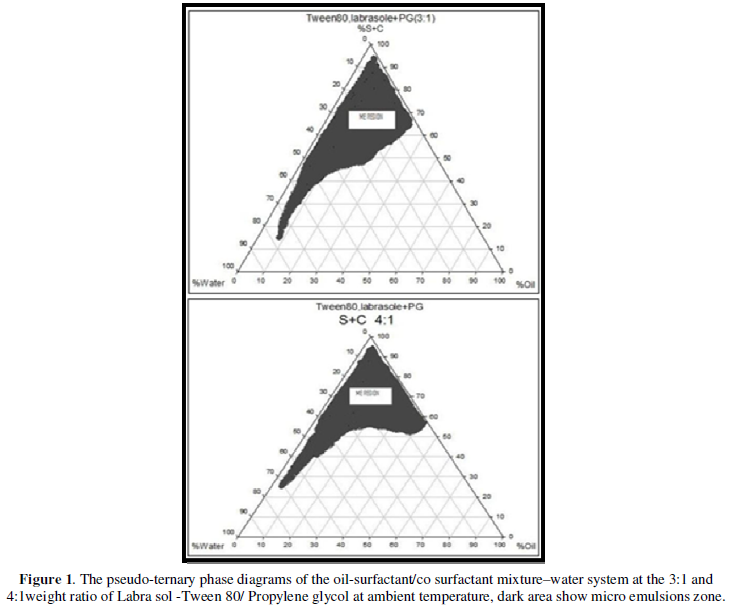


The phase diagrams clearly indicated that ME formulation region increased with an increase in the weight ratio of surfactant/co surfactant [17].
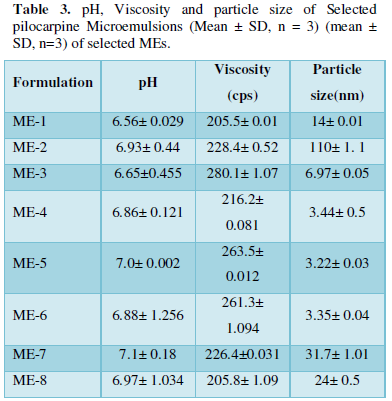
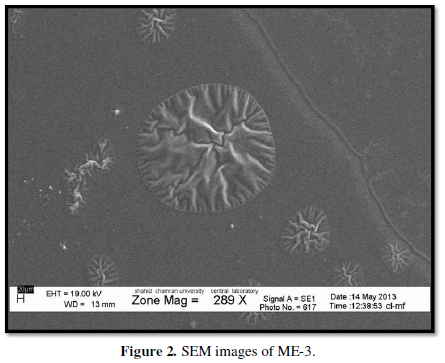
The Viscosity, pH and mean Particle size of Pilocarpine MEs are illustrated in Table 3. ME formulations had particle size in the range of 3.22-110nm. The ME- 5 formulation had the lowest particle size 3.22 nm. The SEM of ME-3 is showed in (Figure 2). There was no significant difference observed in average particle size with S+C amount and oil and water percentages.
The cumulative amount of Pilocarpine that had permeated through the cellulose membrane (Release %) was plotted as a function of time (hours). In this study, ME-1 and ME-6 showed the highest and lowest accumulative release percent, respectively. Table 4 shows release percent and kinetic of release of Pilocarpine ME formulations. In vitro release studies with an artificial hydrophobic membrane can provide information about the diffusion of a drug, which depends on the physicochemical properties of components and the MEs structure [9]. It seems that release percent enhanced with decrease in the S+C percent and increase in S/C ratio. The effectiveness of a variety of Nano careers including Nano micelles, noisome and Nano dispersions in permeation improvement and targeting of drugs have been reported previously [13]. Also, it has been shown that in comparison to a standard solution, formulation of Pilocarpine in ME as vehicle significantly retards the drug release [20]. The releases which may be due to profiles of MEs were calculated by fitting the experimental data to equations describing different kinetic models. Also, the release profile and drug retardation may be affected by the type of micro emulsion [21]. Linear regression analyses were made for zero-order (Mt/M0 = kt), first order (ln (M0–Mt) = kt), Higuchi (Mt/M0 = (kt)1/2), Log Wagner, Linear Wagner, Weibul, second root of mass, Three-Second’s root of mass, and Pepas kinetics. The slowest release was observed for ME-6 with Pepas kinetic and the highest release was observed for ME-1 with Pepas kinetic. The highest release amount percentage was observed for ME-1.



The visual inspection test was performed for 3 months by drawing ME sample at weekly interval for the first month and monthly interval for the subsequent months. The visual observation showed no evidence of phase separation or any flocculation or precipitation. These samples also revealed no sign of phase separation under stress when subjected to centrifugation at 10000 rpm for 30 min. The centrifugation tests revealed that MEs were remained homogenous without any phase separation throughout the test indicates good physical stability of preparations [17].
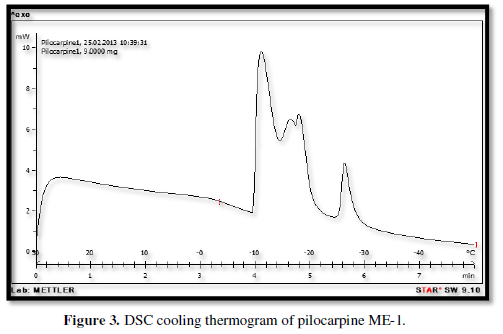
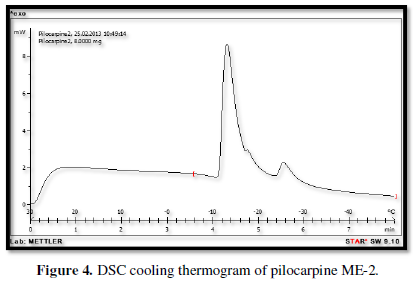
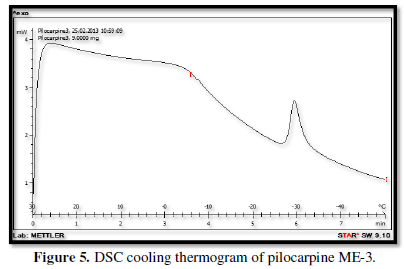
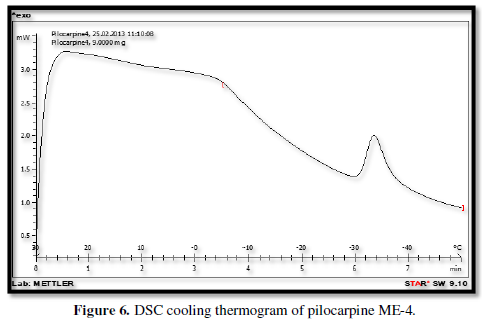
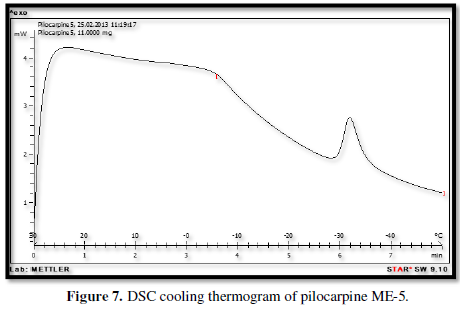
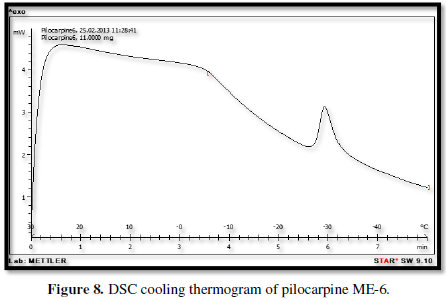
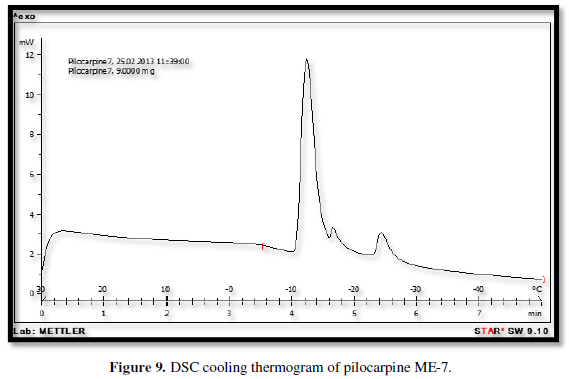
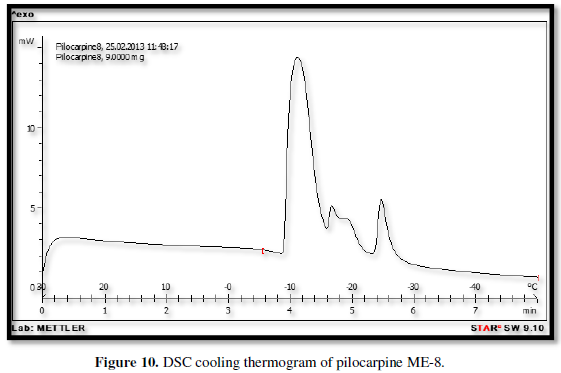
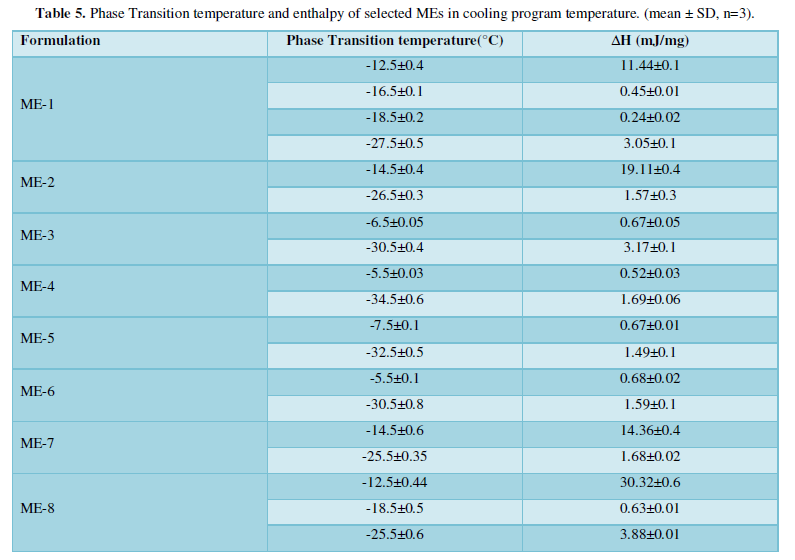
Figures 3-10 represents DSC cooling thermo grams of Pilocarpine MEs. Table 5 showed the results of thermo gram analysis of MEs in cooling program temperature. DSC results indicate important information about water state in MEs. When water is mixed in to a ME system it can be either bound (interfacial) or free (bulk) water depending of its state in the system. In cooling curves of the sample ME-1,
The visual inspection test was performed for 3 months by drawing ME sample at weekly interval for the first month and monthly interval for the subsequent months. The visual observation showed no evidence of phase separation or any flocculation or precipitation. These samples also revealed no sign of phase separation under stress when subjected to centrifugation at 10000 rpm for 30 min. The centrifugation tests revealed that MEs were remained homogenous without any phase separation throughout the test indicates good physical stability of preparations [17].
Figures 3-10 represents DSC cooling thermo grams of Pilocarpine MEs. Table 5 showed the results of thermo gram analysis of MEs in cooling program temperature. DSC results indicate important information about water state in MEs. When water is mixed in to a ME system it can be either bound (interfacial) or free (bulk) water depending of its state in the system. In cooling curves of the sample ME-1, DSC thermo grams showed one exothermic peak at around -10 to -12°C that indicate the freezing of bound water in this formulation and a peak at around -16 to -18°C that indicate the freezing of oil phase in this formulation and a peak at around -26°C that indicate the bound water interacts with surfactant. In ME-2 implies two exothermic peaks at around -14°C (bound water) and a peak around -25 to -26°C (bound water with surfactant). Increase of ∆H of first and last peak with ME-1 was because of increase in water phase in this formulation. In cooling curves of ME-3, DSC thermo grams showed two exothermic peaks at -6°C (free water) and -30°C which indicates bound water with surfactant. In cooling curves of ME-4, DSC thermo grams showed two exothermic peaks at -5°C (free water) and -34°C which indicates bound water with surfactant. DSC thermo grams of ME-5 showed two exothermic peaks at 7°C (bulk water) and -32°C (bound water with surfactant) which means water was bounded to surfactant strongly. DSC thermo grams of ME-6 showed two exothermic peaks at -5°C (bulk water) and -30°C (bound water with surfactant). In cooling curves of the sample ME-7, DSC thermo grams showed one exothermic peak at around 14°C that indicate the freezing of bound water in this formulation and a peak at around -25°C that indicate the bound water interact with surfactant. DSC thermo grams of ME-8 showed one exothermic peak at around -120C that indicate the freezing of bound water in this formulation and a peak at around -18°C that indicate the freezing of oil phase in this formulation and a peak at around -25°C that indicate the bound water interacts with surfactant. The obtained results of DSC experiment give useful information about water state and chemical and physical alterations that affects exothermic or endothermic processes in heat capacity. DSC studies were employed for aqueous mixed behavior of MEs and differentiation between bound (interfacial) water and bulk (free) [22-24].

Pilocarpine belongs to Class-2 of BCS classification system. Because of its low solubility and high permeability, it exhibits shorter half-life. Hence, the patients who are advised for Pilocarpine medications are recommended for repeated dose administrations.
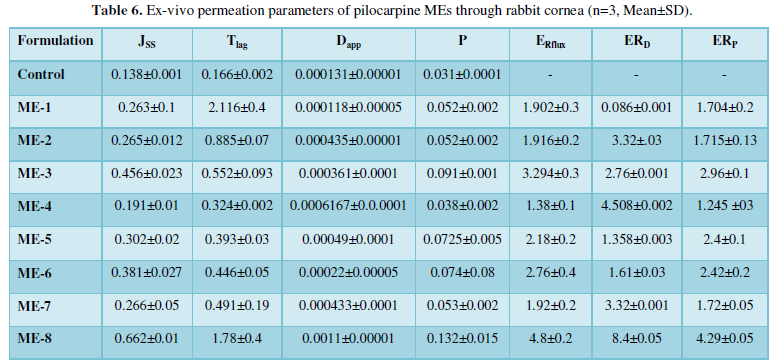
All of MEs increased in Jss parameters and %P6h. The amount of Jss in the ME-8 formulation was 0.665 mg/ cm2h1, which is 4.8 times higher than that of control. The Dap of Pilocarpine from ME-8 was 0.0011 cm2 /h, which is 8.4 times greater than that of control. Table 6 shows Ex-vivo permeation parameters of pilocarpine MEs through rabbit cornea. Therefore, it was concluded that ME carriers could be controlled release behavior and increase flux and apparent diffusivity coefficient (Dapp) in all of the formulations.

Surfactant (Tween 80) phases could enhance the permeation parameters and drug permeated percentage of Pilocarpine MEs. Alternatively, when permeability enhancement is desired, surfactant materials alter membrane properties by negating the protective properties of tear film and mucin, thus disordering the entirety of the epithelial layer by loosening tight junctions or epithelial cell membrane modification [5,25]. The presence of Transcutol P in pilocarpine MEs can increase the corneal permeation of drugs by altering the function of corneal barrier, the effect that is in accordance with Gorantla reports [13]. Kuar and Smitha [26] have indicated that an outer cell membrane composed of a phospholipid bilayer and a lipid membrane containing protein molecules are surrounded corneal epithelial cells. The results of another study indicated that formulation of ribavirin as ME significantly enhances the corneal permeability of the drug as indicated by the higher permeation rate, flux and permeability coefficients [27-30]. TP is a surfactant substance, and the micelles it produces in the epithelial lipid bilayer might affect drug corneal permeation. Specifically, the micelles produced by TP can remove phospholipids from epithelial cell membranes, thereby increasing the trans corneal passage of drugs. High concentrations of TP improve the hydrophilic molecule permeation due to losing the epithelium structure membrane. However, TP might also hinder lipophilic molecule movement by creating a hydration barrier [15-18]. It has been shown that the residence time and therapeutic effect of drugs may be enhanced using a cationic ME system and chitosan-coated cationic microemulsion [4].








CONCLUSION
This study established that physicochemical properties and in vitro release and ex vivo permeability was dependent upon the contents of S+C, oil and water in formulations. Pseudo-ternary Phase diagrams indicated more width ME region with a rise in S/C ratio. By decreasing in S/C ratio and oil percent and increasing in water percent the higher in vitro percentage release was obtained [16]. ME-8 may be preferable for ocular Pilocarpine formulation although the serious work still is needed to be carried out to reveal the mechanisms of drug delivery into the eye.
ACKNOWLEDGMENTS
This paper is extracted from Pharm.D. thesis (Shahin, F) and financial support was provided by Ahvaz Jundishapur University of Medical Sciences. The authors are very thankful to Faratin company executive manager (Taheri, M, Iran) for providing gratis sample of Transcutul P, Labrasol from Gattefosse (France) and also Gattefosse Company (France). The authors are very thankful to Sinadaroo Company for providing Pilocarpine powder.
- Podder SK, Moy KC, Lee VH (1992) Improving the Safety of Topically Applied Timolol in the Pigmented Rabbit through Manipulation of Formulation Composition. Exp Eye Res 54: 747-757.
- Begum G, Leigh T, Courtie E, Moakes R, Butt G, et al. (2020) Rapid assessment of ocular drug delivery in a novel ex vivo corneal model. Sci Rep 10:
- Lynch C, Kondiah PPD, Choonara YE, du Toit LC, Ally N, et al. (2019) Advances in Biodegradable Nano-Sized Polymer-Based Ocular Drug Delivery. Polymers 11: 1371.
- Zamboulis A, Nanaki S, Michailidou G, Koumentakou I, Lazaridou M, et al. (2020) Chitosan and its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments. Polymers 12(7): 1519.
- Moiseev RV, Morrison PWJ, Steele F, Khutoryanskiy VV (2019) Penetration Enhancers in Ocular Drug Delivery. Pharmaceutics 11(7): 321.
- Kompella UB, Domb A, Urtti A, Jayagopal A, Wilson CG, et al. (2019) Ocular drug delivery: Nanotechnology, physical and chemical methods, vitreous drug binding, and aging eye. J Ocul Pharmacol Ther 35(8): 457-465.
- Hsiue GH, Guu JA, Cheng CC (2001) Poly (2-hydroxyethyl methacrylate) lm as a drug delivery system for pilocarpine. Biomaterials 22: 1763-1769.
- El Hoffy NM, Azim EAA, Hathout RM, Fouly MA, Elkheshen SA (2020) Glaucoma: Management and Future Perspectives for Nanotechnology-Based Treatment Modalities. Eur J Pharm Sci 158: 105648.
- Shah J, Nair AB, Jacob S, Patel RK, Shah H, et al. (2019) Nanoemulsion Based Vehicle for Effective Ocular Delivery of Moxifloxacin Using Experimental Design and Pharmacokinetic Study in Rabbits. Pharmaceutics 11(5): 230.
- Okur NU, Çağlar ES, Siafaka PI (2020) Novel Ocular Drug Delivery Systems: An Update on micro emulsions. J Ocul Pharmacol Ther 36(6): 342-354.
- Haβe A, Keipert S (1997) Development and characterization of microemulsions for ocular application. Eur J Pharm Biopharm 43: 179-183.
- Kour J, Kumari N, Sapra B (2020) Ocular Prodrugs: Attributes and Challenges. Asian J Pharm Sci 15(6): 1-17.
- Gorantla S, Rapalli VK, Waghule T, Singh PP, Dubey SK, et al. (2020) Nanocarriers for ocular drug delivery: Current status and translational opportunity. RSC Adv 10: 27835-27855.
- Tartaro G, Mateos H, Schirone D, Angelico R, Palazzo G (2020) Microemulsion Microstructure(s): A Tutorial Review. Nanomaterials 10: 1657.
- Salimi A, Behrouzifar M (2020) Ocular delivery of ketorolac tromethamine using microemulsion as a vehicle: Design, evaluation, and trans corneal permeation. J Res Pharm 24(6): 1-10.
- Soleymani SM, Salimi A (2019) Enhancement of Dermal Delivery of Finasteride Using Microemulsion Systems. Adv Pharm Bull 9(4): 584-592.
- Zhao J, Jiang K, Chen Y, Chen J, Zheng J, et al. (2020) Preparation and Characterization of Microemulsions Based on Antarctic Krill Oil. Drugs 18: 492.
- Salimi A, Zadeh BSM, Moghimipour E (2013) Preparation and Characterization of Cyanocobalamin (Vit B12) Microemulsion Properties and Structure for Topical and Transdermal Application. Iran J Basic Med Sci 16(7): 865-872.
- Paola M (2020) Advantages of the combined use of cyclodextrins and nanocarriers in drug delivery: A review. Int J Pharm 579: 119181.
- Moghimipour E, Salimi A, Saadati Rad A (2013) A Microemulsion System for Controlled Corneal Delivery of Timolol. Int. Res J Pharm. App Sci 3(4): 32-39.
- Ibrahim MM, Maria DN, Di Wang X, Simpson RN, Hollingsworth TJ, et al. (2020) Enhanced Corneal Penetration of a Poorly Permeable Drug Using Bio Adhesive Multiple Micro Emulsion Technology. Pharmaceutics 12(8): 704.
- Hirai M, Kawai-Hirai R, Sanada M, Iwase H, Mitsuya S (1999) Characteristics of AOT Micro emulsion Structure Depending on Apolar Solvents. J Phys Chem B 103(44): 9658-9662.
- Garti N, Aserin A, Tiunova I, Fanun M (2000) A DSC study of water behavior in water-in-oil microemulsions stabilized by sucrose esters and butanol. Colloids Surf Physico chem Eng Aspects 170(1): 1-18.
- Moghimipour E, Salimi1A, Changizi S (2017) Preparation and Microstructural Characterization of Griseofulvin Microemulsions Using Different Experimental Methods: SAXS and DSC. Adv Pharm Bull 7(2): 281-289.
- Notman R, Noro MG, Anwar J (2007) Interaction of Oleic Acid with Dipalmitoyl phosphatidylcholine (DPPC) Bilayers Simulated by Molecular Dynamics J Phys Chem B 111: 12748-12755.
- Kaur IP, Smitha R (2002) Penetration enhancers and ocular bio adhesives: Two new avenues for ophthalmic drug delivery. Drug Dev Ind Pharm 28(4): 353-369.
- Tang‐Liu DD, Richman JB, Weinkam RJ, Takruri H (1994) Effects of four penetration enhancers on corneal permeability of drugs in vitro. J Pharm Sci 83(1): 85-90.
- Makhmalzadeha BS, Salimi A, Niroomand A (2018) Loratadine-Loaded Thermoresponsive Hydrogel: Characterization and Ex-vivo Rabbit Cornea Permeability Iran J Pharm Res 17(2): 460-469.
- Moghimipour E, Salimi A, Yousefvand T (2017) Preparation and Evaluation of Celecoxib Nanoemulsion for Ocular Drug Delivery. Asian J Pharm 11 (3): S543-S550.
- Salimi A, Panahi-Bazaz MR, Panahi-Bazaz E (2017) A Novel Microemulsion System for Ocular Delivery of Azithromycin: Design, Characterization and Ex-Vivo Rabbit Corneal Permeability. Jundishapur J Nat Pharm Prod 12(2): e13938.












