Review Article
Formulation and characterization of Neomycin Sulfate Loaded Ethosomes for Transdermal Delivery
5013
Views & Citations4013
Likes & Shares
Ethosomes are widely used to promote transdermal permeation of both lipophilic and hydrophilic drugs. In this work, Neomycin sulfate loaded ethosomes were prepared and characterised. Vesicle morphology revealed the presence of nanosized spherical or near spherical –shaped vesicular structures. Particle size analysis by Malvern zeta size was observed in nanometer range (500-2000 nm), high negative zeta potential values were also reported and polydispersity index within range 0.281-0.383. Further a high entrapment efficiency from 55.63% to 85.84% was observed. The formulated ethosomes showed enhanced invitro drug release results of 65-80%. The Ethosomal formulae showed lower MIC values (2.44 μg/ml) compared to Neosporin® Antibiotic ointment yielding at (4.88 ug/ml) and finally and high stability stamina after 45 days.
The rationale behind this work was that a formulated permeation enhancing carrier could facilitate the transport of antibacterial molecules through the two biological barriers: stratum corneum of the skin and bacterial membrane/cell wall. Characterization results of formulated ethosomes particle size, zeta potential, entrapment efficiency, in vitro drug release, microbiological assay and stability studies fully satisfy the rationale behind this work.
It can be concluded that the prepared ethosomes can act as an ideal vesicular carrier for transdermal delivery as they are non-staining and easy to prepare.
Keywords: Ethosomes, Ethanol, Phospholipid, Vesicular carriers, Transdermal, Neomycin sulfate, Skin permeation
ABBREVIATIONS
SEM: Scanning electron microscope; PG: Propylene glycol; SPC: Soya phosphahtidyl choline; EE: Entrapment Efficiency
INTRODUCTION
Transdermal delivery has potential advantages over other routes of administration. It could reduce first-pass metabolism associated with oral delivery and is less painful than injections. However, the outermost layer of the skin, the stratum corneum limits passive diffusion to small lipophilic molecules. Therefore, effective methods are needed to safely permeabilize the skin so that ionic and larger molecules may be delivered trans dermally [1,2].
Microbial infections of the skin and underlying tissues are among the most frequent conditions encountered in acute ambulatory care [3,4]. Staphylococcus aureus is the causative agent for the majority of primary skin infections [1,5,6]. Skin infections (such as cellulitis, erysipelas and trauma- and wound-related infections), especially when associated with co-morbid conditions and/or bacteraemia, may lead to severe complications and hospital admission. In some cases they can be a cause of extensive morbidity and mortality. Owing to poor permeation of most antibiotic agents into the deep skin layers and subdermal tissues from conventional topical preparations like ointments etc, deep skin infections generally do not respond to external therapy with antibiotics [1,3,4].
These conditions are therefore usually treated by oral or parenteral antibiotics with high doses of amino glycosides, macrolides, b-lactams or other antibacterial agents [2-4].
During the course of systemic antibiotic treatment there is a significant incidence of side-effects, allergic reactions and patient inconvenience, all of which may highly influence the treatment efficiency. Ethosomes can circumvent this problem by delivering sufficient quantity of antibiotic into deeper layers of skin [5,6,7]. Ethosomes were discovered and developed by research [8]. Specially designed vesicles able to allow transdermal delivery are non-invasive delivery carriers that allow the drug to reach the deeper skin layers and possibly the systemic circulation. They are soft, malleable vesicles, which actively, enhance the delivery of entrapped drugs. Ethosomes are composed mainly of phospholipids, high concentration of ethanol (20-45%) and water. Due to ethosomal high malleability, it may permeate through human skin. It has been proposed that the high concentration of ethanol fluidizes the ethosomal lipids and stratum corneum lipids, thus allowing the soft malleable ethosomes to penetrate the skin layers. The size range of ethosomes varies from tens of nanometers to micrometers [5-9].
Hydrophilic drugs are entrapped in the aqueous core inside lipid carrier while lipophilic drugs are retained in the nonpolar chain. Encapsulation of hydrophilic drug depends on captured volume i.e. volume of water encapsulated per mole lipid whereas entrapment of lipo-philic drug depends on bilayers present in the system [10-12]. Being a hydrophilic drug neomycin sulphate is expected to entrap in the aqueous core inside lipid carrier.
The present study focuses on the preparation and evaluation of neomycin sulfate loaded ethosomes.
MATERIAL & METHODS
Materials
Neomycin sulfate, Propylene glycol, Trioton X-100, Ethanol 99%, Acetic acid Glacial & Sodium Acetate were purchased from Avarice Lab Pvt. Ltd. Lecithin and Dialysis membrane were purchased from Himedia Mumbai.
All the reagents used were of analytical grade.
Preparation of ethosomes
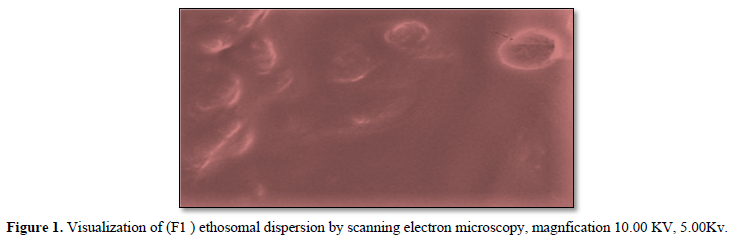
Derivatised neomycin sulfateethosomes was prepared by hot method introduced by Touitoui et al 1997. The ethosomal system of neomycin sulfate was comprised of 0.5 % drug, 1–3 % w/v soya phospholipids (SPC), 40–45 %, v/v of ethanol, propylene glycol 10%v/v and water up to 100% v/v phospholipids were dispersed in water by heating in a water bath (40ºC) until a colloidal suspension was obtained. In a separate vessel, ethanol and propylene glycol were mixed and heated to 40ºC and once it reached the desired temperature the organic phase is added to the aqueous one. The drug was dissolved in the aqueous phase. The mixture was stirred at a speed of 700–2,000 rpm, using a magnetic stirrer for 30-45 min to obtain the required ethosomal suspension. The formulations were stored under refrigeration at 4°C [5-6,13,14] (Table 1).
RESULTS
Characterization of ethosomes vesicle morphology, particle size, zeta potential, polydispersity, entrapment efficiency, invitro permeation, antimicrobial activity.
Vesicle morphology
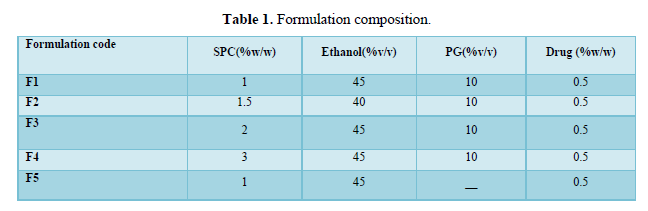
Scanning electron microscopy (SEM) was used to characterize the surface morphology of ethosomal formulation, In SEM image analysis nanosized spherical or near spherical –shaped vesicular structures are clearly observed (Figure 1).


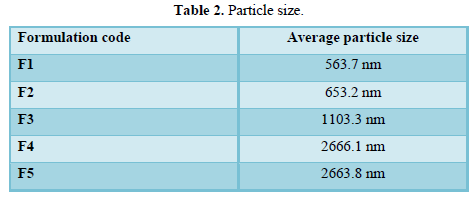
The particle size was measured using a Malvern Zeta sizer 300 HSA (Malvern Instruments, UK) at 25˚C.The samples were diluted with water solution. Zeta potential measurements were determined by analyzing the formulation for 60 s using a Malvern Zeta sizer 300 HSA (Malvern Instruments, UK) at 25˚C as given in Table 2 (Zeta potential and polydispersity measurement).
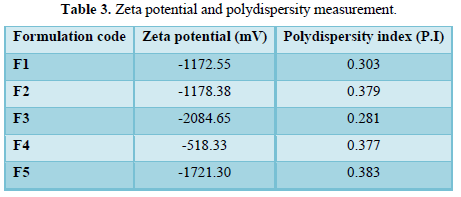
The PDI values of ethosomes formulations lie in the range 0.281- 0.383. With negative zeta potential values as in Table 3.


Entrapment efficiency
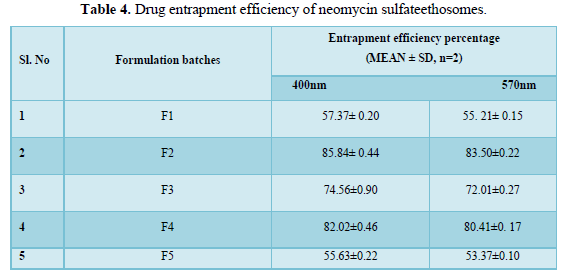
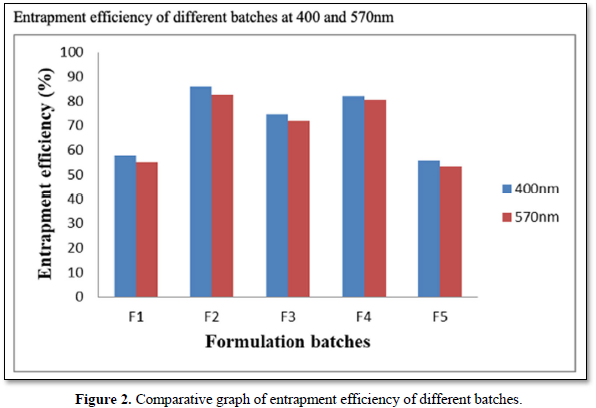
Entrapment efficiency (EE) of the ethosomal vesicular systems was determined by ultracentrifugation method. The sediment was lysed with 0.5% w/w (1ml) Triton X-100) and assayed for the drug content by using a Shimadzu 1800 (Shimadzu, Japan) double beam UV spectrophotometer at λmax of 400nm and 500nm where neomycin sulfate showed absorption peaks. The entrapment efficiency of neomycin sulfate in ethosomes was ranging from from 55.63% to 85.84%. F2 showed the highest entrapment efficiency at 85.84% compared to all other formulations (Table 4 and Figure 2).


In vitro permeation studies using cellophane membrane
Ethosomal formulations (1 ml) were placed in the cellophane membrane dialysis tubing (molecular weight cut off 12,000, Himedia Labs, India), both the ends of which were sealed and suspended in a beaker having 100 ml acetate buffer pH 5.5 at 37±1°C. The buffer in the beaker was stirred with a glass rod at 45 min interval and samples were collected at 2, 4, 6, 8, 10, 12, 18 and 24 h time intervals, replaced with equal quantity of fresh buffer and analysed for the amount of drug released using Shimadzu 1800 (Shimadzu, Japan) double beam UV spectrophotometer.
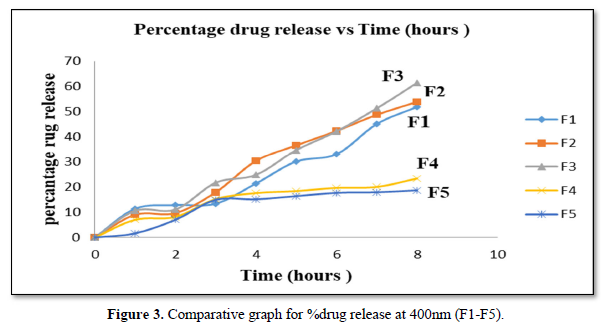
In vitro permeation studies at 400nm (F1-F5)
It can be observed that F1 has the smallest particle size showed the highest initial drug release after the first hour at 11.37% and the lowest permeation activity at 1.63% from F5 with the highest particle size (Figure 3).
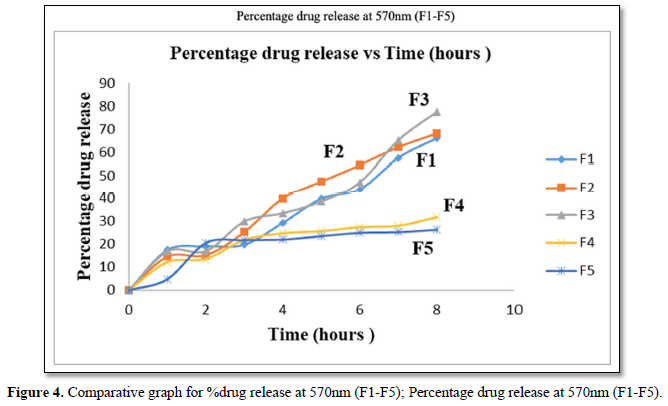
It can be noted that drug release pattern observed was similar to that reported at 400nm in (Figure 4).
Antimicrobial activity
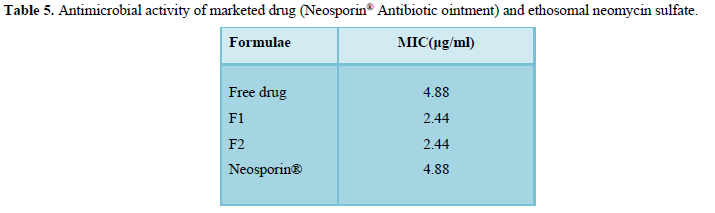
It can be observed from that ethosomal formulations F1 and F2 exhibited a lower minimum inhibitory concentration compared to marketed neomycin sulfate ointment (Neosporin) and the free drug solution of neomycin sulfate (pure drug) (Table 5).
Stability studies after 45 days storage
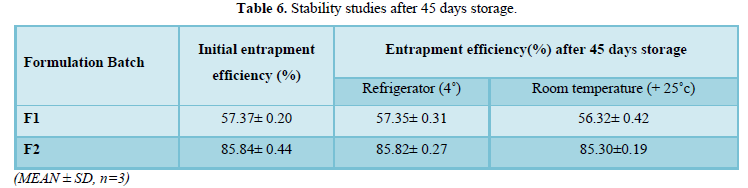
The formulated ethosomes showed greater stability under all storage conditions (Table 6).


DISCUSSION
Vesicle morphology
Visualization by scanning electron microscopy showed that ethosomes have a lamellar vesicular structure and this confirms the existence of vesicular structure at high ethanol concetrations. These results are in accordance with those reported by many scientific researchers in literature [6,15,16].
Particle size
The size of the vesicles was increased with increasing phospholipids concentration. Many studies have shown that increasing phospholipid concentration will increase vesicular size slightly or moderately. This could be attributed to the fact that SPC confers greater rigidity to the vesicle consequently reducing vesicle fusion. As reported by several researchers [5,6,17-19].
F5 with 1% SPC and no PG however, showed a greater particle size this can be explained from the absence of propylene glycol in its composition. Scientific research reports have showed that incorporation of PG resulted in reduction in vesicle size. The opposite is true for formulations without propylene glycol due to the absence of interaction between PG with the phospholipid bilayer where the enhancer interpenetrates the SPC hydrocarbon chain allowing more bilayer flexibility and hence higher vesicle size [20-23].
Zeta potential
Zeta potential is an important parameter that affects the aggregation of vesicles and depicts the physical stability of vesicular systems [24]. Ethosomal formulae exhibited high negative charge ranging from (-518.33 mv to -1721.30 mv). Which indicated good stability as the formulated ethosomes do not aggregate rapidly.
The high zeta potential values are due to the SPC, high ethanol concentration and PG. Ethanol provides a net negative surface charge, SPC provides a greater rigidity to the layers and reduced likelihood of vesicles fusion and carry a negative charge likely due to ionization of phosphate group resulting in a high negative charge [17]. Further studies have shown that PG induces a transition in the charge of the vesicles to more negative values [5-7, 25-26].
Polydispersity index (PDI)
PDI value helps in determining whether the suspension is mono-dispersed or poly-dispersed. The PDI value lies between 0.00-0.5 indicating that formulated suspension was homogeneous ethosomal vesicular system [6-9, 27,28].
Entrapment efficiency percentage
The relatively high entrapment efficiency of neomycin sulfate (55.66% to 85.84%) in ethosomes may be explained by the multilamellarity of ethosome vesicles and the presence high ethanol concentration this may be related to co-solvent effect of ethanol allowing more drug entrapment into the aqueous core of the vesicles [12-15,29].
Increase in SPC concentration showed increase in drug entrapment but only upto1.5% (w/w). Many authors have acknowledged that increasing phospholipid concentration will increase entrapment efficiency significantly only until a certain concentration to which further increment in phospholipid concentration will have no effect on entrapment efficiency. This result also suggests that 2% phospholipid is optimal concentration along with 40% ethanol concentration for better entrapment efficiency [11-15,30,31] F5 exhibited lowest entrapment efficiency compared to all other formulations with PG. indicating that the presence of PG plays a role in enhancing drug entrapment; studies have shown that the presence of ethanol and PG in ethosomes provides better solubility and improved drug distribution throughout the vesicle [22-26, 30-32].
Permeability studies
When SPC concentration increased, there was increase in drug permeation but when SPC concentration exceeded 2% w/w there was a subsequent decrease in permeation of neomycin sulfate from ethosomes. These results indicate that if the percentage of SPC exceeded 2%w/w, bilayer deformability of vesicles occurred with subsequent reduction in permeation drug from ethosomes. This is also similarly reported by other studies [5-9,27,28].
ForulationsF1 to F4 containing showed an increase in drug permeability compared to F5 expalining the permeation enhancing effects of PG by increasing permeability of vesicle through biological membrane due to synergistic effect with ethanol on bilayer of the vesicles. [12-16,33,34].
Antimicrobial activity of marketed drug (Neosporin® Antibiotic ointment) and ethosomalneomycinsulfate
The rationale behind this work was that a permeation enhancing carrier could facilitate the transport of antibacterial molecules through the two biological barriers: stratum corneum of the skin and bacterial membrane/cell wall.
Ethosomal formulae showed lower MIC values (2.44μg/ml) compared to Neosporin® Antibiotic ointment yielding at (4.88ug/ml). Ethosomal formulae also reduced the standard working concentrations for neomycin sulfate (free drug) for S. aureus from 4 to 20microg/ml.
Such enhancement could be explained on the basis of ethosomal formulation composition of ethanol and propylene glycol causing a synergistic permeation enhancement effect through the bacterial membrane compared to neomycin ointment. These results indicate that ethosomes are more efficient carriers for neomycin sulfate delivery for eradication of skin bacterial infections like common staphylococcal infections [5-9,12-15].
Stability studies after 45 days storage
It was observed that on storing the ethosome formulations for 45 days there was no significant decrease in the drug content and presented no changes in visual appearance or aggregation. These results prove the stabilizing effect of higher zeta potential provided by the formulation composition [33-35]. However, the encapsulated drug tends to leak out from the lipid bilayer structure during storage hence the slight observed changes [5-9, 15-18,30-36].
CONCLUSION
The rationale behind this work was that a formulated permeation enhancing carrier could facilitate the transport of antibacterial molecules through the two biological barriers: stratum corneum of the skin and bacterial membrane/cell wall. Results from the evaluation of formulated ethosomes particle size, zeta potential, entrapment efficiency, invitro drug release, microbiological assay and stability studies fully satisfy the rationale behind this work. It can be concluded that the prepared ethosomes can act as an ideal vesicular carrier for transdermal delivery as they are non-staining and easy to prepare compared to neomycin ointment formulations.
ACKNOWLEDGEMENT
I am thankful to Mr Narinder Singh and all other faculty members for the inspiration, help and suggestions received beyond evaluation.
I do thank my research scholar friends in Department of pharmaceutics Mrs Priyanka Sharma and Ms Zeenat Parveen and Department of pharmacology Mamta Kumari, TinkuYadav. Manpreet Singh, Zafar Mehdi, Ranjinderpal Kaur for their timely help and association.
Naimi TS, LeDell KH, Sabetti KC, Borchardt SM, Boxrud DJ, et al. (2003) Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 290: 2976-2984.
-
Fung HB, Chang JY, Kuczynski S (2003) A practical guide to the treatment of complicated skin and soft tissue infections. Drugs 63: 1459-1480.
-
O’Dell ML (1998) Skin and wound infections: an overview. Am Family Physic 57: 2424-2432.
-
Nichols RL (1999) Optimal treatment of complicated skin and skin structure infections. J Antimicrob Chemother 44: 19-23.
-
Touitou E, Alkabes M, Dayan N, Eliaz M (1997) Ethosomes: Novel vesicular carriers for enhanced skin delivery. Pharm Res 14: S305-S306.
-
Godin B, Touitou E (2005) Erythromycin ethosomal systems: Physicochemical characterization and enhanced antibacterial activity. Curr Drug Deliv 2(3): 269-75.
-
Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M, et al. (2000) Ethosomes–novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J Control Release 65: 403-418.
-
Touitou E, Godin B, Weiss C (2000) Enhanced delivery of drugs into and across the skin by ethosomal carriers. Drug Dev Res 50: 406-415.
-
Godin B, Touitou E (2004) Mechanism of bacitracin permeation enhancement through the skin and cellular membranes from an ethosomal carrier. J Control Release 94: 365-379.
-
Horwitz E, Pisanty S, Czerninsky R, Helser M, Eliav E, et al. (1999) A clinical evaluation of a novel liposomal carrier for acyclovir in the topical treatment of recurrent herpes labialis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 88: 700-705.
-
Aktan B, Gundogdu C, Ucuncu H, Unal B, Sütbeyaz Y, et al. (2004) Anti-inflammatory effect of erythromycin on histamine-induced otitis media with effusion in guinea pigs. J Laryngol Otol 118: 97-101.
-
Dubey V, Mishra D, Dutta T, Nahar M, Saraf DK, et al. (2007) Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J Control Release 12: 148-154.
-
Ita K (2016) Current status of ethosomes and elastic liposomes in dermal and transdermal drug delivery. Curr Pharm Des 22(33): 5120-5126.
-
Touitou E, Godin B (2005) Enhanced skin permeation using ethosomes. In: Smith E, Maibach H. Percutaneous Penetration. Enhancers, 2nded. CRC Press, New York, NY, USA.
-
Magdy I, Amna MM, Makky A, Menna M (2012) Abdellatif formulation and characterization of ethosomes bearing vancomycin hydrochloride for transdermal delivery. Int J Pharm Pharm Sci 6(11): 190-194.
-
Zhaowu Z, Xiaoli W, Yangde Z, Nianfeng L (2009) Preparation of matrineethosome, its percutaneous permeation in vitro and anti-inflammatory activity in vivo in rats. J Liposome Res 19(2):155-162.
-
Petty MM, Priyanka S, Gautam S (2017) Ethosomes: A potential transdermal drug delivery system-indepth review. IJPPR Human 11(1): 14-45
-
Confino M, Panayot B (1990) Spectrophotometric determination of amikacin, kanamycin, neomycin and tobramycin, Mikrochim. Acta 102: 305-309.
-
Gerosa V, Melandri M (1956) Farnaco Ed. prat 12: 13-17.
-
Macchi FD, Shen FJ, Keck RG, Harris RJ (2000) Amino acid analysis, using post column in hydrin detection, in a biotechnology laboratory. Methods Mol Biol 159: 9-30.
-
Maehr H, Schaffner CP (1967) These paration and differentiation of the gentamicin complex. J Chromatogr 30: 572-578.
-
Moore S, Stein HW (1954) A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem 211(2): 907-913.
-
Shehzadi N, Hussain K, Khan MT, Salman M, Islam M, et al. (2016) Development of a validated colorimetric assay method for estimation of amikacin sulphate in bulk and injectable dosage form. J Chem Soc Pak 38: 63.
-
O'Keefe AE, Alesi FMR (1949) In: Abstracts 116th Meeting, American Chemical Society P: 63.
-
Pregnollato W, Sabino M, Lutz A (1974) Rev inst 34: 41-46.
-
West R (1965) Siegfried Ruhemann and the discovery of nihydrin. J Chem Educ 42: 386-387.
-
Burns T, Burns D, LioydGH, Walker W (1968) J Lab prac 17:448-449.
-
Verma P, Ram A (2011) Effect of different penetration enhancers on skin permeation of drug using ethosomal carrier systems. J Curr Pharm Res 5(1): 42-44.
-
Pinto JML, Rodríguez MLG, Rabasco AM (2005) Effect of cholesterol and ethanol on dermal delivery from DPPC liposomes. Int J Pharm 298(1):1-12.
-
Liu X, Liu H, Liu J, He Z, Ding C, et al. (2011) Preparation of a ligustrazineethosome patch and its evaluation in vitro and in vivo. Int J Nanomedicine 6: 241-247.
-
Li G, Fan Y, Fan C, Li X, Wang X, et al. (2012) Tacrolimus-loaded ethosomes: Physicochemical characterization and in vivo evaluation. Eur J Pharm Biopharm 82(1): 49-57.
-
Zhang JP, Wei YH, Zhou Y, Li YQ, Wu XA (2012) Ethosomes, binary ethosomes and transfer somes of terbinafine hydrochloride: a comparative study. Arch Pharm Res 35(1): 109-117.
-
Dayan N, Touitou E (2000) Carriers for skin delivery of trihexyphenidylHCl: Ethosomesvs liposomes. Biomaterials 21(18): 1879-1885.
-
Touitou E (1996) Inventor and assignee. Compositions for applying active substances to or through the skin. United States patent US 5540934 A.
-
Touitou E, Godin B, Dayan N, Weiss C, Piliponsky A, et al. (2001) Intracellular delivery mediated by an ethosomal carrier. Biomaterials 22(22): 3053-3059.
-
Touitou E, Godin B, Weiss C (2000) Enhanced delivery of drugs into and across the skin by ethosomal carriers. Int J Drug Develop Res 50: 406-415.






