Review Article
Nipah: A Deadly Zoonotic Disease
2981
Views & Citations1981
Likes & Shares
Nipah virus infection [Niv] is an emerging zoonotic infection which presents with Acute Encephalitis and Respiratory Distress Syndrome. Nipah virus is a member of Paramyxoviridae family and belongs to genus Henipavirus. The name of disease was originated from Kampung Sungai Nipah village, state of Perak, Malaysia in 1998. It shows high mortality rate and classified as Biological Safety Level 4 [BSL] in view of its features which make it a potential agent for Bioterrorism. Nipah virus first emerged in Malaysia, outbreak continue to occur in Bangladesh and India. In Malaysia-Singapore outbreak primarily occurs through pigs where as in Bangladesh and India it is related with consumption of date palm sap and human-to-human transmission. Nipah is an envelope, negative sense non segmented single stranded RNA genome with base pairs of 18252. The incubation period is reported after 45 days of exposure. Clinical presentation is from Asymptomatic to Fatal Encephalitis. Some people can also experience atypical pneumonia and severe respiratory problems. Bats are main reservoir for this virus, which can cause disease in both animals and humans. Dogs, cats, goats and horses are sensible to NiV infection. This infection is mostly risk for hospital workers and caretakers of infected persons. Rapid diagnosis and implementation of infection control measures are essential to contain outbreaks. A number of serological and molecular diagnostic techniques have been developed for diagnosis and surveillance. Intensive supportive care with treatment of symptoms is the main approach to managing the infection in people. Ribavirin and favipiravir are the only anti-virals with some activity against Nipah virus. Ribavirin reduces the mortality rate but its usage was not clear. Monoclonal antibodies and vaccines against NiV have been found effective in animals but are yet to be tested in humans. Standard precautions, good hygiene and personal protective equipment’s are helpful in prevention and control strategy.
Keywords: Nipah virus, Transmission, Diagnosis, Treatment
INTRODUCTION
Nipah viral infection is known as Niv and caused by Nipah virus. Nivisa deadly zoonotic disease and seen in both Humans and Animals. Niv causes inflammation of brain and respiratory problems. Nipahvirus is a member of Paramyxoviridae family, orders Mononegavirales and belongs to genus Henipavirus which also contains Hendra virus [Hev] and Cedar virus. Cedar virus is recently discovered and it is non-pathogenic to animals which are found in Australian bats. Niv is transmitted by Pteropus species, mainly fruit bats or flying fox. According to WHO, Pteropus species are fruit eating bats and acts as natural and animal reservoir of Niv. Niv was transmitted through animal-animal, animal-human and human-human [1]. Niv consists of negative ss RNA virus and shows more mortality rate from 40% to 100% in both animal and human because lack of efficacious treatment or vaccines against this infection, so it is called Global Threat. Neurologic and Respiratory disorders were observed in patients who are infected with this type of virus. Recent studies show that it causes more outbreaks in Kerala but most of the cases were diagnosed difficulty in many countries due to lack of equipments. Niv is used as potential agent for bioterrorism and classified as Biological Safety Level 4 [BSL] pathogen. Currently there is no prophylactic and therapeutic treatment against this serious infection. Niv is categorized as C by US National Institute of Allergy and Infectious Diseases and CDC. In Malaysia, it was first detected as Japanese encephalitis later it was identified as the new zoonotic disease.
The name of disease was originated from Kampung Sungai Nipah village, state of Perak, Malaysia in 1998 where pig farmers ill with encephalitis. Similarly, in pigs it was confused with classical swine flu. Before 1999, the zoonotic potential of Niv was unknown till Malaysia get experienced. Such an outbreak had created alarming situation in public health community globally as well as viral distribution in wide spread. Nipah virus circulates in bats and they spill over into pigs and horses. The main manifestation of this infection is Acute Encephalitis [1]. In most of patients, a syncytium forming virus was isolated from cerebrospinal fluid. Electron microscopy [EM] shows an enveloped virus with filamentous nucleocapsid. The virus showed a herringbone structure, characteristic of the family Paramyxoviridae on negative staining. The major target was the central nervous system in preliminary autopsy findings. Niv viruses, infect humans either directly or through adaptation of animal host and most of infections caused by humans is directly. They initially infect susceptible animals which further infection to humans. Niv infect the human through flying fox without involvement of pigs. The person-person transmission is less documented. Pigs get infected indirectly in endemic regions. Niv is one of the pathogens in WHO priority list of pathogens. It needs urgent research and developmental actions because these viruses lead to more outbreaks. Sometimes this type of infection may be Asymptomatic. Myoclonus [segmental] along with Tachycardia may be evident. In this infection brain stem is involved which is responsible for vital functions is probably responsible for death and mortality rate may vary between 32% and 92%. The complications of this infection are encephalitis along with seizures. Symptoms of neurological functions such as encephalopathy, cerebral atrophy, change in behaviour, ocular motor palsies, cervical dystonia, weakness and facial paralysis which remain for several years. For diagnosis serological tests are helpful but discrete, high signal lesions are visualised by Fluid-Attenuated Inversion Recovery [FLAIR] where the effect of cerebrospinal fluid is reduced so that the enhanced MRI image can be obtained [2].
EPIDEMIOLOGY
Nipah virus infection is an Emerging Fatal Viral Zoonotic disease. The epidemiology of this infection has not been understood as Bio safety Level-4 [BSL-4] which is required for virus research. In humans and animals, the Niv infection seen in organs like respiratory tract, brain, kidneys or can also affect central nervous system.276 cases were noted in Malaysia and Singapore from September 1998 to May 1999 starts with acute encephalitis. In Malaysia, who were contact with swine population or directly contact with Niv infected pigs or pig farmers and their products and observed Niv in both humans and animals. In Singapore, this infection was due to abattoir workers may be through urine exposure of pigs. In March 1999, one dead due to import of pig meat from Malaysia and Niv was isolated from brain and spinal fluid in Ipohin victims-22. The incubation period of pigs was 4to14 days [3]. In Malaysia, the Niv was isolated from urine of bats and antibodies against Nipah virus have been detected in 23 species of bat across Asia and also bats in Ghana and Madagascar. Most of the outbreaks were seen in rural or semi-rural locations. During 2001, Niv was saw in Siliguridt of west Bengal with 74% mortality but laboratory confirmed in 2006and Nadiadt of west Bengal with 100% fatality in 2007. At that time the bats are hanging near the patient and it is useful for identifying infection directly from bats to humans. Two case reports were reported in India, West Bengal through person-person transmission and neighbouring Bangladesh. Total 639 human cases of Niv infection reported from Bangladesh due to consumption of date palm which is infected by bats. Other countries show Niv were India (85 cases), Singapore (11 cases), Philippines in 2014 (17 cases) and Malaysia (265 cases), with a mortality rate of about 59%. In Bangladesh, mostly person-person transmission is observed but not from pigs and bats. Philippines outbreak was due to Niv or Nipah like virus. Recent 2018 outbreak in perambra, Kerala, the patient experienced both neurological and respiratory symptoms through human-human transmission and 17 people dead. Kerala outbreak team doctors identified this infection in second patient itself when compared to other outbreaks where it took several months to found out causative organism. Early diagnosis of Nipah virus helps effectively to implement preventive measures. On 17 May 2018, Kozhikode dt through local fruit bats, kerala in 28 years male patient with Encephalitis. During 2-29 May 23 cases Of Niv were identified with index cases [not laboratory confirmed], 18 confirmed cases and 4 probable cases. Transmission of NiV occurred in 3 hospitals, all in Kozhikkode District: Taluk Headquarters hospital, Perambra (hospital 1); Government Medical College, Kozhikode (hospital 2); and the Community Health Centre, Balussery. Five countries were affected with Niv until 2018.They was Malaysia, Singapore, Bangladesh, India and Philippines.643 laboratory confirmed patients and atleast 380 [59%] human deaths. Niv were found only in flying foxes and no Niv infected human case was reported in countries of Cambodia and Thailand. The Epidemiology of India and Bangladesh outbreaks were less defined [4].
IDENTIFICATION
On 11th march 1998, after 3 days presence of virus the inactivated Vero cells which are lineage of cells used in cell cultures, kidney epithelial cell extracted from African green monkey. Vero cells infected with virus and were ready to view under microscope but it was not possible due to lack of proper working of electron microscope. Dr. Chau took slides prepared by Ms. Elsie Wong of Department of Pathology to the Institute of Higher Learning of the University but the microscope was not maintained well. The only few blurred images shows 100+ to 200 +nm size of enveloped viral particles with thickening of infected cell membrane were visible, finally no information obtained. In USA, Dr. Chau met Dr. Nick of Centre of Disease Control and Prevention on 14th March. Dr. Nick handle the antibodies against arbovirus of immune fluorescence assay technique. Dr. Chau used the antigen slide he brought along, 2 hours. Dr. Nick and Dr. Chau are observed that none of antibodies react with virus. Then Dr. Cropp handed microscope to Dr. Chau but is old version which needs liquid nitrogen for cooling. Dr. Chau recognised “concrete ring like” structures of paramyxovirus nucleocapsids. Dr. Chau cutted the transverse section of viewed structure and again seen under microscope. He sends the images to CDC and Malaysia with the help of Dr. Cropp which results in structures found were new type of paramyxovirus immediately teleconference occurs between CDC, USA and CDC, Atlanta happened and make a decision. They decision was taken to transfer all things carried by Dr. Chau to CDC, Atlanta for observation. Nipah virus was identified after observing slides of Dr. Chau, the CDC team of Atlanta confirmed that results obtained by Dr. Chau are positive [5].
CAUSES
Nivis caused by various factors [5].
- Drinking of contaminated raw date palm juices/sap/toddy
- Consumption of infected fruits by bats
- Exposure to saliva, droplets, excreta including contaminated especially palm trees sap.
- Close contact with infected persons through secretions
- Undercooked meat of infected animals
- Uncovered containers used for collecting raw date palm sap
- People working at date palm trees
Nipah Virus Structure
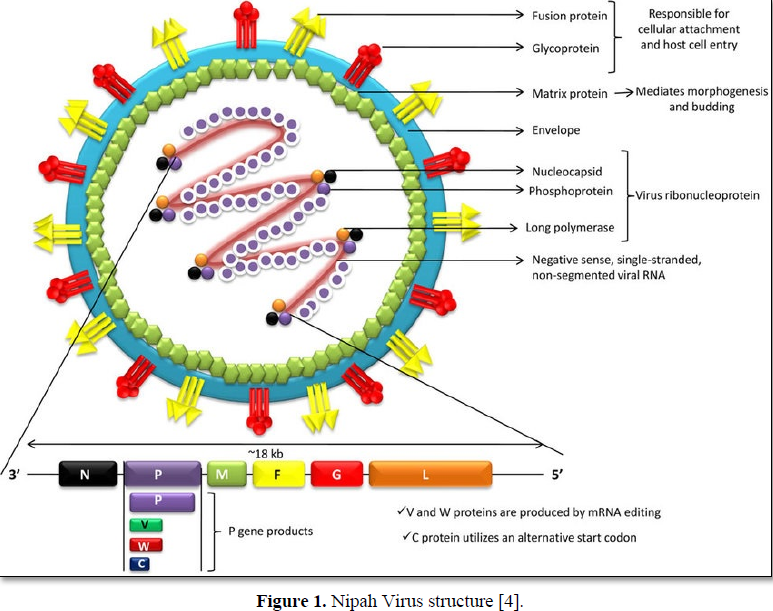
The Exact structure of Niv was not found. Its structure is nearly similar to Hendra virus and it is hypothesized. The Nipah virus is a type of RNA virus belongs to genus Henipa Virus. The core of virion contains a linear Ribonucleo protein [RLN] comprising of negative sense non segmented single stranded RNA genome with base pairs of 18252.The shape of this virus was pleomorphic in nature [altering from spherical to filamentous]and its size is from 40-600nm in diameter. [6]. The RLN consists of three critically important proteins: Nucleocapsid protein [N], Phosphoproteins [P] and large polymerase proteins[L]. The N proteins are tightly bound to various nucleotides of RNA strand with P and L proteins which provide polymerase activity during viral replication in cytoplasm. It helps in arrangement of capsid structure with helical symmentry. P gene produces nonstructural accessory proteins such as C, V and W are used in virulence of Nivand blocks interferons signalling is unknown. Niv also contain matrix protein [M] which preserves the virion structure, contact between the Ribonucleo protein [RNP] complex and surface glycoprotein and exhibits Morphogenesis and Budding process. It is enveloped by lipid bilayer which is single but are spiked with fusion and receptor binding glycoproteins. The viral proteins present inside of this virus are fusion proteins [F] and attachment glycoproteins [G]. G protein are responsible for cellular attachment of virion through EFNB2, a highly conserved protein mostly in mammals. The F protein involves in host cell entry and for induces neutralizing antibodies. The phosphoprotein [P] act as polymerase co-factor [5]. It also acts as Immuno suppressor by binding to STAT-1 for blocking signalling of interferons. Although members of the paramyxovirus family typically contain genomic length of 15,550nuclotides. The enzyme protease present in host cell cleaved the newly produced precursor F protein [F0] into F1 and F2. It is susceptible to common soaps, disinfectants and lipid solvents like alcohol, ether and sodium hypochlorite solutions for outbreak for disinfection and is inactivated for 60 minutes at 60 degree centigrade. Niv survive up to 3 days in fruit juices or mango fruits and for at least 7 days in artificial date palm sap. Niv life span was 18 hours in urine of bats. Like paramyxovirus, Niv does not have hemagglutinin and neuraminidase proteins. Unlike other paramyxoviruses, it has cytoplasmic inclusions closely related to endoplasmic reticulum. Niv is average larger than other paramyxoviruses [4] (Figure 1).
INCUBATION PERIOD AND SIGNS AND SYMPTOMS
Human infections were from Asymptomatic to Fatal Encephalitis. Niv infection is associated with Encephalitis. After exposure of Niv and incubation period of 4-14 days. The incubation periods had been reported after 45 days of exposure. A small percent people show Asymptomatic. It starts with high fever, headache, myalgia. The respiratory and neurological symptoms start about 4 days after onset of fever. The neurological symptoms like dizziness, vomiting, in spatial perception, myoclonus, altered consciousness, drowsiness, seizure, disorientation, mental confusion, abnormal plantar response and sore throat. The others signs and symptoms are flexia, hypnotonia, gazeplasy, limb weakness, nausea, loose stools, vision disturbances, stomach disorders, anxiety, shortness of breath, fatigue and flu like symptoms like cold, cough, nystagmus, abnormal chest, abdominal pain, Diarrhoea, anorexia, hyperthermia, constipation and renal impairment. These signs and symptoms lead to coma within 24-48 hr suggest that Acute Encephalitis. Hypertension and Tachycardia which are signs of brain stem dysfunction like abnormal doll’s eye reflex, pupillary reflexes, vasomotor changes, seizures, and myoclonic jerks were also present. Signs of cerebellum are relatively common and upper cervical spinal cord. Long Term exposure of this infection shows persistant convulsions and personality changes. Latent infection cause death and it was reported months and several years ago. Recovered patients may relapse year’s later [7]. The potentially fatal complication of Niv is Encephalitis. Insiliguri outbreak, the patient shows symptoms like fever, headache, myalgia, vomiting, altered sensorium, respiratory symptoms like tachycardiato acute respiratory distress or atypical pnemoniae and involuntary movements or convulsions. The gastrointestinal l [bleeding] is less commonly affected. It shows disturbances like nausea, vomiting, diarrhoea, abdominal pain, anorexia [8] (Table 1).
IN ANIMALS
In Pigs, this disease is also called as porcine respiratory and encephalitis syndrome [PERS], barking pig syndrome [BPS] which was saw in peninsular Malaysia or one- mile cough. An acute febrile illness has been reported in pigs below 6 months of age. Pigs initially suffered from respiratory illness associated with non-productive cough. In nervous system, twitching of muscles, weakness of hind legs, tremors, paresis either flaccid or spastic, nystagmus along with seizures in boars as well as sows’ in boars and sows, acute febrile illness with laboured respiration, increased salivation and nasal discharge, accompained neurological signs such as agitation, head pressing or knocking, clamping of mouth, nystagmus, tetanus-like spasm and seizures. In Dogs, inflammation of lungs along with necrosis of glomeruli as well as tubules with formation of syncytia in kidneys. In Cats, development of endothelial syncytia along with vasculopathy in multiple organs. In Experimental, hamster, guinea-pig, chick embryo and African green monkey [squirrel monkey] which results in development of lesions in the parenchyma in the CNS along with vasculopathy and other organs like lungs, kidneys, liver, urinary bladder, female genital tract, muscles, lymphoid organs. In Mice, clinical signs are absent for unknown reason [10] (Figure 2).
POST MORTEM FINDINGS
Magnetic Resonance Imaging [MRI] shows an involvement of cortex, pons and temporal bones of brain. May be bilateral abnormalities in the white matter of the brain Cerebral cortex shows more than 1 hyperintensities [t1 weighted] which are similar to necrosis of laminar cortex, Lesions in corpus callosum, brain stem and cortex of the cerebrum. It may be presence of disseminated micro infarction in the brain due to thrombosis induced by vasculitis. Neurons get involved directly. Vasculiticlesions also found in kidney, heart and respiratory tract. Medium and small sized blood vessels are get involved which results in development of syncytia (multinucleated) along with fibrinoid necrosis. Consodilation with haemorrhages (either petechiae or ecchymosis) in the lungs of affected pigs at necropsy. Froth-filled bronchi along with trachea. May be presence of blood-stained fluids in the trachea and bronchi. Congestion along with generalized oedema in kidneys and brain. Cortex and surface of kidney become congested. pneumonia (moderate to high) along with formation of syncytial cells in the endothelial cell lining of the blood vasculatures. In CNS and other major organs like lungs and kidneys there may be development of small vessel vasculopathy [acute infection-disseminated]. Generalized vasculitis with fibrinoid necrosis and mononuclear cell infiltration may be identified in the brain, kidneys and lungs. Viral antigens are appeared in blood vascular endothelial cells [lungs] In pigs, viral antigens are seen at upper respiratory tract due to admit the cellular debris and spreads the disease through exhalation. Lesser number of pigs shows meningeal inflammatory infiltrates. In dogs, congestion with haemorrhage seen in kidneys. Exudate may be in bronchi and trachea [4].
REPLICATION
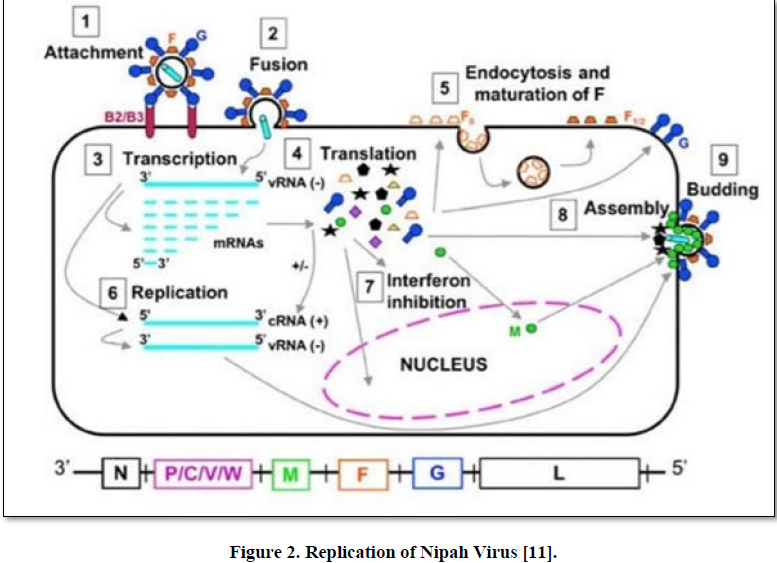
During nucleocapsid assembly, the interaction of Nipah virus Nucleocapsid [N] protein with phosphoprotein [P] which is essential process in viral life cycle. The encapsidated RNA genome can be used for replication [11].
PATHOLOGY
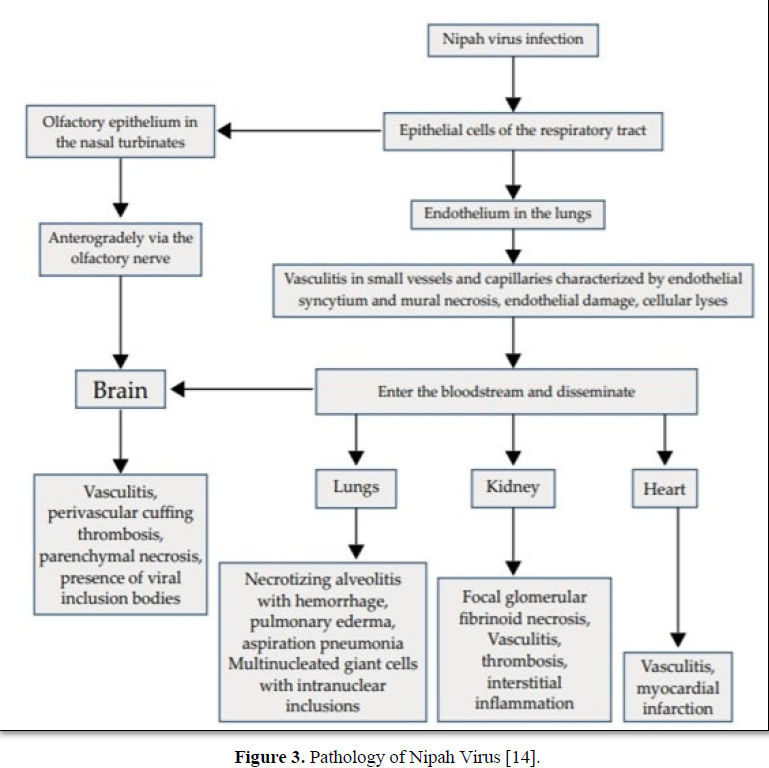
Nipah virus infection cause vasculitis and endothelial damage it leads to cellular lyses arterioles, venules and capillaries of various organs. Organs like brain, lungs and kidney produce various clinical features due to vasculitis and cellular damage [12].
RISK
Mostly it has risk for Hospital workers and Caretakers of infected persons. In Malaysia and Singapore, the Niv infection occurs through infected pigs. In Bangladesh and India, the Niv occurs through consumption of raw date palm [toddy]and contact with bats [11].
POOR PROGNOSIS
Age, Thrombocytopenia, Elevation levels of aminotransferases on admission, Brainstem involvement and seizures, Reduced the levels of consciousness, Vomiting, Abnormal doll’s eye reflex, Abnormal pupils, Hypertension, Tachycardia, Diabetes mellitus [13].
RESERVOIRS
Natural reservoir
Fruit Bats are responsible for cause infection. They are known as Flying Foxes or old-world fruit bats and belongs to Pteropodidae family. Under this family 41 genera and about 170 species are present. Pteropodids life span is about at least 30 years both in captivity and in wild. The Pteropus bats such as P. vampyrus, P. hypomelanus, P. lyleiand P. giganteus were cause infection in various countries of South Africa, islands of the Indian ocean to the northern and western coast of Australia along with across South Asia. Bats carrying this virus were with Asymptomatic. Recently African fruit bats which belong to family pteropodidae, genus Eidolonis cause for infection. Pteropushypomelanus and Pteropusvampyrus having the highest rates of seroprevalence with the infectious virus being identified in their urine and saliva. In South and South East Asia Pteropushypomelanus and Pteropuslylei were isolated as being carriers. There is some evidence the female bats which are in the states of pregnancy and lactation, are more susceptible to infection [9].
INTERMEDIATE HOST
Pigs are act as intermediate host between bats and humans. Pigs spread this disease to other domesticated animals like horse, goats, sheep [not confirmed it is in controversial], cat and dogs and first described in Malysian outbreak in 1999. Niv also infects guinea pigs, hamsters, ferrets and African green monkeys. Most of pigs do not show any symptoms with neurological and respiratory involvement but others show feverish illness, laboured breathing and neurological symptoms like trembling, twitching and muscle spasms. In young piglet’s mortality rate was low. If pigs having unusal barking cough, it was suspected that Niv infection. Nipah virus is highly contagious in pigs and produce diseases like porcine respiratory and neurologic syndrome, porcine respiratory and encephalitic syndrome (PRES), and barking pig syndrome (BPS) [14]. (Figure 3).
MODES OF TRANSMISSION
Animal-to-human transmission
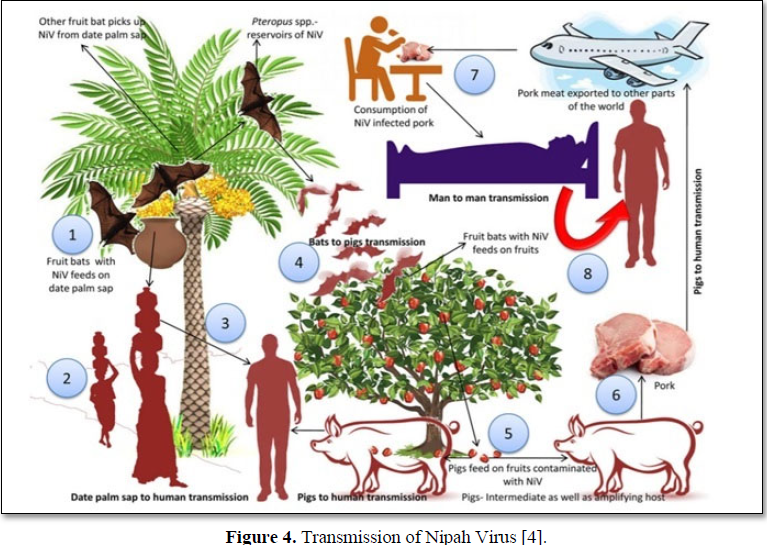
Niv is caused by Fruit bats through saliva, feces and urine [kidney] of bats, excreates and secretions of bats. When bats are symptoms less infected the humans as well as animals like pigs, dogs, cows etc. [4].
Bats-to-humans through date palm sap
Fruit Bats, mainly petropusgiganteus infect the date palm sap by licking date palm juice-tari most frequently during extraction of palm trees [15]. Upon consumption of palm sap, the infection can spread to humans. The virus can survive in sugar rich like Fruit pulp [4]. The recent outbreak of Nipah virus occurred on May 2018 due to consumption of fruits which are bitten by fruit bats and cause Niv spreads to humans. Bats spill over humans and cause infection but it is limited transmission [11] Owners of date palm sap heard bats at night. They thought that bats are nuisance because the owners drank sap directly from tap or clay pots. Excretions of bats present on surface of pot or floating on sap. Sometimes death bats were floating on sap [2].
PIGS-TO-HUMANS
Niv is caused by pigs. Infected pig’s meat consumption spreads this disease to humans. The pork meat imported to other continents which leads to spread of disease from one part of world to other part of globe and cause infection. People who are contact with secretions of saliva and other areas of pigs fell sick through touching, feeding and air droplets. The virus spreads from animal to humans. Recent investigations show that close contact of Syrian hamster with pig’s cause Niv through air droplets [aerosol exposure [16].
ANIMAL-TO-ANIMAL TRANSMISSION
Pigs-to-pigs
Niv is caused by pigs.Pigs spreads this infection to other pigs as well as domesticated animals through fruits, water or aborted bat foetuses or birth products [3].
Bats-to-pigs
Pigs ate the fruits which are infected by bats and cause infection.It is reported that the pigs which are ate the infected fruits contain saliva of bats. The transmission of Niv infection between pigs through urine or saliva or cough [6].
Human-to-human transmission
Niv infection is transmitted from person to person through nasal discharges, body fluids and fomites [urinary] such as clothing, shoe and equipment. It is spreads through coughing and air droplets [aerosol] [18]. The persons who received Niv infection treatment shows Nipah virus RNA in semen. The RNA detected at 26th day following onset of illness but not at days 42 or 59. These findings suggest that there is a potential risk of getting this infection through sexual transmission but the presence of RNA in individual semen is unknown and sexual transmission of Niv has not been described to date the incubation period is 6-11 days in person-person. It spreads through sharing food and beds. Niv was identified in saliva or urine of infected person s [11] (Figure 4).
Transmission of the Nipah virus 1. Fruit bats with NiV feeds on date palm sap 2.consumption of date palm sap 3.Fruit bats of Pteropus spp. which are contaminate the farm soil and fruits 4. Contaminated fruits areate by pigs and other animals 5. Niv transmit to pigs through infected fruits6.Nivinfection transmit to pigs or other animal’s 6.Infected Pork meat are exported to other parts 7.Consumption of infected pork cause infection to human 8.Close contact with infected human can lead to spread of NiV to other persons.
Malaysia
Niv outbreaks in Malaysia reported that pigs act as intermediate hosts as well as amplifying hosts for spreading of disease. Case report showed that most of patients suffered from this infection through close contact of pigs and 8% of cases had no contact with pigs. The outbreaks were stopped after the pigs in affected areas were slaughtered [13].
Bangladesh
The three transmission pathways of this infection have been identified after investigation carried out in Bangladesh through fermented date palm juice-tari, mainly fruit bat-P.gingateus. The outbreak seen in tangaildt was found to be associated with drinking of raw date palm sap. Symptoms have been identified during the season of date palm collection i.e; December to March. Another route of transmission of virus through domesticated animals by eating infected fruits. It is reported that niv is spreads from sick cows, dogs and catsin 2001 Meherpur. Infected pigs, saliva of goats and secretion of bats spread this infection in Naogaon (International Centre for Diarrhoeal Disease Research). The mostly seen in family and care takers of infected persons. It occurs through respiratory droplets, contact with throat or nasal secretions from pigs or contact with tissue of pigs [17]. People who work at trees also get infected. In 2004 the outbreak occurs in faridpur through person-person transmission [15].
India
InSiliguri, 75% cases were seen in hospital staff or visitors by close contact with infected people [8].
DIAGNOSIS
Initial signs and symptoms are non-specific and accurate diagnosis challenge during outbreaks. Specimens are collected from symptomatic patient or at post mortem examination [18].
Laboratory Data [17]
- Serology
- Histopathology
- PCR and virus isolation [culture tests]
- Samples are collected from throat and nasal for laboratory investigations
- Blood samples
- Fluids taken from cerebrum, spinal cord and urine
- IgG and IgMantibodies are detected after recovery to confirm Niv infection
- Viral RNA can be isolated from saliva of infected persons
- Immuno histochemistry studies on tissue collecting during autopsy-confirmation
- Electron microscopy
- Viral RNA rarely detects in oropharyngeal swabs and rectal swabs from naturally or from infected bats
- Quantitative real-time polymerase chain reaction (QrtPCR) as immune histochemistry
- Tissue biopsy
Others [13]
- Blood urine, throat scrub and CSF sample are collected for diagnostic testing
- From brain, lung, kidney and spleen samples virus isolation can be done using African green monkey kidney (vero) and rabbit kidney (RK-13) cell culture
- For detection of anti-nipah virus antibodies for reference by serum neutralization test
- Detection of IgM and Ig G antibodies by ELISA
Confirmation Test [19]
- Serum Neutralization Test[sn]
- ELISA
- RT-PCR
- Nucleophilic amplification test
- Serology
- Histopathology
- Virus Isolation [culture tets]
- Viral RNA can be isolated from saliva of infected persons
- Immunohistochemistry studies on tissue collecting during autopsy
Bangladesh, India and Thailand have developed well laboratories for diagnostic and research purposes. There are few laboratories are developed for study of virus safety without a risk of infecting more people [6].
DIFFERENTIAL DIAGNOSIS
Niv is difficult to distinguish from host of other febrile illness during its onset. Most common causes of viral pneumonia include adenovirus and influenza and viral encephalitis like Japanese encephalitis which is also transmitted by swine need to be excluded. Insuspected patients the differentials should be considered in patients. They are a) Japanese encephalitis (JE), b) Measles, c) Rabies, d) Dengue encephalitis, e) Cerebral malaria, f) Scrub typhus, g) Leptospirosis, h) Herpes encephalitis and i) Bacterial meningitis [13].
LABORATORY
During convalescent phase of disease [20]
- Routine blood examination may show leucopenia and thrombocytopenia.
- Elevation of liver enzymes
- Respiratory symptoms show atypical pneumoniae and acute respiratory distress syndrome
- CSF shows elevation of blood cell count and protein for encephalitis but not confirm niv infection.
- CT brain shows normal while MRI shows focal lesions in the sub corticol and deep white matter.
DETECTION OF AGENT
It was done by polymerase chain reaction. Tissue samples, swabs, CSF and urine are the specimen’s used in animals. It may be difficult as virus detection has low sensitivity [21].
PCR
Conventional PCR targeting nucleocapsid [N] gene is developed by US Centers for Disease Control and Prevention [CDC]. NiVRNA can be identified from respiratory secretions, throat swabs, nasal swabs, blood, urine or cerebrospinal fluidby Real Time PCR (RT-PCR) [taqman] [16]. A different region of the N gene can be targeted by SYBR Green-based assay also developed. A positive test shows presence of virus in body. In any outbreaks this test carried out at National Institute of Virology, pune which is national reference laboratory for viral diseases in India [10].
IMMUNOHISTO CHEMISTRY
In this Formalin fixed-tissues are used because of viral replication occurs at site of vascular endothelium. The tissues like brain, lung, spleen, kidney and lymph nodes. In pregnant animals analysed the uterus, placenta and products of conception. Convalescent human serum was used in this previously but now it is replaced by rabbit serum against Niv [22].
VIRUS ISOLATION
Virus can be separated from respiratory secretions, urine, brain, spleen, lung, cerebrospinal fluid or other tissue specimens but it must be done in a BSL-4 laboratory. The cell line of this Niv infection is vero cell line from kidney of African green monkey. Pteroid bat cell lines have also been developed. Within 3 days, cytopathic effects can be observed. The cells form syncytial and subsequently lead to formation of punctate holes in monolayer. Niv forms larger syncytia and there is difference in nucleus distribution used for identification from other viruses like Hev. Identification of virus from cell culture by PCR or Immunohisto chemistry [23].
ANTIBODY DETECTION
CSF or IgM antibodies are detected. IgG anti bodies detection are used as good test for surveillance in humans and for identification of reservoir animal during epidemiological investigations. It used as identifying in human during outbreaks [21].
ELISA
In Malaysia the diagnosis was confirmed by detection of IgG and IgM antibodies which are developed by CDC. During Niv outbreaks, this test is used as surveillance in Bangladesh. Other tests like recombinant proteins by using conserved N antigen. On the illness 50% patients observed IgM antibodies. Most of the patients [100%] IgG antibodies are observed after the day 18 and persistants for several months. Antibodies are produced against virus and circulate in patients’ blood. When blood sample is taken from patient who are infected with Niv shows elevation levels of antibodies. Based on level of antibodies the amount of virus present in body can be estimated [21]. Table 2.
SERUM NEUTRALIZATION TEST
It used the pseudo typed particles. It is considered as gold standard test but it requires BSL-laboratory. In this test, the test sera are incubated with virus and allowed to infect Vero cells. Cytopathic effects are blocked by positive sera and it is seen after 3 days. The modified neutralization test can be seen in 24 hr. After adsorption, virus-sera mixture is removed and immunostaining is used for virus detection. A pseudo typed virus is an enveloped virus with one or more foreign envelope proteins and perform a surrogate neutralization test. These viruses safely handled in BSL laboratory and Niv envelope proteins get neutralise by positive sera. In non-endemic areas Niv infection is not seen [10].
ELECTRON MICROSCOPY
Electron Microscopy [EM] shows an enveloped virus with filamentous nucleocapsid. The virus showed a herringbone structure, characteristic of the family Paramyxoviridaeon negative staining. The major target was the central nervous system in preliminary autopsy findings [2]. Visualization of virus by electron microscopy and detection of antibodies by immune electron micros copy rapidly provides the information about virus structure and its antigenic reactivity [23]. BSL-4 lab at National Institute of Virology (ICMR) in India, pune got all preparedness diagnosis of Niv in India. For exotic animal disease diagnosis high security animal disease laboratory, Bhopal with BSL3+ facility caters are required [21].
MRI
It shows multiple discrete lesions seen in sub cortical and deep white matter of cerebral hemisphere due to Micro Infraction [13].
MANAGEMENT
Patients must be isolated and start control practices. Primary treatment is managing the symptoms such as fever and neurological symptoms if any [13]. The treatment is supportive maintenance of airway, breathing and circulation, balance the fluid and electrolytes level. Patients with severe pneumonia and acute respiratory failure are treated with mechanical ventilation. Hospitalized persons provide with ventilator support [7]. Niv virus encephalitis transmitted from person-person. Standard infection control practices and proper barrier nursing techniques are important in preventing hospital acquired infections (nosocomial transmission) [23].
ANTIVIRAL CHEMOTHERAPY
Currently there is no treatment of Niv. The treatment of Niv infection is supportive care and consisted of anticonvulsants. The treatment of secondary infection is mechanical ventilation and rehabilitation. There is no approved or licensed therapeutic for treating Niv infection [9]. There is no specific antivirals or vaccines are present. During past outbreaks, ribavirin and acyclovir are used for treatment of Niv. Acyclovir has been used to treat nausea, headache and vomiting [24].
RIBAVIRIN
Ribavirin is Guanosine Analouge, Ribavirin was given orally or Intravenously to patients with Niv Encephalitis in Malaysian outbreak which is used to treat hepatitis c and shown effective in invitro and it is broad spectrum activity against DNA and RNA viruses which had ability to cross the blood brain barrier [orally CSF/plasma ratio of 0.7] for treatment of viral Encephalitis. The treatment of ribavirin reduced the mortality rate upto 36% in open label trial and without any neurological deficits [18]. Ribavirin is most effective against other paramyxoviruses such as respiratory syncytial virus [RSV] and it is ineffective in test animal models. Ribavirin is used in inhalation solution to treat lower respiratory tract infection in young children caused by RSV. Ribavirin reduces the ventilator support and hospital stay. Ribavirin studied in less number of people but its usage correct or not was unknown until 2011.Clinical use of ribavirin remains uncertain. According to WHO, ribavirin dose for lassa fever of 30 mg/kg for children and 2,000 mg/kg for adults, followed by 10 days of therapy (4 g in divided doses for first four days and 2 g in divided doses for next six days). Ribavirin adverse effects are Neutropenia, anemia (11% to 35%; children & adolescents: 11%), lymphocytopenia and suicidal ideations. In long term use of ribavirin cause most of side effects. The Infectious Diseases Society of America has recommended in 2008 the use of ribavirin to treat NiV infections. During the absence of effective antivirals ribavirin is used to treat all confirmed cases through oral or parenteral route. Ribavirin is not used as chemoprophylasix [22].
ACYCLOVIR
Acyclovir was given to patients with Niv encephalitis in Singapore outbreak and only one dead reportdue to Niv infection but the role of acyclovir drug is still unclear [22].
CHLOROQUINE
Chloroquine was reported well in cell culture but it is fail to prevent death in hamster model used in isolation or in combination with ribavirin. Chloroquine is ananti-malarial drug that blocks the functions for maturation in Nipah virus [3].
FAVIPIRAVIR
Favipiravir (T-705,6-flouro-3- hydroxy-2- pyrazinecarboxamine) antiviral drug which is licensed in japan by toyama chemical company for treatment of influenza shows promising results when tested on Niv infected golden hamsters in recent in vivo studies. Favipiravir inhibits Niv replication and transcription in syrian hamster model on oral administration for 14 days with twice a daily. Favipiraviris RNA dependent-RNA polymerase [RdRp]. Nivoutbreaks often lead to high mortality rate and cause more impact on public health [24].
OTHERS
There is a strong need of specific viral agents for early treatment of Niv. Innon-human primate model, neutralizing human monoclonal antibody has been found to be effective. In India use of anti-G and anti-F monoclonal antibodies during emergency. Patients are discharged only after negative result of RT-PCR which is based on results of throat swab/blood obtained. Therefore, the patients who are going to be discharged also advised to remain isolation until 21 days after confirmation of infection [24]. Adenosine nucleoside analogue GS441524, its monophosphate prodrug G S - 5 7 3 4 and another nucleoside analogue, R1479 (balapiravir) was also used against Niv in various studies [12]. Neutralizing human mechanical antibody, the m102.4 which act as the receptor binding domain of the Nipahvirus G glycoprotein is successfully tested in animal like ferret [19]. m102.4 prevented infection and death after injection of a lethal dose of NiV in 12 AGM subjects in 14 African green monkeys [AGM]. AGM controlled subjects contracted severe infection and developed Encephalitis and ARDS. Till now effective treatment of Niv was not found [13].
VACCINE
There is no vaccine against this infection either in humans or animals. Vaccine for Niv is in pre-clinical studies, most of them tested in hamsters, ferret’s aorta gonad mesonephros [AGM]. In infected hamsters, use of vaccines against Niv infection shows promising results. A number of vaccine procedures have been developed for Niv which are tested in domesticated animals. A vaccine has been made in monkey against Niv but its use not reported in humans [21]. The most studied vaccine based on G glycoprotein (sG) of Nivand Hev. In Hev and Niv shows a cross protective immune response to Hev-sG. It is put into a horse vaccine Equivac against Hev which is registered by Australia. Virus vector based recombinant vaccines are also developed. F and G glycoproteins are expressed on their surface of mammals in recombinant vaccines and a mammalian virus like particle vaccine is produced [25]. All these approaches lead to complete protection aganistoro-nasal Niv challenge after a single dose in various animal models. The success of sG vaccine in horses are attractive for eventual use in humans. Vesicular stomatitis virus vector vaccine protective against hamsters, ferrets and African green monkey. Creating vaccine is difficult because mutations in RNA zoonotic diseases.9different antibodies are found in pteropid species in Malaysia, Cambodia, Thailand and Bangladesh [8].
PREVENTIVE MEASURES AND CONTROL
Controlling Niv infection in animals reduces the further spreading of this disease and prevents outbreaks. Awareness to public and help them to avoid virus for control of this infection [12].
PREVENT THE INFECTION IN HUMANS
Avoid direct contact with infected persons and host organisms like fruit bats and pigsor with their secretions. Avoid consumption of contaminated food by saliva or droplets of bats. Fruits or other products from trees should be checked and washed and peeled before consumption [8]. Proper precautions should take from infected persons because Niv can easily spreads through respiratory droplets or by contact transmission. Standard operating procedures like frequent hand washing, sanitization with 70% ethanol and avoid of direct contact with body fluids like urine, saliva, blood etc., in Niv suspected workers. Boil fluids before consumption. Awareness about consuming infected date palm sap/juice/toddy [22]. Boiling of date palm juice can reduce the chances of Niv transmission. Wear gloves, masks, goggles, boots and protective clothing before touching infected pigs to avoid Niv transmission during slaughtering [killed] and culling procedures. Maintain a good hygiene. Health workers undergo tests for suspected Niv infection. Avoid fruits which are fallen from trees. Avoid raw date palm nearer infected bat areas. Avoid contaminated endemic area for visiting. Avoid drinks that are made near the palm trees. To cover the date palm sap and prevent contact with bats by using bamboo sap skirts. Avoid prolong close contact with infected person; the person should be counseled [14]. Avoid usage of water from wells which are infected by bats. Bats are drink toddy and urinate in it an open containers and cause infection. Physical barriers are used to avoid bats from contaminating sap. Avoid exposure of sick pigs and bats. Develop quality control for laboratory testing. Research is needed with investigating questions such as the seasonality of disease with reproductive cycles of bats for better understanding of ecology of bats and Niv. Raising awareness and educate the people to take measures to prevent infection. Warned against eating half-eaten fruits, entering abandoned wells, and to properly handle dead bodies. Avoid consumption of date palm sap [14].
PREVENT THE INFECTION IN ANIMALS
Cleaning pig farms by using disinfectants like sodium hypochlorite [bleaching], chlorinated lime and other detergents the environment and equipments in which infections can be seen. Farmers who have domesticated animals should avoid the pets to eat fruits which are infected [26]. Farms are built with more space to avoid disease between animals and should not near the trees that attract bats. Vaccination to pigs and horses used to avoid animal-animal transmission. Reliable laboratory assays for early detection of disease in communities and livestock should be used for surveillance [2]. Disinfectant the pig farms and equipments. Restrict the transfer of infected animals to other areas for the prevention of disease. Avoid consumption of infected fruits of other animals. Using screens at open air access for the prevention of pig farms contact with fruit bats and their secretions [16,4].
CONCLUSION
Nipah virus is a deadly zoonotic disease and spreads easily. It belongs to genus Henipavirus and Paramyxoviridae Family. Niv become rising disease in worldwide without proper treatment and preventive measures only to avoid this infection. Therefore, conduct several researches on this virus for effective treatment to prevent Niv. Niv cause encephalitis and respiratory problems and shows high mortality rate. Effective vaccines strategies should be developed for future purpose. Various laboratories with equipment’s should be developed. It was first identified in Malaysia and name was originated from Kampung Sungai Nipah village. Recently it shows serious outbreaks in kerala. To prevent this infection, several awareness programs should be conducted.
- Angeletti S, Presti AL, Cella E, Ciccozzi M (2016) Molecular epidemiology and phylogeny of nipah virus infection: A mini review. Asian Pac J Trop Med 9(7): 630-634.
- Narang R (2018) Nipah virus: Biology, disease, treatment, control, and prevention. Journal of Mahatma Gandhi Institute of Medical Sciences 23(2): 65-68.
- Kuldip D, Rakesh W, Divya T, Priya S (2018) Nipah Virus - InfectiousAgent: An Overview. World J Pharm Pharm Sci 7(8): 379-385.
- Singh RK, Dhama K, Chakraborty S, Tiwari R, Natesan S, et al. (2019) Nipah virus: Epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies–a comprehensive review. Vet Q 39(1): 26-55.
- Chanchal DK, Alok S, Sabharwal M, Bijauliya RK, Rashi S (2018) Nipah: Silently rising infection. Int J Pharm Sci Res 9(8): 3128-3135.
- Monika, Priyanka, Shashikala, Wati L (2018) Nipah virus: A Review. Int J Curr Microbiol Appl Sci 7(6): 3056-3063.
- Wahed F, Kader S, Nessa A, Mahamud M (2011) Nipah virus: An emergent deadly Paramyxovirus infection in Bangladesh. Journal of Bangladesh Society of Physiologist 6(2): 134-139.
- Nagaraju GV, Kumar GVP, Subrahmanayam NS, Malyadri Y, Reddy SPK, et al. (2018) A brief description on fact sheet and pharmacoepidemology diagnosis, transmission, management and prevention of nipah virus. European Journal of Pharmaceutical and Medical Research 5(11): 622-629.
- Ochani RK, Batra S, Shaikh A, Asad A (2019) Nipah virus-the rising epidemic: A review. Infez Med 27(2): 117-127.
- Kulkarni DD, Tosh C, Venkatesh G, Kumar DS (2013) Nipah virus infection: current scenario. Indian J Virol 24(3): 398-408.
- Onkar D, Sagar K, Begum MF, Priyanka M, Tejashri M (2019) A report on Nipah Virus. JDDT 9(2): 449-452.
- Raveendran AV, Sadanandan S, Thulaseedharan NK, Kg SK, Pallivalappil B, et al. (2018) Nipah Virus Infection. J Assoc Physicians India 66 (2018): 58.
- Banerjee S, Gupta N, Kodan P, Mittal A, Ray Y, et al. (2019) Nipah virus disease: A rare and intractable disease. Intractable Rare Dis Res 2018-01130.
- Ang BSP, Lim TCC, Wang L (2018) Nipah virus infection. J Clin Microbiol 56(6): 01875-17.
- Siddique AB, Fardows J, Farhana N, Mazumder M (2016) Nipah virus: A public health concern. J Enam Med Coll 6(2): 101-105.
- Areshkumar M, Divya S (2018) Nipah Virus-Overview. Int J Curr Microbiol Appl Sci 7(6): 2840-2844.
- Islam MMZ, Rahman MM (2016) Nipah virus Infection: A fatal Emerging disease. North Int Med Coll J 7(2): 146-148.
- Shrestha D, Bhattachan B (2018) Nipah virus (NiV) infection: Is nepal prepared for the possible outbreak? NJB 6(1): 69-73.
- Arora A, Dogra A, Dogra A, Goyal B, Sharma AM (2018) Nipah virus: An Outbreak of Deadly Paramyxvirus. Biomed Pharmacol J 11(3): 1177-1185.
- Ramphul K, Mejias SG, Agumadu VC, Shaheen S, Ruhi S, et al. (2018) The Killer Virus Called Nipah: A Review. Cureus 10(8): e3168.
- Prarthana MS (2018) Nipah virus in India: past, present and future. Int J Community Med Public Health 5(9): 3653-3658.
- Aditi, Shariff M (2019) Nipah virus infection: A review. Epidemiol Infect 147: e95.
- Sureshkumar M (2018) Nipah virus: A Journey from Bats to Humans. National Journal of Basic Medical Sciences 8(4): 189-198.
- Sharma V, Kaushik S, Kumar R, Yadav JP, Kaushik S (2019) Emerging trends of nipah virus: A review. Rev Med Virology 29(1): e2010.
- Saravanan R, Senthilkumar K, Dhachinamoorthi D, Chellaram S, Reddy M (2010) Virosomes: As a versatile carrier for delivery system. J Pharm Res 3(6): 1-7.
- Vandali V, Biradar RB (2018) Nipah virus (Niv) infection: A systematic review. JOJ Nurse & Health Care 8(1): 2018.