979
Views & Citations10
Likes & Shares
A 56 year old CKD 5 patient
due to diabetic nephropathy (biopsy proved) presented to our hospital in
transplant clinic for transplant assessment. She is not yet on dialysis and her
current eGFR is 20 ml/min. She was diagnosed at the age of 29 to have diabetes.
It is not clear from notes whether she is type 1 or type II DM. She is
currently on insulin with frequent episodes of hypoglycemia unawareness,
particularly during last three years. Her kidney function started to
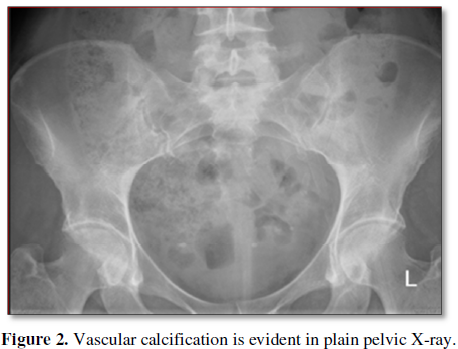
deteriorate 9 years from diagnosis of her diabetes. Her plain X-Ray pelvis is
shown below. She has no DSA and has a family member (28 years old lady whose
blood pressure is well controlled by one agent, but no more available
information) who expressed her interest to donate a kidney for her.
Our team is intended to
discuss her possible options of diabetic control and the outcome of each
option, addressing graft (s) survival, patient survival, as well as the
postoperative complications. We need also to council the potential donor
regarding the procedures of kidney donation and to outline the workup of this
potential donor. Furthermore, workup of this prospective recipient, as well as her
prospective follow-up plan, might be generally outlined.
Keywords: Diabetic
complications, Pre-emptive renal transplantation, Simultaneous pancreas and
kidney transplant (SPK)
CASE ANALYSIS
1.
Post-menopausal
lady 54 years old.
2.
Stage 5 CKD with
estimated glomerular filtration rate (eGFR)=20 ml/min. Not yet on dialysis
(DX).
3.
Possibility of
pre-emptive renal transplantation (RTx).
4.
Type I/II DM on
insulin therapy with hypoglycemia unawareness for 3 years.
5.
Plain-x-ray:
evidence of vascular calcification in her deep pelvic vessels.
6.
Absence of
evidence of DSA.
7.
Recipient data:
female, 28 years old, with controlled hypertension (HT) with single agent.
DISCUSSION
Possible options of diabetic control: the outcome of each option
addressing graft (s) survival, patient survival and postop complications:
This post-menopausal diabetic lady, with CKD 5, possibly suffered from
type I prolonged and uncontrolled DM as appeared in her recurrent episodes of
unawareness of hypoglycemia due to associated autonomic neuropathy. Episodes of
hypoglycemia appeared due to decreased insulin requirements owing to her
progressing renal failure (eGFR)=20 ml/min). So, this lady is currently in need
for a healthy kidney associated with a suitable option for her diabetic
complications. Fortunately, one of her relatives is ready to offer her a
kidney. Consequently, the following therapeutic options may be offered for
diabetic control [1]:
1.
SPK
(Simultaneous kidney-pancreatic transplantation).
2.
PAK (Pancreas
after kidney transplantation) (poor option).
3. Pre-emptive kidney transplantation (KTx) alone
(indirect therapy).
The American Diabetes Association (ADA) have addressed the following
criteria [2]
·
SPK
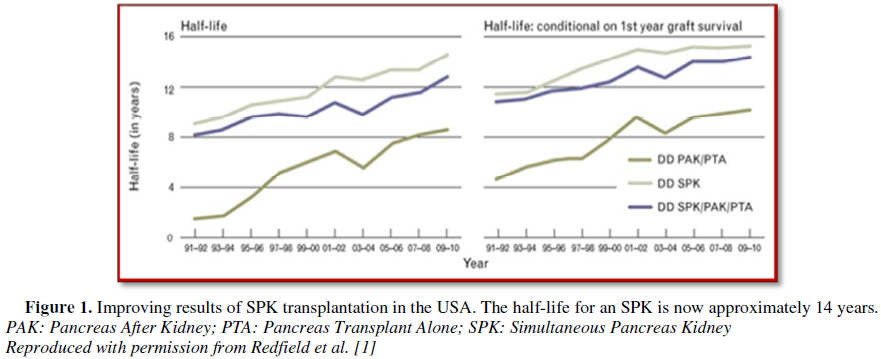
(Figure 1): for type I DM, transplant
recipient (TR) with ESRD who have had or plan to have a KTx are candidates for
pancreas transplant. Successful transplant of a pancreas will definitely
improve glycaemia levels and may improve also kidney allograft survival. Most
pancreatic transplants are performed in patients with DM complicated with ESRD.
Majority of these patients receive SPK rather than PAK [3].
The
current status of the techniques in SPK transplantation is yielding superior
and continuous improving results. So, the first option for this patient is SKT.
PAK and Islets after kidney are poorer option but pancreas alone or Islets
alone NOT an option. Apart from SPK transplantation, other treatment options
include:
§ Live donor
kidney transplant (pre-emptive transplantation):
A kidney transplant from a young relative live donor is a very good option, as
they tend to work straight away, and usually work for longer than a kidney from
a deceased donor. However, without a pancreas, in addition to diabetic
complications, our patient will still have diabetes. Furthermore, the impact of
the immunosuppressive medications post-transplant may make the recipient’s
blood sugar control even worse.
§ Live donor
kidney transplant followed by a pancreas transplant (PAT):
This is a poor option. A pancreas transplant from a deceased donor can take
place 12 to 18 months after a live donor kidney transplant. Considering that
the transplanted pancreas and kidney came from different donors, the risk of
the expected rejection for the pancreas is currently higher. The average
survival of a pancreas transplanted after a live donor kidney transplant is 3-5
years. This is much less than a pancreas transplanted as a part of an SPK
transplant (10-14 years) (Figure 1)
[2].
Outcomes: Mortality, morbidity, and results of transplant may vary
with the operative experience as well as with patient selection.
Patient survival:
1. According
to 2004 to 2015 registry data, patient survival rates for SPK, PAK, or PTA
ranged from: 96-99% within 1 year, 89-91% at 5 years, at 70-80% at 10 years
postoperatively [4-6]. Most deaths that occur within the first 3 months
post-transplant were due to cardiovascular (CVS) or cerebrovascular disc.
2. Few
data exist about survival benefit for transplant compared with waitlisted
patients. The following data relies on retrospective studies of transplantation
registries from 1995 to 2003:
·
SPK
survival of TR was much better than that of waitlisted patients who remain on
DX [7]. The decreased mortalities is partially due to the apparent survival
benefit conferred by KTx alone (KTA; even without pancreas transplant) compared
with DX.
Graft survival: According to 2004-2015 registry data, early
allograft failure (within 90 days) reported in about 8-9.4% of patients [4]. 5 years
pancreas graft survival for SPK, PAK and PTA was approximately 73, 65 and 53%,
respectively [5]. Pancreas graft survival is reported to be inversely related
to several donor varieties, including: age, body mass index as well as CVS
death. TR of pancreas alone whose organs came from donors with poor donor risk
indices usually experience lower rate of graft survival as compared to TR of
SPK (77 vs. 88% at one year) [6,8]. Recognition of pancreas graft survival has
been variably defined by different transplantation centers (e.g. complete
insulin independence, continuity of C-peptide production) [5]. A stable
universal definition may help the evolution of robust future outcomes studies.
In US, the United Network for Organ Sharing has postulated a new definition of
graft failure that includes: use of insulin ≥ 0.5 units/kg/days for 90
consecutive days [5,9]. In 2018, a classification of graft function was
addressed by the International Pancreas and Islet Transplant Association and
the European Pancreas and Islet Transplant Association [10].
Vascular calcification (VCL): One of the remarkable finding in this
recipient preparation is the presence of evident VCL in her radiographic
examination. VCL can be simply assessed by plain radiology of the aorto-pelvic
area (Figure 2). Considering the
silent nature of this disease as well as its devastating Sequalea in renal TR,
this investigation has gaining much popularity [11]. Moreover, VCL is
categorized as a strong predictor of post-transplant all-cause and CVS
mortality. Arterial calcification can be seen in the intima or the media.
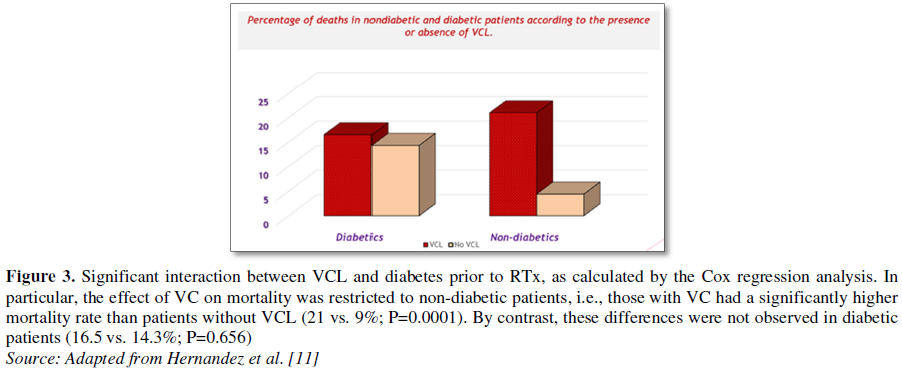
Sequalea and difference between both locations are summarized (Table 1 and Figure 3).
Post-operative complications
Graft loss: Causes of pancreas graft loss vary with the time after
transplant. Early graft loss, that can be defined as loss occurring within
hours or days post operatively, it is usually results from (thrombosis, leaks,
bleeding, infection and pancreatitis) (these complications are usually called
technical failures). One series reported 211 TR undergoing pancreas transplant,
technical graft failure was observed in 23 TR (11%), with the most common
reported cause was due to thrombosis. Risk factors for technical failure
include [obese donor/recipient and delayed preservation time of the donor
organ]. Later graft loss, i.e., several weeks later, is more common and usually
attributed to immunologic rejection [6,12,13].
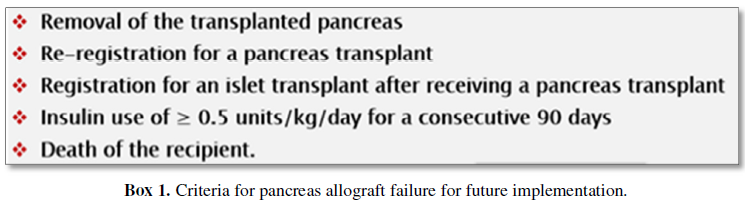
The absence of a uniformly
agreed criteria of allograft failure is currently impeded the proper estimation
of pancreatic graft outcome. While some centers deny the failure of the
allograft as long as C-peptide production persists, other centers document
graft failure only with loss of recipient’s independence on insulin. However,
the OPTN/UNOS Pancreas Transplantation Committee has currently summarized the
following criteria for pancreas allograft failure, to be implemented in the
near future (Box 1) [14,15].
Rejection: In old reports pancreas transplantation may be rejected
within few days or after many years post-transplant. Incidence of acute
allograft rejection of pancreas may approach 60-80% of pancreatic grafts.
However, Alemtuzumab and Tacrolimus based steroid free regimes are reported to
have very low early rejection rates [16-18]. Management of rejection includes
patient hospitalization with intensifying the immunosuppressive load. Methodologies
applied to manage acute rejection of pancreas transplant alone are similar to
that used in kidney-pancreas transplant.
Indices of rejection, however,
include:
1. Increasing
blood glucose levels.
2. Increasing
serum amylase levels.
3. Diminished
urinary amylase excretion (pancreatic exocrine function).
These aforementioned markers are
less sensitive than a rise in S. Cr if a concurrent renal allograft is
transplanted. Raised fasting blood glucose, however, is considered a relatively
late indicator of graft deterioration, and the elevated serum enzymes, e.g.
amylase, are nonspecific indicators of rejection. If rejection is a
possibility, a cystoscopic-guided transduodenal pancreatic biopsy is ultimately
the preferred technique for a definite diagnosis.
Workup of the prospective recipient: Diabetic nephropathy is proved
to be the most common cause of ESRD in the western countries. In the US
diabetic nephropathy is the etiology of ESRD in about 23% of kidney TR.
v Kidney
transplantation is generally the optimal therapy for diabetics with ESRD and is
generally preferred than commencing dialysis.
v One
of the vital factors affecting patient’s outcome is the timing of
transplantation. Patients proceeded into transplantation with no previous
history of dialysis (preemptive transplantation) usually show decreased
mortality rates as compared to those who have experienced dialysis before
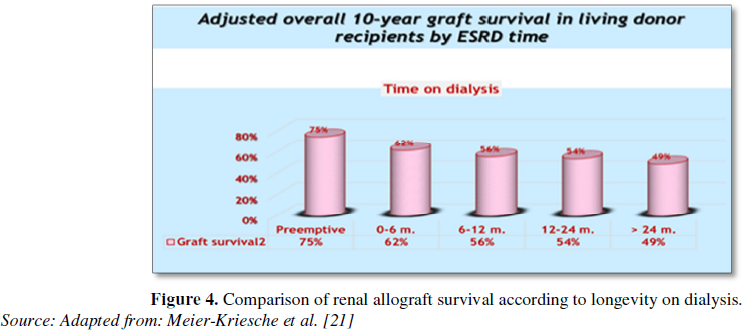
transplantation (Figure 4).
v Diabetics
with ESRD and candidate for transplantation: it is recommended that if
possible, pre-emptive kidney transplantation rather than commencing dialysis
followed by transplantation. Living-donor kidneys are generally superior to
deceased-donor kidney.
v In
view of considering pre-emptive transplant as the goal, diabetics should be
referred to the responsible transplant center when (eGFR) approached <30
mL/min. So, our patient is little late, but generally she is candidate to
proceed in pre-emptive transplantation.
v For
cardiac clearance, coronary heart disease assessment is advised and to limit
the risk of toxic effects of immunosuppression, diabetics with ESRD are better
be evaluated for the presence of underlying coronary heart disease. Optimal
approach is unclear. Accordingly, the following steps are suggested:
1. Diabetic
TR should be thoroughly evaluated as regard full detailed history, physical
examination, ECG, and chest radiography.
2. Diabetic
with symptoms and signs suspicious for coronary heart disease or myocardial
infarction should perform cardiac catheterization unless revascularization has
been previously performed.
3. If
initial screening for coronary angiography has not been performed, patient
should proceed with screening dobutamine stress echocardiography. If positive,
decision to perform angiography and possible angioplasty or surgery is usually
made in collaboration with her cardiologist.
Pre-emptive transplantation and living-donor versus deceased kidneys:
Pre-emptive (i.e., before dialysis is indicated) KTx is generally recommended
whenever possible, rather than commencing DX followed by transplantation after
dialysis (Figure 4). Robust evidence
suggests that pre-emptive KTx can result in substantial improvement in patient
survival as compared to transplantation following a period of DX [19,20].
Moreover, limited evidence also suggests that diabetics with CKD have a
survival advantage with pre-emptive transplantation [20].
Thorough analysis of 73,103
patients registered in USRDS database that include 20,000 diabetics, compared
with preemptive transplantation, there was a relative increase in
post-transplant mortality risk of 21, 28, 41, 53 and 72% among those with
waiting times of 6 to 12, 12 to 24, 24 to 36, 36 to 48 and over 48 months,
respectively [19]. Similarly, relative to pre-emptive transplantation, waiting
times of 0 to 6, 6 to 12, 12 to 24 and over 24 months conferred a 17, 37, 55
and 68% relative increase in post-transplant risk for death-censored graft
loss, respectively. The association between mortality risk/allograft loss and increased
time on DX was observed among all subgroups recognized by etiology of ESRD,
including diabetics.
The workup of the potential donor: Fortunately, this lady has a
family member, 28 years old pre-menopausal female who’s enthusiastic to donate
her one kidney. The only positive information with this lady (the donor), is
that she has hypertensive disease that is well controlled by only one
medication. However, evaluation of this donor includes:
·
The
presence of past history of hypertension:
This mandates thorough evaluation and detailed questionnaire. The
hypertensive donor on two or more medications is generally excluded [20]. But
if she is controlled by one medication, she may continue in the process of
donor workup only with absence of any evidence of end organ damage. Of note, a
2007 survey, 41% of centers may consider donors with well-controlled HT on one
medication and only 8% will consider donors on two medications [22]. For donors
on one antihypertensive drug, the following
conditions should be guaranteed:
1. Absence
of microalbuminuria.
2. Absence
of obesity and dyslipidemia.
3. Cardiac
clearance with absent left ventricular hypertrophy (LVH).
4. Clearance
of ophthalmologic changes characteristic of HT in funduscopic examination.
Control of blood pressure (BP)
should be documented at least in the last six months prior to the start of evaluation
with availability of strict follow up of her BP after donation. Considering
that evolution of HT and CKD in African Americans as well as Hispanics
ethnicities post-donation [23], hypertensive disease may show poorer outcomes
in these ethnicities, so that exclusion of large percentage of this population
should be expected.
There is universal agreement the
(2017 KDIGO guidelines) that the definition of normal blood pressure is
confined by the local guidelines related to the general population in region/country
where the donation is arranged [24]. Diagnosis of hypertension, however, is
mainly established by the OPD measurement of BP and strict follow up. In case
of reluctant diagnosis about HT diagnosis (variable readings or high normal),
further evaluation can be accomplished through ambulatory blood pressure
monitoring (ABPM) or through repetition of the standard measuring [24].
According to the OPTN policy
(The Organ Procurement and Transplantation Network), there is general agreement
that uncontrolled hypertension, or the presence of HT that is associated with
an evidence of end-organ damage (mentioned above), is considered an absolute
contraindication to living kidney donation [25]. Our donor here, in this
scenario has her BP controlled with only one medication, so it is mandatory to
clear her target end-organs from any HT detrimental complications. The
criterion of acceptance of a donor with controlled HT, however, is not
universal. One series in 2005 showed that 47% and 41% of kidney centers in the
US have excluded donors taking any antihypertensive drug or treated with more
than one drug, respectively [26].
For donors, at least two BP
readings on two separate occasions should be performed [27]. If she has any
elevated BP reading she must sent for 24 h ABPM to rule out white coat
hypertension or to confirm the abnormal finding.
Kidney function: OPTN policy requires estimation of kidney
function, either by measured GFR or a 24 h Cr Cl (creatinine clearance) [25].
KDIGO recommends the use of eGFR from serum creatinine concentration (S. Cr)
for initial assessment, which should be followed by confirmation with one or
more additional estimations: measured GFR, 24 h Cr Cl, eGFR from the
combination of S. Cr. and cystatin C (eGFRcr-cys) or repeat eGFR [24]. The last
option (i.e., repeat eGFR) is the least preferred one. However, utilizing eGFR
alone is not recognized by OPTN policy, but screening with eGFR confirmed by 24
h Cr Cl or measured GFR can be efficacious and more policy compliant.
Identification of renal anatomy: accepting donor with anatomical
aberrations is now considered only a relative CI by most transplant centers.
Renal imaging prior to nephrectomy can be performed through US, DSA, CT and
MRA. With final evaluation, all donors should have a full-detailed assessment
of vascular/ureteric anatomy, usually CT or MRI testing.
Informing the risk: An honest and deep discussion with the donor
with clear explanation by the transplant team in regard to the potential risks
of donation as well as her health status if she had pregnancy with a solitary
kidney and if she currently had children or not [28].
Post-transplantation care and follow up
Several complications involving
kidney TR may occur in all diabetics that include: allograft rejection,
increased risk of infection and malignancy. We will focus here in this scenario
on some of these issues related to diabetics after SPK. Further complications
affecting all diabetics, e.g. gastroparesis, autonomic neuropathy, peripheral
neuropathy, and foot ulcers, are discussed elsewhere.
Allograft rejection: The actual incidence of allograft rejection in
diabetic TR has not been well evaluated. In small series, risk of acute
allograft rejection was reported to be similar among diabetic and non-diabetic
TR [29,30]. However, Alemtuzumab and Tacrolimus based steroid free regimes have
been reported to have very low early rejection rates [16-18].
Malignancies: Despite paucity of data about the real incidence of
malignancies, it appears to be similar in TR with and without diabetic disease
[31]. However, some series reported an increased incidence of malignancies in
TR receiving SKP as compared to kidney transplant alone. It is not clear
whether this may be related to the increased immunosuppressive burden among
such cohort of recipients.
Viral infection: Viral infections in diabetics post-transplant are
discussed elsewhere.
Urinary tract infection: Although the use of post-transplant
prophylactic antibiotics is widely applied, UTI still represents a common
complication among TR. However, the incidence of post-transplant UTI is
reported to be more common in diabetics as compared to non-diabetics [32,33].
This may be attributed in part to the high incidence of neurogenic bladders
among diabetic TR. Prophylactic therapy among diabetic TR is appeared to be
similar to that in non-diabetic recipients. Among TR with or without diabetes,
it is recommended to cover with an antimicrobial agent to guard against UTI.
Recurrent diabetic nephropathy: Most diabetic TR may develop
histological changes of diabetic nephropathy recurrence that appear in some
recipients within one-year post-transplant. However, diabetic nephropathy is
rarely complicated by graft failure [34]. Disease recurrence in the allograft
can be theoretically prevented through optimizing glycemic control.
Interestingly, one single randomized trial of type 1 diabetic recipient
reported that intensive insulin therapy at time of transplant was associated
with less pathological alterations related to diabetic nephropathy on 5-years
follow up kidney biopsies. Recurrent diabetic nephropathy, however, can be
prohibited through successful kidney-pancreas transplant.
Glycemic control: The optimum glycemic control may be hampered
immediately post-transplant, partly due to insulin resistance as well as due to
diminished insulin secretion induced by steroids therapy in addition to the
effect of other immunosuppressive medications. The importance of glycemic
control on TR outcomes has been elucidated in a study of type I DM TR who
underwent SKP or living-donor kidney between 1983 and 2012 [35]. In a median
follow-up of about 8 years, the adjusted hazard ratio (HR) for CVS
disease-related death in SPK compared with living-donor kidney was 0.63. This
outcome has been exaggerated in those with a functioning SPK transplant.
CONCLUSION
With the development of
devastating diabetic complications, management of which should take the first
priority. With a pancreatic graft life span approaching 14 years, this lady
would get many benefits with SKP transplant. However, if early deceased donor
transplantation is not available due to, for example, a very long waiting list,
a preemptive living related donor transplant will be a reasonable therapeutic
option, unless there was a clear contraindication. PAK and Islets after KTx are
alternative options, but pancreas alone or Islets alone would be inadvisable in
this case due to its associated sensitization which would make future kidney
transplantation difficult.
1.
Redfield RR, Scalea JR, Odorico JS (2015) Simultaneous
pancreas and kidney transplantation: current trends and future directions Curr
Opin Organ Transplant 20: 94-102.
2.
Robertson RP, Davis C, Larsen J, Stratta R, Sutherland DE, et al. (2006) Pancreas and islet transplantation
in type 1 diabetes. Diabetes Care 29: 935.
3.
https://www.guysandstthomas.nhs.uk/resources/patient-information/kidney/having-a-simultaneous-pancreas-kidney-(spk)-transplant.pdf
4.
Kandaswamy R, Stock PG, Gustafson SK, Skeans MA, Curry MA, et al. (2018) OPTN/SRTR 2016 Annual Data Report: Pancreas. Am J
Transplant 18: 114-171.
5.
Dean PG, Kukla A, Stegall MD, Kudva YC (2017) Pancreas
transplantation. BMJ 357:
j1321.
6.
Gruessner AC, Gruessner RW (2016) Pancreas
Transplantation of US and Non-US Cases from 2005 to 2014 as Reported to the
United Network for Organ Sharing (UNOS) and the International Pancreas
Transplant Registry (IPTR). Rev Diabet Stud 13: 35.
7.
Gruessner RW, Sutherland DE, Gruessner AC (2004)
Mortality assessment for pancreas transplants. Am J Transplant 4: 2018.
8.
Axelrod DA, Sung RS, Meyer KH, Wolfe RA, Kaufman DB (2010) Systematic evaluation of pancreas allograft
quality, outcomes and geographic variation in utilization. Am J Transplant 10:
837-845.
9.
https://optn.transplant.hrsa.gov/media/1116/03_pa_graft_failure_definition.pdf
10.
Rickels MR, Stock PG, de Koning EJP, Piemonti L,
Pratschke J, et al. (2018)
Defining outcomes for β-cell replacement therapy in the treatment of diabetes:
A consensus report on the Igls criteria from the IPITA/EPITA Opinion Leaders
Workshop. Transplantation 31: 343-352.
11.
Hernández D, Rufino M, Bartolomei S, González-Rinne A,
Lorenzo V, et al. (2005)
Clinical impact of preexisting vascular calcifications on mortality after renal
transplantation. Kidney Int 67: 2015-2020.
12.
London GM (2003) Cardiovascular calcifications in uremic
patients: Clinical impact on cardiovascular function. J Am Soc Nephrol 14: S305-S309.
13.
Moe SM, O'Neill KD, Resterova M, Fineberg N, Persohn S, et
al. (2004) Natural history of vascular calcification in dialysis and transplant
patients. Nephrol Dial Transplant 19: 2387-2393.
14.
Kandaswamy R, Stock PG, Gustafson SK, Skeans MA, Curry
MA, et al. (2018) OPTN/SRTR 2016 Annual Data Report: Pancreas. Am J Transplant 18: 114-171.
15.
Pancreas Transplantation Committee (2018) Proposal for
the definition of pancreas graft failure. Organ procurement and transplantation
network. Available at https://optn.transplant.hrsa.gov/governance/public-comment/proposal-for-the-definition-of-pancreas-graft-failure/
16.
Uemura T, Ramprasad V, Matsushima K, Shike H, Valania T, et al. (2011) Single dose
of alemtuzumab induction with steroid-free maintenance immunosuppression in
pancreas transplantation. Transplantation 92: 678-685.
17.
Burke GW 3rd, Kaufman DB, Millis JM, Gaber AO, Johnson CP, et al. (2004)
Prospective, randomized trial of the effect of antibody induction in
simultaneous pancreas and kidney transplantation: Three-year results.
Transplantation 77: 1269-1275.
18.
Kaufman DB, Burke GW III, Bruce DS, Gaber AO, Johnson CP, et al.
(2003) Prospective, randomized, multi-center trial of antibody induction therapy
in simultaneous pancreas-kidney transplantation. Am J Transplant 3: 855-864.
19.
Meier-Kriesche HU, Port FK, Ojo AO, Rudich SM, Hanson JA,
et al. (2000) Effect of waiting time on renal transplant outcome. Kidney Int
58: 1311-1317.
20.
Gill JS, Tonelli M, Johnson N, Pereira BJ (2004) Why do
preemptive kidney transplant recipients have an allograft survival advantage?
Transplantation 78: 873-879.
21.
Meier-Kriesche HU, Kaplan B (2002) Waiting time on
dialysis as the strongest modifiable risk factor for renal transplant outcomes:
A paired donor kidney analysis. Transplantation 74: 1377-1381.
22.
Mandelbrot DA, Pavlakis M, Danovitch GM, Johnson SR, Karp SJ, et al. (2007) The medical evaluation of living
kidney donors: A survey of US transplant centers. Am J Transplantat 7: 2333-2343.
23.
Lentine KL, Schnitzler MA, Xiao H, Saab G, Salvalaggio PR, et al. (2010) Racial
variation in medical outcomes among living kidney donors. N Engl J Med 363: 724-732.
24.
Lentine KL, Kasiske BL, Levey AS, Adams PL, Alberú J, et
al. (2017) KDIGO Clinical Practice Guideline on the evaluation and care of
living kidney donors. Transplantation 101: S1-S109.
25.
OPTN (Organ Procurement and Transplantation Network)/UNOS
(United Network for Organ Sharing) (2014) OPTN Policies, Policy 14: Living Donation. Available at: http://optn.transplant.hrsa.gov/ContentDocuments/OPTN_Policies
26.
Mandelbrot DA, Pavlakis M, Danovitch GM, Johnson SR, Karp
SJ, et al. (2007)
The medical evaluation of living kidney donors: A survey of US transplant
centers. Am J Transplant 7: 2333-2343.
27.
AST/ASTS/NATCO/UNOS Joint Societies Work Group (2015)
Evaluation of the living kidney donor - A consensus document from the
AST/ASTS/NATCO/UNOS Joint Societies Work Group (2011). Available at: http://optn.transplant.hrsa.gov/PublicComments/pubcommentPropSurveyExhibit_38.pdf
28.
https://bts.org.uk/wp-content/uploads/2018/01/BTS_LDKT_UK_m
Guide-lines_2018.pdf
29.
Schiel R, Heinrich S, Steiner T, Ott U, Stein G (2005) Post-transplant
diabetes mellitus: Risk factors, frequency of transplant rejections and
long-term prognosis. Clin Exp Nephrol 9: 164-169.
30.
Schiel R, Heinrich S, Steiner T, Ott U, Stein G (2005) Long-term prognosis of
patients after kidney transplantation: A comparison of those with or without
diabetes mellitus. Nephrol Dial Transplant 20: 611-617.
31.
Bastos M, Baptista C, Campos MV, Alves R, Freitas L, et al. (2003) Kidney
transplantation and diabetes: Post-transplantation
malignancy. Transplant Proc 35: 1098-1099.
32.
Valera B, Gentil MA, Cabello V, Fijo J, Cordero E, et al. (2006)
Epidemiology of urinary infections in renal transplant recipients. Transplant
Proc 38: 2414-2415.
33.
Alangaden GJ, Thyagarajan R, Gruber SA, Morawski K,
Garnick J, et al. (2006) Infectious complications after kidney transplantation:
Current epidemiology and associated risk factors. Clin Transplant 20: 401-409.
34.
Siddqi N, Hariharan S, Danovitch G (2005) Evaluation and
preparation of renal transplant candidates. In: Handbook of Kidney
Transplantation. 4th Edn. Lippincott Williams & Wilkins, Philadelphia.
35.
Lindahl JP, Hartmann A, Aakhus S, Endresen K, Midtvedt K,
et al. (2016) Long-term cardiovascular outcomes in type 1 diabetic patients
after simultaneous pancreas and kidney transplantation compared with living
donor kidney transplantation. Diabetologia 59: 844-852.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Alcoholism Clinical Research
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- Dermatology Clinics and Research (ISSN:2380-5609)
- Oncology Clinics and Research (ISSN: 2643-055X)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Journal of Clinical Trials and Research (ISSN:2637-7373)