1308
Views & Citations308
Likes & Shares
Post-transplant lymphocele has a reported incidence of 0.6-34%. While numerous surgical and non-surgical risk factors have been described, the incidence remains fairly constant among different transplant settings. The majorities of such lymphoceles are asymptomatic and are incidental findings on routine imaging of the allograft. Large volume lymphoceles and those in relation to the graft hilum may exert pressure effects causing potential graft dysfunction. Local symptoms may develop if venous or lymphatic outflow of gonads or lower limb is impinged. Such symptomatic lymphoceles require definitive treatment. This review looks at the risk factors for lymphocele formation along with different management options and their outcomes.
Keywords: Renal transplant, Lymphocele, Lymph leak, Fluid collections, Laparoscopic fenestration
INTRODUCTION
A lymphocele is an abnormal collection of lymphatic fluid that lacks an epithelialized cover, usually occurring at a site of extensive surgical dissection. In renal transplantation, a lymphocele may occur adjacent to the graft, due to multiple factors including damage to host retroperitoneal lymphatics as well as donor lymphatics accompanying the allograft. A peri-graft lymphocele is well-recognised morbidity following renal transplantation and can manifest in a broad spectrum of clinical presentations. This can range from indolent collections detected merely as incidental findings on or those that cause graft dysfunction, vascular compromise or sepsis.
PATHOPHYSIOLOGY AND RISK FACTORS
Several other factors surrounding the recipient and donor operation have also been recognized as potential risk factors for the incidence of lymphocele. Laparoscopic donor nephrectomy has been implicated with a higher incidence of lymphocele compared to open nephrectomy. Saidi et al. [6] reported a significantly higher incidence of lymphocele with laparoscopic live donor nephrectomy compared to deceased donor transplants. Mazzucchi et al. [7] reported that donor kidneys with complex arterial anatomy carried a higher risk of lymphocele (12.5%) compared to grafts with single renal artery (3.1%).
Sansalone et al. [8] demonstrated that the ipsilateral placement of the kidney and implantation to the common iliac vessels compared to contralateral iliac fossa placement and implantation to external iliac vessels was associated with a lower incidence of lymphocele (2.1% vs. 8.5%). The authors postulated that there is higher lymphatic disruption associated with dissection around the external iliac compared to common iliac vessels. However, other studies that compared the incidence of lymphocele based on different surgical approaches, the degree of iliac dissection and level of surgeons’ experience failed to show any significant difference [9,10]. Minimal disruption of lymphatics is the key.
Non-surgical risk factors
Multiple non-surgical factors have also been implicated as possible risk factors in the formation of lymphocele. Ulrich [11] studied the potential non-surgical risk factors for lymphocele in over 420 transplants performed over five years. Use of tacrolimus, the incidence of acute rejection and diabetes in the recipient were all found to be significant in univariate analysis. However, during the multivariate analysis, only diabetes in the recipient proved to be an independent risk factor.
Adult polycystic kidney disease in the recipient has also been described as a potential risk factor [12]. The possible explanation has been the external pressure on the inferior vena cava by the polycystic kidney resulting in impaired lymphatic drainage from the allograft and iliac region. Obesity in the donor (BMI>30) has also been described as an independent risk factor for the occurrence of lymphocele [13].
The correlation between the incidence of lymphocele and the use of different immunosuppressive agents is controversial. Goel et al. [13] demonstrated a significantly higher incidence of lymphocele with the use of sirolimus, mycophenolate and prednisolone combination. Furthermore, Langer [14] also demonstrated that the use of sirolimus was an independent risk factor for lymphocele formation. However, a subsequent study by Tondolo et al. [15] failed to show any correlation based on different immunosuppressive agents including sirolimus. Benavides [16] described the higher incidence of lymphocele with the use of rabbit anti-thymocyte globulin induction.
Lundin et al. [17] demonstrated a significantly higher incidence of lymphocele with the use of low molecular weight heparin after transplantation. The authors postulated that the increased anticoagulant effect impaired sealing of damaged lymphatics resulting in higher incidence of lymphocele. This is difficult to accept because the suggestion that anti-coagulation would affect patency of opened up lymphatics, does not sound a logical argument. Other risk factors implicated with lymphoceles in different studies include: increased recipient age, increased warm ischaemia time [18], acute tubular necrosis and delayed graft function [19], prolonged pre-transplant dialysis [20] and re-transplantation [21].
Goel et al. [13], Khauli [19] and many authors have independently described the acute rejection of the graft as an independent risk factor for the incidence of lymphocele. Veeramani et al. [22] also demonstrated that patients with symptomatic lymphoceles had a significantly higher incidence of acute rejection compared to those who had no lymphoceles (51% vs. 20%). The intense inflammatory process during an episode of acute rejection is involved with increased lymphangiogenesis and lymph flow, possibly explaining its association with lymphocele.
CLINICAL PRESENTATION
The vast majority of lymphoceles are asymptomatic and are detected as an incidental finding on imaging. Depending on the size, extent and location in relation to the allograft, lymphoceles may exert pressure effects causing symptomatic presentation. Pressure on the hilar vessels can lead to impaired graft function and may even lead to catastrophic renal artery or vein thrombosis in rare instances where it goes undetected. Pressure on the ureter may lead to hydroureter or hydronephrosis of the graft. Pressure on the recipient iliac vein or compression of lymph drainage may lead to unilateral limb oedema, scrotal or vulval oedema and deep vein thrombosis of the iliac veins.
Large lymphoceles may cause abdominal discomfort, pain, urgency (due to bladder compression) and backache (sacral nerve compression). Association with wound dehiscence can lead to sepsis or lympho-cutaneous fistula. The latter presentation requires careful assessment to differentiate from urinary leakage and needs prompt intervention to prevent septic complications.
DIAGNOSIS
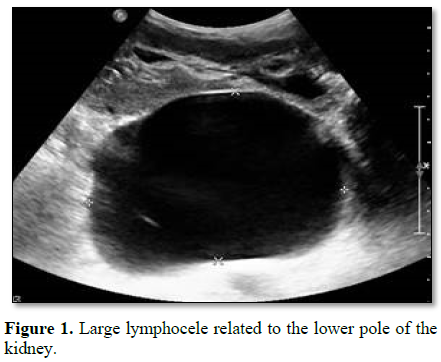
The primary modality in diagnosis is imaging. USS can determine the collection as well as its dimensions, location in relation to the graft and possible effects on the graft vessels and ureter (Figure 1). USS guided aspiration, and biochemical analysis of contained fluid allows differentiation from urinary leak and urinoma. The biochemical analysis should be done for creatinine, electrolytes, protein content, gram stain and culture. Comparison with simultaneous samples taken from serum and urine for creatinine and electrolytes often become invaluable in differentiating from urinoma.
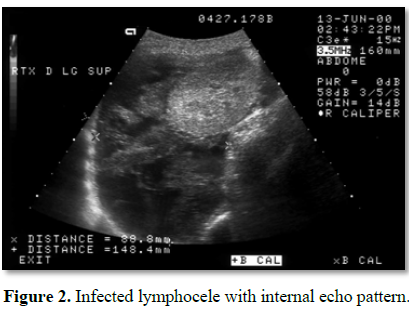
USS appearance can also indicate the possible presence of infection within the collection. Complex echo pattern with internal debris within the collection is more indicative of complicated infected lymphocele (Figure 2) [23]. An uncomplicated lymphocele appears hypoechoic or anechoic compared to the hyper-echoic appearance of an infected lymphocele. Further imaging with computerised tomography can also assist in differentiating innocuous lymphoceles from infected ones and other collections such as hematomas.
Once a lymphocele has been confirmed by the above tests, some authors have recommended further testing to evaluate the origin of the lymph; donor or recipient. Pacovsky et al. [24] described distinct difference in the creatine kinase (CK) levels of the fluid based on its source, with recipient origin lymph demonstrating higher levels of CK. However, the clinical utility of this analysis from a management perspective remains unproven.
TREATMENT
The vast majority of asymptomatic lymphoceles are self-limiting and do not require specific treatment. Once they are detected, further testing is done to establish any pressure effects on the vasculature or ureter. In the absence of any demonstrable pressure effects or evidence of infection, such lymphoceles can be safely left alone with periodic imaging surveillance. Notably, small lymphoceles located cephalad to the graft, away from the vasculature and ureter are unlikely to cause pressure effects and rarely need intervention. The decision to intervene depends on definitive pressure effects causing symptoms, graft dysfunction, evidence of sepsis or fistula formation. The reported incidence of lymphoceles requiring definitive intervention varies between 0.04-14.6% [4,5].
Intra-operative drain placement
The placement of retroperitoneal drains adjacent to the graft at the time of transplantation is a practice performed by many surgeons. These are usually removed once the drainage becomes negligible or before hospital discharge. Some studies have shown that drains placed intra-operatively decrease the incidence of lymphocele [25]. However, other authors have reported contrary outcomes where drain placement showed no benefit in reducing post-transplant lymphocele [26]. Hence, this practice remains an individual choice of the surgeon based on individual practice and patient characteristics.
Percutaneous aspiration and sclerotherapy
Symptomatic lymphoceles can be aspirated under USS guidance and remain the safest mode of intervention where needed. It also allows for sampling of the collection to establish its true nature and rule out infection. Percutaneous aspiration can, at the best, is a diagnostic step that helps in differentiation from urinoma. Seroma may not appear again, if aspiration is really indicated. However, lymphocele would not be treated just by aspiration. Some clinicians recommend placement of a percutaneous drain to minimise re-accumulation, but external drainage always get infected. Furthermore, some studies have documented the advantage of performing percutaneous drainage followed by sclerotherapy to sclerose open lymphatics, but it is mentioned here only for condemnation.
A systematic review by Lucewicz et al. [4] looking at over 20 studies, reported that simple aspiration alone was associated with a recurrence rate between 10-95% (mean 59%), compared to 50% with percutaneous drain placement. The eventual success rate also depends on the size and volume of lymphoceles. Krol et al. [25] demonstrated that lymphoceles with a volume >140 ml were symptomatic and those >500 ml were unlikely to resolve with percutaneous aspiration, sclerotherapy or drain placement.
Different sclerosing agents have been described in various studies with varying degrees of success. These include; povidone iodine, fibrin glue, 95% ethanol, fibrinogen, sodium tetradecyl sulphate and tetracycline [4,27,28]. The sclerosing agent has been instilled and kept in situ for varying periods ranging from 5 min to 24 h. The review by Lucewicz et al. [4] reported the recurrence rate after sclerotherapy was 31% over 14 studies. Placement of a percutaneous drain allows for continuous drainage as well as repeated instilling of sclerosants if needed. However, the chief drawbacks of repeated installation of sclerosants are the risk of introducing infection. Furthermore, several case reports have reported direct graft injury and graft loss as a result of sclerosant installation [27,29]. At the cost of repetition, it is worthwhile emphasizing that external drainage or sclersing therapy are not correct options.
Laparoscopic fenestration
Large lymphoceles can be opened into the peritoneal cavity by making fenestrations in the lymphocele capsule. This allows for lymph to be internally drained to the peritoneal cavity whereby the peritoneal lymphatics would drain it into thoracic duct. In patients who are fit to undergo general anesthesia, this can be performed by laparoscopic fenestration. However, the presence of infection in the lymphocele needs to be carefully excluded before this procedure. Laparoscopic fenestration has shown high rates of success with a minimal rate of recurrence (4-8%) [30,31]. It carries lower procedure-related morbidity, and reduced overall hospital stay compared to open surgical drainage. In the review by Lucewicz et al. [4], (total of 322 patients), 26 patients (12%) required conversion to open drainage. The indications for conversion included technical difficulty in reaching the lymphocele, peritoneal adhesions, thick, impenetrable lymphocele capsule and injury to abdominal viscus.
Open surgery
Open surgical drainage of lymphocele is required in the presence of infection (external drainage) or where laparoscopic fenestration is not possible (internal drainage to the peritoneum). In this era of laparoscopy, open drainage is only of historical importance. Lymphoceles located in relation to the lower pole of the graft or complex lymphoceles causing vascular compromise is best treated by open de-roofing. However, open drainage carries a significantly higher risk of ureteric damage and needs to be performed with the utmost care in order to minimise additional morbidity. The reported recurrence rate following open surgical drainage to the peritoneal cavity is 16% [3].
CONCLUSION
Peri-graft lymphocele is fairly common morbidity following renal transplantation. However, the vast majority of these remains asymptomatic and is self-limiting. Nevertheless, close surveillance is required to rule out pressure effects on the graft and possible secondary infection. Multiple risk factors have been described which helps in identifying patients at a higher risk of lymphocele. Symptomatic or complicated lymphoceles require prompt intervention with minimal morbidity to the graft as well as the patient. Small volume collections, only if there is clinical indication, may be treated with percutaneous techniques to allow resolution. Recurrent collections or large volume lymphoceles are best treated by laparoscopic fenestration into the peritoneal cavity. Open surgical de-roofing is of historical importance.
1.
Ebadzadeh MR, Tavakkoli M (2008) Lymphocele after kidney transplantation: Where are we
standing now? Urol J 5: 144-148.
2.
Ziȩtek Z, Sulikowski T, Tejchman K, Sieńko J, Janeczek M,
et al. (2007) Lymphocele after
kidney transplantation. Transplant Proc 39: 2744-2747.
3.
Allen RDM
(2014) Vascular and lymphatic complications after kidney transplantation. In:
Peter J Morris, Stuart J Knechtle, editors. Kidney Transplantation - Principles and Practice. 7th Edn. Content Repository Only! pp:
435-463.
4.
Lucewicz A, Wong G, Lam VWT, Hawthorne WJ, Allen R, et
al. (2011) Management of primary
symptomatic lymphocele after kidney transplantation: A systematic review.
Transplantation 92: 663-673.
5.
Minetti EE
(2011) Lymphocele after renal transplantation, a medical complication. J Nephrol
24: 707-716.
6.
Saidi RF, Wertheim JA, Ko DSC, Elias N, Martin H, et al. (2008) Impact of donor kidney recovery method on
lymphatic complications in kidney transplantation. Transplant Proc 40: 1054-1055.
7.
Mazzucchi E, Souza AA, Nahas WC, Antonopoulos IM,
Piovesan AC, et al. (2005) Surgical complications after renal
transplantation in grafts with multiple arteries. Int Braz J Urol 31: 125-130.
8.
Sansalone CV, Aseni P, Minetti E, Di Benedetto F,
Rossetti O, et al. (2000) Is lymphocele
in renal transplantation an avoidable complication? Am J Surg 179: 182-185.
9.
Hamza A, Fischer K, Koch E, Wicht A, Zacharias M, et al. (2006) Diagnostics and therapy of lymphoceles after
kidney transplantation. Transplant Proc 38: 701-706.
10.
Cash H, Slowinski T, Buechler A, Grimm A, Friedersdorff
F, et al. (2012) Impact of
surgeon experience on complication rates and functional outcomes of 484
deceased donor renal transplants: A single-centre retrospective study. BJU Int 110: E368-373.
11.
Ulrich F, Niedzwiecki S, Fikatas P, Nebrig M, Schmidt SC,
et al. (2010) Symptomatic
lymphoceles after kidney transplantation - Multivariate analysis of risk
factors and outcome after laparoscopic fenestration. Clin Transplant 24: 273-280.
12.
Martínez-Ocaña JC, Lauzurica R, Castellote E, Bonet J,
Tenesa M, et al. (1995) Adult polycystic kidney disease: A risk factor for
lymphocele formation after renal transplantation? Transplant Proc 27: 2246-2247.
13.
Goel M, Flechner SM, Zhou L, Mastroianni B, Savas K, et
al. (2004) The influence of
various maintenance immunosuppressive drugs on lymphocele formation and
treatment after kidney transplantation. J Urol 171: 1788-1792.
14.
Langer RM, Kahan BD (2002) Incidence, therapy and consequences of lymphocele after
sirolimus-cyclosporine-prednisone immunosuppression in renal transplant
recipients. Transplantation
74: 804-808.
15.
Tondolo V, Citterio F, Massa A, Salerno MP, Romagnoli J,
et al. (2006) Lymphocele after
renal transplantation: The influence of the immunosuppressive therapy. Transplant
Proc 38: 1051-1052.
16.
Benavides C, Mahmoud KH, Knight R, Barcenas C, Kahan BD, et al. (2005) Rabbit anti-thymocyte globulin: A postoperative risk factor
for sirolimus-treated renal transplant patients? Transplant Proc 37: 822-826.
17.
Lundin C, Bersztel A, Wahlberg J, Wadström J (2002) Low molecular weight heparin prophylaxis
increases the incidence of lymphocele after kidney transplantation. Ups J Med
Sci 107: 9-15.
18.
Zagdoun E, Ficheux M, Lobbedez T, Chatelet V,
Thuillier-Lecouf A, et al. (2010)
Complicated
lymphoceles after kidney transplantation. Transplant Proc 42: 4322-4325.
19.
Khauli RB, Stoff JS, Lovewell T, Ghavamian R, Baker S (1994) Post-transplant lymphoceles: A critical look
into the risk factors, pathophysiology and management. J Urol 150: 22-26.
20.
Mokos I, Basic-Jukic N, Kastelan Z, Kes P, Pasini J (2010) Influence of long-term dialysis treatment on
operative complications after renal transplantation. Transplant Proc 42: 2531-2533.
21.
Stephanian E, Matas AJ, Gores P, Sutherland DE, Najarian
JS (1992) Retransplantation as a
risk factor for lymphocele formation. Transplantation 53: 676-678.
22.
Desai M, Veeramani M, Mishra S, Kurien A, Ganpule A, et al. (2010)
Does
rejection has a role in lymphocele formation post renal transplantation? A
single centre experience. Indian J Urol 26: 193.
23.
Moreno CC, Mittal PK, Ghonge NP, Bhargava P, Heller MT (2016) Imaging complications of renal transplantation.
Radiol Clin North Am 54: 235-249.
24.
Pacovsky J, Hyspler R, Navratil P, Ticha A, Brodak M (2010) The estimation of post-transplant lymphocele
origin using creatine kinase activity. Ups J Med Sci 115: 187-192.
25.
Król R, Kolonko A, Chudek J, Ziaja J, Pawlicki J, et al. (2007) Did volume of lymphocele after kidney
transplantation determine the choice of treatment modality? Transplant Proc 39: 2740-2743.
26.
Cimen S, Guler S, Tennankore K, Imamoglu A, Alwayn I (2016) Surgical drains do not decrease complication
rates but are associated with a reduced need for imaging after kidney
transplant surgery. Ann Transplant 21: 216-221.
27.
Tasar M, Gulec B, Saglam M, Yavuz I, Bozlar U, et al.
(2005) Post-transplant symptomatic
lymphocele treatment with percutaneous drainage and ethanol sclerosis. Clin
Imaging 29: 109-116.
28.
Shaver TR, Swanson SJ, Fernandez-Bueno C, Kocandrle V (1993) The optimal treatment of lymphoceles following
renal transplantation. Transpl Int 6: 108-110.
29.
Manfro RC, Comerlato L, Berdichevski RH, Ribeiro AR,
Denicol NT, et al. (2002) Nephrotoxic
acute renal failure in a renal transplant patient with recurrent lymphocele
treated with povidone-iodine irrigation. Am J Kidney Dis 40: 655-657.
30.
Fuller TF, Kang SM,
Hirose R, Feng S, Stock PG, et al. (2003) Management of lymphoceles
after renal transplantation: Laparoscopic versus open drainage. J Urol 169: 2022-2025.
31.
Iwan-Ziętek I, Ziętek Z, Sulikowski T, Nowacki M, Zair L,
et al. (2009) Minimally invasive
methods for the treatment of lymphocele after kidney transplantation. Transplant
Proc 41: 3073-3076.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Stem Cell Research and Therapeutics (ISSN:2474-4646)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- International Journal of AIDS (ISSN: 2644-3023)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Journal of Clinical Trials and Research (ISSN:2637-7373)