1080
Views & Citations80
Likes & Shares
Background: Autophagy has a paradoxical role in cancer. Autophagy-related 16-like 1 (ATG16L1) and the light chain 3 B (LC3B) are proteins that form an essential part of the membranes of the autophagosomes. To date, the significance of ATG16L and LC3B expression in OSCC has not been fully clarified.
Aim: Aim of the current study was to investigate ATG16L and LC3B tissue protein expression levels in tissues which were taken from OSCC patients then correlate our results with pathological and clinical patients’ data.
Methods: ATG16L and LC3B expression was assessed in 40 patients with OSCC using immunohistochemistry. We followed the patients for 5 years for progression and recurrence of the disease and for survival.
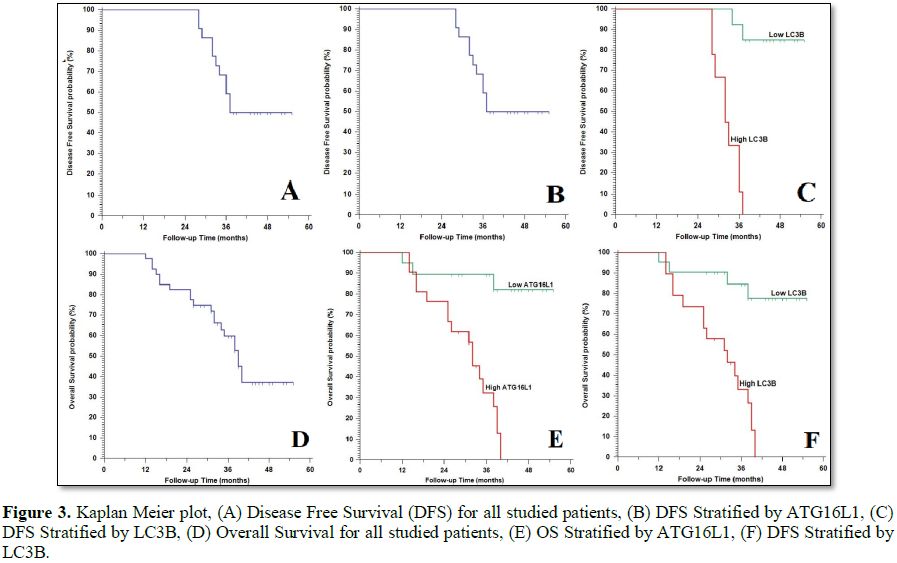
Results: High ATG16L1 and LC3B expression was correlated with higher grade (p=0.021 and 0.049, respectively), advanced stage of the tumor (p=0.003 and 0.005, respectively), presence of lymph node metastases (p=0.007 and 0.033, respectively), distant metastases (p=0.045 and 0.040, respectively), poor response to therapy (p=0.018 and 0.050, respectively), higher incidence of tumor recurrence, worse 5 year DFS and OS rates (p<0.001).
Conclusion: ATG16L and LC3B overexpression are markers of poor prognosis in OSCC patients.
Keywords: ATG16L, LC3B, OSCC, Autophagy, Immunohistochemistry, Prognosis
INTRODUCTION
Therefore, we aimed in the current study to investigate ATG16L and LC3B tissue protein expression levels in tissues which were taken from OSCC patients then correlate our results with pathological and clinical patient’s data.
PATIENTS AND METHOD
The current prospective cohort study is a study where we have included sections from formalin fixed paraffin embedded 40 tissue blocks that collected from samples of 40 cases of OSCC that have been surgically operated in General Surgery Hospitals, Oncology unit, Faculty of Medicine, Zagazig University. Specimens were sent to Pathology department Faculty of Medicine, Zagazig University for further processing, accurate diagnosis, grading and staging. We followed our patients for 5 years till death or their most recent medical examination for response to therapy, progression, and recurrence of the disease after successful therapy and survival of patients in Clinical Oncology and Nuclear Medicine Department and in Medical Oncology Department, Faculty of Medicine, Zagazig University for 5 years in the period between December 2013 to December 2018. We include all cases of OSCC and the World Health Organization classification was used for pathologic grading. The study complied with the guidelines of the Local Ethics Committee of Faculty of Medicine, Zagazig University.
Immunohistochemical analysis
Formalin-fixed paraffin-embedded blocks from malignant tissues were cut into 4 μm sections, deparaffinized and rehydrated in xylene and graded alcohols, respectively. Antigen retrieval was done by heating in a microwave in citric acid buffer; we incubated the sections with 3% H2O2 for half an hour at room temperature so as to antagonize endogenous peroxidase activity. We have incubated all sections with normal serum for half an hour then incubated them with the primary antibodies; rabbit polyclonal anti-ATG16L1 antibody and anti-LC3B (Novus Biologicals, CO, Littleton) at a dilution of 1:250 for 30 min at room temperature.
Evaluation of the ATG16L and LC3B cytoplasmic immunoreactivity in OSCC tissues
Immunoreactivity score (IRS), was obtained by multiplying the scores of stain intensity and scores of stain extent. Regarding stain intensity, negative cytoplasmic staining was scored as 0, weakly positive as 1, moderately positive as 2 and strongly positive as 3. Regarding extent score, negative stains were scored as 0, 1-10%, as 1, 11-50% as 2 and as 3>50%. We assigned the final IRS as ranges from 0 to 9, with 0-4 were defined as being low expression and 6-9 as high expression. Cervical adenocarcinoma cells and breast cancer cells were used as positive control for ATG16L and LC3B, respectively. Negative control was obtained by replacing the primary antibody with non-immune serum [9,10].
All slides were evaluated independently by 2 senior pathologists who were blinded to the patients’ clinical information and follow-up data.
STATISTICAL ANALYSIS
The categorical variables were compared through using Fisher's exact test or Pearson’s Chi-square test when was appropriate. Trend of change between ordinal data were compared using Chi-square test for trend. Strength of relationship between IHC of ATG16L1 and LC3B were determined by computing phi coefficient with (+) sign was indicator for direct relationship and (-) sign was indication for inverse relationship. Overall Survival (OS) was calculated as the time from diagnosis to death or the most recent follow-up. Disease Free Survival (DFS) was calculated as the time from starting treatment to date of relapse or the most recent follow-up contact that patient was known as relapse free. Stratification of OS and DFS was done according to ATG16L1 and LC3B. These time-to-event distributions were estimated using the method of Kaplan-Meier plot. A p-value
RESULTS
Patient characteristics
40 samples collected from OSCC patients. The clinical characteristics of the 40 patients with OSCC that are included in the study are present in Table 1.
Immunohistochemical results
ATG16L, LC3B expression are positively correlated with each other phi coefficient r= +0.905 (p<0.001).
Follow-up and survival results
After a median follow-up period of 38.79 months with range (33.98-43.61) months, OS rates were 97.5%, 82.5%, 59% and 37% in the 1st, 2nd, 3rd and 4th years, respectively. 16 (61.5%) patients showed response to therapy, 13 (54.2%) of patients showed recurrence of the disease.
DISCUSSION
OSCC is the most serious cancer which leads to marked disfigurement and it is considered a serious health problem, particularly in the developing countries. OSCC pathogenesis is complex and had many factors for its pathogenesis. Previous reports have pointed to that autophagy dysregulation could alter the metabolic process of the cells and it has significant consequences which is related to plethora of human cancer, including OSCC [8,11], the role of autophagy in cancer progression is found as there is altered expression of many ATGs has been reported in many human malignancies [8]. The dysregulation of different autophagy related proteins have been found to correlate with clinico-pathological parameters and cancer patients outcomes. Such findings highlighted the vital roles of autophagy in carcinogenesis. Despite numerous studies which assessed the associations between autophagy and cancer progression; the role of autophagy in cancer remains controversial as it has cancer inhibiting and stimulating properties [1]. As cancer initiating agent, autophagy gives amino acids as an alternating source of energy for proliferation of the malignant cells and it provides cancer cells resistance toward radiotherapy and chemotherapy [12].
In the current study we tried to clarify the role of expression of 2 proteins which have association with autophagy in OSCC; ATG16L and LC3B.
We have noticed that ATG16L1 is considered one of the least clarified autophagy related proteins in human oncogenesis. ATG16L1 is a part of a large protein complex which is essential for autophagy [13]. We have proved that overexpression of ATG16L1 was significantly associated with advanced stage and higher grade of the tumor. Moreover, we found that ATG16L1 was increased in OSCCs with the presence of lymph node metastasis. Regarding the Kaplan-Meier plots analysis showed that patients with ATG16L1 overexpression had a poor OS and DFS. All these results were similar to Tang et al. [8], who proved similar results in OSCC and CHEN et al. [13], in osteosarcoma.
Moreover, the results of our current study are in line with previous results proved by Nomura et al. [14] who proved that increased ATG16L1 expression was associated with presence of lymph node metastasis in patients with OSCC. Tang et al. [8] study clarified the role of overexpression of ATG16L1 in OSCC pathogenesis in the survival and recurrence of patients of OSCC. Our present study and results of previous studies provided additional evidences of dysregulation of autophagy in OSCC which suggested that overexpression of ATG16L1 have prognostic significance in OSCC and raised the benefit of considering autophagy in the targeted therapy against OSCC. Previous studies have demonstrated that autophagy is mostly activated as a protective mechanism in cancer cells against several chemotherapeutics [8], which explain our results regarding the association between autophagy activation and OSCC progression. Autophagy is responsible for degradation of intracellular components so as to regenerate metabolites for energy and growth by the lysosomal machinery [15]. But, the molecular mechanisms which are underlying autophagy-mediated resistance to chemotherapy in cancer cells remain unknown [13].
Chen et al. [13] have stated that miR-410 could enhance the chemosensitivity by inhibition of autophagy through targeting ATG16L1 in osteosarcoma cells which provided an insight into a promising approach for future osteosarcoma treatment.
As the results of previous studies regarding prognostic role of ATG16L1 in OSCC we assessed the expression of another autophagy related marker which is LC3B that participates in elongation of autophagosome membrane formation, when it is activated it strongly binds to the membranes of the pre-autophagosomes and autolysosomes membranes [12,16] and we have proved that LC3B overexpression in OSCC was markedly linked to advanced disease stage, higher incidence of metastases of lymph node, and higher histological grade. Moreover, Kaplan- Meier plots showed that LC3B overexpression was related to poor patients’ outcome in terms of shorter OS and DFS rates in OSCC patients.
LC3B is an autophagosome marker which was demonstrated to be an effective prognostic marker in many cancers including oral SCC [1,11,17]. Increased expression of LC3B is positively related to progression and poor prognosis of many cancers [18,19].
Liu et al. [17], proved that increased LC3B tissue expression was related to poor DFS in patients complaining with oral SCC which is similar to ours, but, a more definitive assessment of LC3B prognostic role in cancer could lead to establishment of its expression with the autophagy activity of cancer cells and thus assess the benefits of considering autophagy as a management strategy for OSCC patients.
Similarly, high LC3B expression is related to poor OS and DFS rates in locally advanced cancer breast and in TNBC [20,21]. In astrocytoma, marked increase of LC3B expression is related to dismal outcome and poor OS rate [22], additionally, in hepatocellular carcinoma and esophageal carcinoma high LC3B expression is related to advanced TNM stages, presence of vascular invasion, lymph node metastasis and poor OS rate [23,24]. In prostate adenocarcinoma, increased LC3B expression is associated with a high Gleason scores [25]. The present study showed that increased autophagy related proteins expression, which denotes activated autophagy, is correlated with dismal outcome of OSCC, but it was previously found that autophagy can be involved in either cancer promotion or inhibition. The importance of assessment of the role of autophagy in OSCC progression is that, when autophagy is considered in the therapeutic management strategies for these tumors.
The inhibition of the autophagy process by therapeutic specific inhibitors and RNA related interference of genes which are related to autophagy might lead to enhancement of malignant cells chemo-sensitivity and photosensitivity [12]. Recent clinical trials which investigated the values of using inhibitors of autophagy in combination with the currently used immunotherapies, targeted therapies and chemotherapeutic agents in malignant tumors have been used since 2010 and give encouraging primary results [7].
Moreover, LC3 gene deficiency could enhance the HCC cells sensitivity to Epirubicin [26]. Additionally, 3-Methyladenine (3-MA), that, inhibits autophagy by preventing formation of autophagosome through inhibition of phosphatidylinositol 3-kinase (PI3K), might enhance the cytotoxicity of many chemotherapy agents like; Cisplatin, Tamoxifen, 5-fluorouracil (5-FU), Camptothecin and Trastuzumab [8,9]. Radiotherapy (RT) could lead to induction of autophagy in cancer cells which has a huge role in radio-resistance. Suppression of autophagy by using CQ and/or RNAi could elevate the radio-sensitivity and chemo-radio-sensitivity in malignant cell lines [29,30]. Although the therapeutic significance of autophagy inhibition in response to RT is yet to be functionally tested on cells of OSCC, the activity of autophagy has been reported to be increased in irradiated OSCC cells [23]. The current study proved that autophagy is associated with progression of OSCC; it will be better that more recent studies should assess the benefits of inhibition of autophagy in OSCC cell lines in response to chemotherapy and radiotherapy which could help to classify other patient into subgroups for variable therapeutic approaches [31,32].
CONCLUSION
1. Lai K, Matthews S, Wilmott JS, Killingsworth MC, Yong JL, et al. (2018) Differences in LC3B expression and prognostic implications in oropharyngeal and oral cavity squamous cell carcinoma patients. BMC Cancer 18: 624.
2. Moro JDS, Maroneze MC, Ardenghi TM, Barin LM, Danesi CC (2018) Oral and oropharyngeal cancer: Epidemiology and survival analysis. Einstein (São Paulo) 16: eAO4248.
3. Lu J, Ma H, Lian SH, Huang D, Lian M, et al. (2017) Clinical significance and prognostic value of the expression of LAMP3 in oral squamous cell carcinoma. Dis Markers 2017: 1218254.
4. Langer R, Neppl CH, Keller MD, Schmid RA, Tschan MP, et al. (2018) Expression analysis of autophagy related markers LC3B, p62 and HMGB1 indicate an autophagy-independent negative prognostic impact of high p62 expression in pulmonary squamous cell carcinomas. Cancers (Basel) 10: E281.
5. Aqbi HF, Tyutyunyk-Massey L, Keim RC, Butler SE, Thekkudan T, et al. (2018) Autophagy-deficient breast cancer shows early tumor recurrence and escape from dormancy. Oncotarget 9: 22113-22122.
6. Sun Y, Chen Y, Zhang J, Cao L, He M, et al. (2017) TMEM74 promotes tumor cell survival by inducing autophagy via interactions with ATG16L1 and ATG9A. Cell Death Dis 8: e3031.
7. Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, et al. (2011) Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res 17: 654-666.
8. Tang JY, Hsi E, Huang YC, Hsu NC, Yang WC, et al. (2014) Overexpression of autophagy-related 16-like 1 in patients with oral squamous cell carcinoma. Pathol Oncol Res 21: 301-305.
9. Lee YJ, Hah YJ, Kang YN, Kang KJ, Hwang JS, et al. (2013) The autophagy-related marker LC3 can predict prognosis in human hepatocellular carcinoma. PLoS One 8: e81540.
10. Kimmelman AC (2011) The dynamic nature of autophagy in cancer. Genes Dev 25:1999-2010.
11. Tang JY, Hsi E, Huang YC, Hsu NC, Chu PY, et al. (2013) High LC3 expression correlates with poor survival in patients with oral squamous cell carcinoma. Hum Pathol 44: 2558-2562.
12. Lai K, Killingsworth MC, Lee CS (2014) The significance of autophagy in colorectal cancer pathogenesis and implications for therapy. J Clin Pathol 67: 854-858.
13. Chen R, Li X, He B, Hu W (2017) MicroRNA-410 regulates autophagy-related gene ATG16L1 expression and enhances chemosensitivity via autophagy inhibition in osteosarcoma. Mol Med Rep 15: 1326-1334.
14. Nomura H, Uzawa K, Yamano Y, Fushimi K, Ishigami T, et al. (2009) Overexpression and altered subcellular localization of autophagy-related 16-like 1 in human oral squamous-cell carcinoma: Correlation with lymphovascular invasion and lymph-node metastasis. Hum Pathol 40: 83-91.
15. Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132: 27-42.
16. Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, et al. (2010) LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J 29:1792-1802.
17. Liu JL, Chen FF, Lung J, Lo CH, Lee FH, et al. (2014) Prognostic significance of p62/SQSTM1 subcellular localization and LC3B in oral squamous cell carcinoma. Br J Cancer 111: 944-954.
18. Chen HI, Tsai HP, Chen YT, Tsao SC, Chai CY (2016) Autophagy and apoptosis play opposing roles in overall survival of esophageal squamous cell carcinoma. Pathol Oncol Res 22: 699-705.
19. Liao W, Sun L, Wang C, Huang H, Liu J, et al. (2014) LC3A-positive “stone-like” structures predict an adverse prognosis of gastric cancer. Anat Rec (Hoboken) 297: 653-662.
20. Chen S, Jiang YZ, Huang L, Zhou RJ, Yu KD, et al. (2013) The residual tumor autophagy marker LC3B serves as a prognostic marker in local advanced breast cancer after neoadjuvant chemotherapy. Clin Cancer Res 19: 6853-6862.
21. Zhao H, Yang M, Zhao J, Wang J, Zhang Y, et al. (2013) High expression of LC3B is associated with progression and poor outcome in triple-negative breast cancer. Med Oncol 30: 475.
22. Winardi D, Tsai HP, Chai CY, Chung CL, Loh JK, et al. (2014) Correlation of altered expression of the autophagy marker LC3B with poor prognosis in astrocytoma. Biomed Res Int 2014: 723176.
23. Wu DH, Jia CC, Chen J, Lin ZX, Ruan DY, et al. (2014) Autophagic LC3B overexpression correlates with malignant progression and predicts a poor prognosis in hepatocellular carcinoma. Tumor Biol 35: 12225-12233.
24. El-Mashed S, O'Donovan TR, Kay EW, Abdallah AR, Cathcart MC, et al. (2015) LC3B globular structures correlate with survival in esophageal adenocarcinoma. BMC Cancer 15: 582.
25. Giatromanolaki A, Koukourakis MI, Harris AL, Polychronidis A, Gatter KC, et al. (2010) Prognostic relevance of light chain 3 (LC3A) autophagy patterns in colorectal adenocarcinomas. J Clin Pathol 63: 867-872.
26. Peng W, Du T, Zhang Z, Du F, Jin J, et al. (2015) Knockdown of autophagy-related gene LC3 enhances the sensitivity of HepG2 cells to epirubicin. Exp Ther Med 9: 1271-1276.
27. Claerhout S, Verschooten L, Van Kelst S, De Vos R, Proby C, et al. (2010) Concomitant inhibition of AKT and autophagy is required for efficient cisplatin-induced apoptosis of metastatic skin carcinoma. Int J Cancer 127: 2790-2803.
28. Liu D, Yang Y, Liu Q, Wang J (2011) Inhibition of autophagy by 3-MA potentiates cisplatin-induced apoptosis in esophageal squamous cell carcinoma cells. Med Oncol 28: 105-111.
29. He G, Wang Y, Pang X, Zhang B (2014) Inhibition of autophagy induced by TSA sensitizes colon cancer cell to radiation. Tumor Biol 35: 1003-1011.
30. Schonewolf CA, Mehta M, Schiff D, Wu H, Haffty BG, et al. (2014) Autophagy inhibition by chloroquine sensitizes HT-29 colorectal cancer cells to concurrent chemoradiation. World J Gastrointest Oncol 6: 74-82.
31. Spector ME, Gallagher KK, Light E, Ibrahim M, Chanowski EJ, et al. (2012) Matted nodes: poor prognostic marker in oropharyngeal squamous cell carcinoma independent of HPV and EGFR status. Head Neck 34: 1727-1733.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Journal of Alcoholism Clinical Research
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- Journal of Spine Diseases
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- Ophthalmology Clinics and Research (ISSN:2638-115X)